-
PDF
- Split View
-
Views
-
Cite
Cite
Yochun Jung, Byoung Hee Ahn, Kyo Seon Lee, In Seok Jeong, Kye Hun Kim, Kook Joo Na, Sang-Wan Ryu, Sang Gi Oh, A novel solution to prosthetic valve dehiscence after aortic valve surgery in Behçet’s disease, Interactive CardioVascular and Thoracic Surgery, Volume 24, Issue 3, March 2017, Pages 342–347, https://doi.org/10.1093/icvts/ivw361
Close - Share Icon Share
Abstract
OBJECTIVES: Prosthetic valve dehiscence after aortic valve surgery in Behçet’s disease patients is common. We aimed to validate the usefulness of our new technique ‘subannular endomyocardial implantation of valve prosthesis’ designed to prevent prosthetic valve dehiscence.
METHODS: Subannular endomyocardial implantation of valve prosthesis involves suturing the sewing cuff of the valve prosthesis in the endomyocardium below the aortic annulus, which is based on the idea that annular tissue should be excluded from the suture line in Behçet’s disease patients. Medical records of 7 patients in whom the new technique was performed between 2002 and 2014 were reviewed.
RESULTS: Five men and two women were included (median age, 44 years). Aortic root replacement was performed in 6 cases, and aortic valve replacement in 1. No operative mortality occurred. Postoperatively, complete atrioventricular block developed in 3 cases, and permanent pacemakers were implanted in 2. No reoperation was performed for prosthetic valve dehiscence during the median 7.8-year follow-up. One late death occurred due to sudden cardiac arrest 8.4 years after surgery. One additional permanent pacemaker was implanted for complete atrioventricular block, which developed at 4.2 years postoperatively. The last echocardiography (median, 6.7 years after surgery) revealed no paravalvular leakages.
CONCLUSIONS: Subannular endomyocardial implantation of valve prosthesis seems useful for preventing prosthetic valve dehiscence after aortic valve surgery for Behçet’s disease. It poses a risk of complete atrioventricular block, but considering the high reoperation rate and mortality due to prosthetic valve dehiscence after conventional aortic valve surgery, this risk seems reasonable.
INTRODUCTION
Surgical treatment of aortic regurgitation (AR) in Behçet’s disease (BD) patients causes a high risk of postoperative morbidity due to prosthetic valve dehiscence because of the fragility of the aortic annulus caused by tissue inflammation [1]. The incidence of prosthetic valve dehiscence after aortic valve surgery in BD patients is 40.0–78.9% [2, 3]. To reduce this risk, several methods such as using homografts [4, 5]; reinforcing the suture line with Teflon felt, normal cusps [6], or subannular ring [7]; and performing modified valve replacement [8] have been suggested. However, there is no widely accepted solution.
We experienced the first clinical case of aortic valve surgery in a BD patient in 1998. Revision surgery was performed three times during a 2.5-year period due to repeated prosthetic valve dehiscence after conventional aortic valve replacement (AVR). After this challenging experience, we developed a new surgical technique, subannular endomyocardial implantation of valve prosthesis (SEIV), based on the assumption that completely excluding annular tissue from the suture line is the only way to avoid annular inflammation due to BD and the resulting prosthetic valve dehiscence.
After confirming a favourable outcome following the second case of aortic valve surgery in a BD patient, for whom SEIV was performed initially using a stentless bioprosthetic valve in March 2002, we have performed SEIV as the treatment of choice for AR in all BD patients. The present study aimed to validate the usefulness of SEIV by analyzing the mid-term outcomes of aortic valve surgery by SEIV in BD patients.
MATERIALS AND METHODS
Patients
Among 584 cases of aortic valve surgery performed at our hospital between January 2002 and December 2014, the medical records of 7 consecutive patients who underwent SEIV were reviewed. All seven procedures were performed by one surgeon (BH Ahn). Five men and 2 women were included (median age, 44 years [range, 21–58 years]). Four patients underwent aortic valve surgery by SEIV as the first operation following preoperative BD diagnosis. One patient (Case 3) underwent AVR initially for infective endocarditis, but the diagnosis was changed to BD postoperatively according to pathological and clinical findings. He began steroid treatment on postoperative day 3, but finally underwent revision surgery with SEIV due to prosthetic valve dehiscence at 12 months postoperatively. The other 2 patients (Cases 1 and 5) who underwent conventional AVR at our hospital had not been suspected to have BD. However, after identifying severe prosthetic valve dehiscence, which occurred within 1 year postoperatively, they underwent revision surgery by SEIV due to suspicion of BD. The second surgical findings and pathological results confirmed BD in these 2 patients (Table 1). BD was diagnosed based on clinical, echocardiographic and pathological findings. In this study, an experienced cardiologist (K.H. Kim) reviewed the preoperative echocardiography images and summarized the characteristic findings of BD. This study was approved by Chonnam National University Hospital’s Institutional Review Board, with patient consent waived (IRB no. CNUH-2015-214).
| Case . | Sex/age . | Timing of diagnosis of BD . | Diagnosis before 1st op . | Previous op (prosthesis) . | Op by SEIV (prosthesis) . | Interval (months) . | Medication before SEIV . |
|---|---|---|---|---|---|---|---|
| 1 | M/52 | Before 2nd op | AR, MR, IE | AVR (Me), MAP | ARR (St) | 11 | |
| 2 | M/58 | 1 month before op | AR | ARR (St) | Steroid, colchicine | ||
| 3 | M/21 | After 1st op | AR, IE | AVR (Me) | ARR (St) | 12 | Steroid |
| 4 | F/41 | 3 years before op | AR, MR, VSA | ARR (St) | Methotrexate | ||
| 5 | M/44 | Before 2nd op | AR | AVR (Me) | ARR (MC) | 11 | |
| 6 | M/52 | 12 years before op | AR, VSA | ARR (MC) | |||
| 7 | F/41 | 20 years before op | AR | AVR (Me) | Steroid, colchicine, dapsone |
| Case . | Sex/age . | Timing of diagnosis of BD . | Diagnosis before 1st op . | Previous op (prosthesis) . | Op by SEIV (prosthesis) . | Interval (months) . | Medication before SEIV . |
|---|---|---|---|---|---|---|---|
| 1 | M/52 | Before 2nd op | AR, MR, IE | AVR (Me), MAP | ARR (St) | 11 | |
| 2 | M/58 | 1 month before op | AR | ARR (St) | Steroid, colchicine | ||
| 3 | M/21 | After 1st op | AR, IE | AVR (Me) | ARR (St) | 12 | Steroid |
| 4 | F/41 | 3 years before op | AR, MR, VSA | ARR (St) | Methotrexate | ||
| 5 | M/44 | Before 2nd op | AR | AVR (Me) | ARR (MC) | 11 | |
| 6 | M/52 | 12 years before op | AR, VSA | ARR (MC) | |||
| 7 | F/41 | 20 years before op | AR | AVR (Me) | Steroid, colchicine, dapsone |
AR: aortic regurgitation; ARR: aortic root replacement; AVR: aortic valve replacement; BD: Behçet’s disease; F: female; IE: infective endocarditis; M: male; MC: mechanical valved conduit; Me: mechanical valve; MR: mitral regurgitation; Op: operation; SEIV: subannular endomyocardial implantation of valve prosthesis; St: stentless bioprosthetic valve; VSA: Valsalva sinus aneurysm.
| Case . | Sex/age . | Timing of diagnosis of BD . | Diagnosis before 1st op . | Previous op (prosthesis) . | Op by SEIV (prosthesis) . | Interval (months) . | Medication before SEIV . |
|---|---|---|---|---|---|---|---|
| 1 | M/52 | Before 2nd op | AR, MR, IE | AVR (Me), MAP | ARR (St) | 11 | |
| 2 | M/58 | 1 month before op | AR | ARR (St) | Steroid, colchicine | ||
| 3 | M/21 | After 1st op | AR, IE | AVR (Me) | ARR (St) | 12 | Steroid |
| 4 | F/41 | 3 years before op | AR, MR, VSA | ARR (St) | Methotrexate | ||
| 5 | M/44 | Before 2nd op | AR | AVR (Me) | ARR (MC) | 11 | |
| 6 | M/52 | 12 years before op | AR, VSA | ARR (MC) | |||
| 7 | F/41 | 20 years before op | AR | AVR (Me) | Steroid, colchicine, dapsone |
| Case . | Sex/age . | Timing of diagnosis of BD . | Diagnosis before 1st op . | Previous op (prosthesis) . | Op by SEIV (prosthesis) . | Interval (months) . | Medication before SEIV . |
|---|---|---|---|---|---|---|---|
| 1 | M/52 | Before 2nd op | AR, MR, IE | AVR (Me), MAP | ARR (St) | 11 | |
| 2 | M/58 | 1 month before op | AR | ARR (St) | Steroid, colchicine | ||
| 3 | M/21 | After 1st op | AR, IE | AVR (Me) | ARR (St) | 12 | Steroid |
| 4 | F/41 | 3 years before op | AR, MR, VSA | ARR (St) | Methotrexate | ||
| 5 | M/44 | Before 2nd op | AR | AVR (Me) | ARR (MC) | 11 | |
| 6 | M/52 | 12 years before op | AR, VSA | ARR (MC) | |||
| 7 | F/41 | 20 years before op | AR | AVR (Me) | Steroid, colchicine, dapsone |
AR: aortic regurgitation; ARR: aortic root replacement; AVR: aortic valve replacement; BD: Behçet’s disease; F: female; IE: infective endocarditis; M: male; MC: mechanical valved conduit; Me: mechanical valve; MR: mitral regurgitation; Op: operation; SEIV: subannular endomyocardial implantation of valve prosthesis; St: stentless bioprosthetic valve; VSA: Valsalva sinus aneurysm.
Operative technique
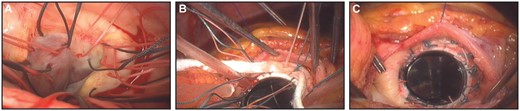
Aortic valve replacement by SEIV. (A) Subannular multiple interrupted sutures were in place. (B and C) After the Teflon felt strip was interposed between the sewing ring and the endomyocardium, suture threads were tied.
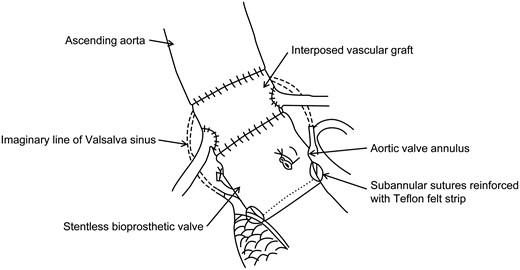
Schematic drawing of aortic root replacement with stentless bioprothetic valve by SEIV.
Statistical analysis
All numeric variables are presented as medians with ranges. Differences between the preoperative and final echocardiographic parameters were analyzed using the Wilcoxon signed-rank test. A P value < 0.05 was considered statistically significant. Statistical analysis was performed using IBM SPSS, version 19.0 (IBM Corp., Armonk, NY).
RESULTS
Preoperative status
All patients experienced exertional dyspnoea preoperatively; 4 patients were categorized as a New York Heart Association (NYHA) functional class II, 2 as III and 1 as IV. The BD-related clinical findings are summarized in Table 2. Four patients underwent SEIV during immunosuppressive therapy, 3 of whom received steroids (Table 1); in cases where secondary adrenal insufficiency could occur due to the interruption of steroid treatment, we maintained steroid treatment throughout the hospital stay. In other cases, steroid treatment was used only when necessary to control the BD symptoms during the perioperative period; we did not use steroids to control the ‘disease activity’, which is believed to be reflected by the level of erythrocyte sedimentation rate (ESR) or C-reactive protein (CRP). The median values of preoperative ESR and CRP levels were 21.5 mm/h (range, 8.0–46.0 mm/h) and 0.97 mg/dl (range, 0.03–2.90 mg/dl), respectively. Preoperative electrocardiography (ECG) revealed normal sinus rhythm in all cases. Severe AR was observed on echocardiography performed before SEIV in all patients. Two cases had concomitant aneurysmal dilatation of a single Valsalva sinus, and 1 had concomitant moderate mitral regurgitation (Table 1). Near-total dehiscence of the prosthetic valve was observed in all 3 cases who had undergone AVR previously. The median values of preoperative ejection fraction (EF), left ventricular end-diastolic dimension (LVEDD) and left ventricular end-systolic dimension (LVESD) were 54.0% (range, 29.1–74.0%), 68.0 mm (range, 56.9–77.5 mm) and 49.0 mm (range, 31.9–62.9 mm), respectively. Three cases showed left ventricular dysfunction of EF <45% and distention of LVEDD ≥ 68 mm. All echocardiographic images obtained before the first operations were reviewed again for this study; coaptation failure due to cusp abnormalities was observed in all cases, and echocardiographic-free space was observed in 2. The frequency of each abnormal echocardiographic finding is summarized in Table 3.
| Case . | Recurrent oral ulcer . | Genital ulcer . | Eye lesion . | Skin lesion . | Pathergy test . | Others . |
|---|---|---|---|---|---|---|
| 1 | Yes | No | No | No | NR | Gastritis |
| 2 | Yes | Yes | No | Yes | Yes | Laryngitis, gastritis |
| 3 | Yes | Yes | No | Yes | NR | |
| 4 | Yes | Yes | No | Yes | NR | Arthritis |
| 5 | Yes | No | No | No | No | |
| 6 | Yes | Yes | No | Yes | NR | |
| 7 | Yes | Yes | Yes | Yes | Yes | Arthritis, gastritis |
| Case . | Recurrent oral ulcer . | Genital ulcer . | Eye lesion . | Skin lesion . | Pathergy test . | Others . |
|---|---|---|---|---|---|---|
| 1 | Yes | No | No | No | NR | Gastritis |
| 2 | Yes | Yes | No | Yes | Yes | Laryngitis, gastritis |
| 3 | Yes | Yes | No | Yes | NR | |
| 4 | Yes | Yes | No | Yes | NR | Arthritis |
| 5 | Yes | No | No | No | No | |
| 6 | Yes | Yes | No | Yes | NR | |
| 7 | Yes | Yes | Yes | Yes | Yes | Arthritis, gastritis |
NR: no record.
| Case . | Recurrent oral ulcer . | Genital ulcer . | Eye lesion . | Skin lesion . | Pathergy test . | Others . |
|---|---|---|---|---|---|---|
| 1 | Yes | No | No | No | NR | Gastritis |
| 2 | Yes | Yes | No | Yes | Yes | Laryngitis, gastritis |
| 3 | Yes | Yes | No | Yes | NR | |
| 4 | Yes | Yes | No | Yes | NR | Arthritis |
| 5 | Yes | No | No | No | No | |
| 6 | Yes | Yes | No | Yes | NR | |
| 7 | Yes | Yes | Yes | Yes | Yes | Arthritis, gastritis |
| Case . | Recurrent oral ulcer . | Genital ulcer . | Eye lesion . | Skin lesion . | Pathergy test . | Others . |
|---|---|---|---|---|---|---|
| 1 | Yes | No | No | No | NR | Gastritis |
| 2 | Yes | Yes | No | Yes | Yes | Laryngitis, gastritis |
| 3 | Yes | Yes | No | Yes | NR | |
| 4 | Yes | Yes | No | Yes | NR | Arthritis |
| 5 | Yes | No | No | No | No | |
| 6 | Yes | Yes | No | Yes | NR | |
| 7 | Yes | Yes | Yes | Yes | Yes | Arthritis, gastritis |
NR: no record.
| Characteristic finding . | Number of cases (%) . |
|---|---|
| Cusp perforation | 5 (71.4) |
| Redundant motion of the elongated cusp | 4 (57.1) |
| Motion limitation of the thickened cusp | 5 (71.4) |
| Echocardiographic-free space | 2 (28.6) |
| Aneurysmal change of a single Valsalva sinus | 2 (28.6) |
| Characteristic finding . | Number of cases (%) . |
|---|---|
| Cusp perforation | 5 (71.4) |
| Redundant motion of the elongated cusp | 4 (57.1) |
| Motion limitation of the thickened cusp | 5 (71.4) |
| Echocardiographic-free space | 2 (28.6) |
| Aneurysmal change of a single Valsalva sinus | 2 (28.6) |
| Characteristic finding . | Number of cases (%) . |
|---|---|
| Cusp perforation | 5 (71.4) |
| Redundant motion of the elongated cusp | 4 (57.1) |
| Motion limitation of the thickened cusp | 5 (71.4) |
| Echocardiographic-free space | 2 (28.6) |
| Aneurysmal change of a single Valsalva sinus | 2 (28.6) |
| Characteristic finding . | Number of cases (%) . |
|---|---|
| Cusp perforation | 5 (71.4) |
| Redundant motion of the elongated cusp | 4 (57.1) |
| Motion limitation of the thickened cusp | 5 (71.4) |
| Echocardiographic-free space | 2 (28.6) |
| Aneurysmal change of a single Valsalva sinus | 2 (28.6) |
Early outcome
ARR was performed in the first 6 patients, among whom a stentless bioprosthetic valve was used in 4 and a valved conduit composed of a mechanical valve and a vascular graft was used in 2. AVR using a mechanical valve was performed in the seventh patient. The diameters of the prosthesis were 21 mm in 1, 23 mm in 3, 25 mm in 2 and 27 mm in 1. Vascular graft interposition was required for anastomosis of the coronary button(s) in 3 of 4 cases in whom a stentless bioprosthetic valve was used. In 3 cases, surgery was completed with ventricular pacing due to persistent complete atrioventricular block (CAVB) after weaning of CPB. The median values of the CPB and aortic cross-clamp (ACC) time were 374 min (range, 209–444 min) and 236 min (range, 170–348 min), respectively. There was no operative mortality but 1 patient underwent reoperation for bleeding. Among 3 patients in whom CAVB had occurred in the operating room, CAVB resolved spontaneously and ventricular pacing was discontinued on postoperative day 4 in 1 patient (Case 3); ECG at discharge showed sinus rhythm with right bundle branch block (RBBB). In the other 2 patients (Cases 1 and 6), a permanent pacemaker was inserted on postoperative days 41 and 62, respectively. Of the 4 cases in whom sinus rhythm was maintained postoperatively, left bundle branch block (LBBB) was observed in 1 case and RBBB in another. Two cerebrovascular complications occurred postoperatively. One experienced a generalized tonic–clonic seizure during the immediate postoperative period and left leg weakness thereafter. Brain magnetic resonance imaging (MRI) showed an acute embolic infarction in the right frontal lobe. In another case, delayed left haemiparesis occurred on postoperative day 11; brain MRI revealed no acute infarction, but stenosis of the left and right middle cerebral arteries was present, presumably associated with BD. One recurrent sternal wound infection occurred. The median durations of the intensive care unit and hospital stay were 132 h (range, 38–1320 h) and 30 days (range, 12–117 days), respectively.
Long-term outcome
No cases were lost to follow-up and the median postoperative follow-up was 7.8 years (range, 1.4–8.5 years). One patient exhibited exertional dyspnoea of NYHA functional class II, but the others were categorized as NYHA functional class I. No residual disability was observed at the last visit in the 2 patients who had experienced a cerebrovascular complication postoperatively. We had no fixed protocol for postoperative steroid treatment for BD, so treatment was personalized. Steroid treatment was maintained from the immediate postoperative period to the last outpatient visit in 2 cases. In the other three cases, steroid treatment was initiated to control postoperative BD symptoms, which was discontinued and resumed repeatedly according to changes in symptoms; at the last outpatient visit, a steroid was given to all three patients. One patient began steroid treatment for gout arthritis 16.6 months postoperatively, which was maintained for 6.3 months and then discontinued. No steroid was used postoperatively in the last case. Median values of the last ESR and CRP level were 42 mm/h (range, 10–61 mm/h) and 0.41 mg/dl (range, 0.16–2.69 mg/dl), respectively.
During follow-up, one late death, one delayed CAVB and one bleeding complication associated with anticoagulation occurred. The deceased patient (Case 1) had preoperative severe left ventricular dysfunction (EF 29.1%) and dilatation (LVEDD 68.0 mm, LVESD 54.5 mm). Postoperatively, he experienced CAVB and intermittent ventricular arrhythmia; a permanent pacemaker was inserted on postoperative day 41 and discharge was delayed until postoperative day 115. After discharge, although exertional dyspnoea of NYHA functional class II persisted, the permanent pacemaker functioned well and ventricular arrhythmia did not occur until his last outpatient visit. Echocardiography performed at 8.0 years (i.e. 4 months before death) postoperatively showed well-functioning prosthetic valve with no paravalvular leakage, but exacerbated left ventricular dysfunction (EF 26.9%) and distention (LVEDD 86.3 mm, LVESD 75.0 mm). He was admitted after cardiopulmonary resuscitation for sudden cardiac arrest at 8.4 years postoperatively and died of hypoxic brain injury and multiorgan failure. In a case of delayed CAVB (Case 2), sinus rhythm with RBBB persisted postoperatively. ECG performed for palpitation and dizziness at 4.2 years postoperatively showed CAVB; thus, a permanent pacemaker was inserted. In another patient (Case 5), exploratory laparotomy was performed for postoperative haemoperitoneum, which developed after resuming anticoagulation therapy following laparoscopic cholecystectomy at 4.0 years after SEIV.
The last echocardiography performed at a median of 6.7 years postoperatively (range, 1.0–8.2 years) showed no paravalvular leakage in all cases, and the median value of the transvalvular mean pressure gradient was 11.5 mmHg (range, 4.7–22.8 mmHg). There was no case of severe patient–prosthesis mismatch. All cases, except for Case 1, showed EF and LVEDD within normal ranges. Moderate mitral regurgitation shown on preoperative echocardiography improved to a mild degree in Case 4, even without mitral valve surgery. Compared with the preoperative echocardiographic measurements, there was an increase in the median value of EF (58.0% [range, 26.9–64.9%]) and a decrease in the median values of LVEDD and LVESD (50.5 mm [range, 42.3–86.3 mm] and 35.1 mm [range, 27.4–75.0 mm], respectively); however, the difference did not reach statistical significance.
DISCUSSION
During a median 7.8-year follow-up, no prosthetic valve dehiscence occurred in the 7 BD patients who underwent aortic valve surgery by SEIV. These results contradict the reported outcomes of aortic valve surgery in BD patients. The fundamental concept of SEIV is that because the pathophysiology of BD involves systemic vasculitis, prosthetic valve dehiscence seems unavoidable unless the aortic annular tissue is excluded from the suture line; therefore, valve prostheses should be implanted in the endocardium of left ventricular outflow tract. Since such an idea for aortic valve surgery in BD patients has not yet been reported, it may be controversial. The first question is whether the myocardium, which is more fragile than the annulus, is appropriate for valve prosthesis implantation. We were also concerned about the possibility of cut-through of the endomyocardium at the suture line when the valve prosthesis was exposed to high pressure. However, considering that the patch covering the ventricular septal defect, which was also fixed to the endomyocardium, had no issue with enduring the systemic pressure, SEIV was attempted in the first case. Since we have accumulated surgical experience with favourable outcomes, we are confident that the endomyocardium is strong enough to hold the valve prosthesis. The second issue is the risk of postoperative CAVB. When the valve prosthesis is implanted below the annulus, suturing in the dangerous zone of the membranous septum is unavoidable. Theoretically, SEIV cannot be free from CAVB, and a permanent pacemaker was inserted in 3 of 7 (42.9%) cases. RBBB and LBBB were observed in the other 2 cases. Therefore, SEIV is not applicable in usual circumstances of aortic valve surgery. However, considering the high reoperation rate due to prosthetic valve dehiscence after conventional aortic valve surgery for BD, about 50% risk of CAVB by SEIV seems acceptable. The third issue is the possibility of increases in postoperative mitral regurgitation due to deep implantation of aortic valve prostheses, especially in cases where the aorto-mitral curtain or anterior mitral leaflet is relatively short. However, there were no such cases in this study. Final echocardiography revealed 3 cases of mitral regurgitation, all of which were in mild degree; compared with preoperative examination, mitral regurgitation had improved (Case 4) or not changed (Cases 1 and 7) after SEIV. Based on our experience, therefore, the possibility of disturbing mitral valve function by SEIV seems low. The last concern is difficulties with the technique itself. As SEIV involves suturing the endomyocardium 3–4 mm deeper than the annulus, the surgical field is narrower than in other conventional aortic valve surgeries. Additionally, in cases of ARR using a stentless bioprosthetic valve, additional vascular graft interposition is occasionally needed for naturally aligned anastomoses of the coronary buttons. The valve sutures are tied deeper than normally, which requires careful attention to avoid cut-through due to the fragility of the endomyocardium. The prolonged CPB and ACC times noted in this study seem to reflect such surgical difficulties. However, there were no difficulties in CPB weaning in any case, and it seems unreasonable to associate long CPB and ACC times directly with postoperative morbidities. Therefore, the surgical difficulties of SEIV may be overcome by carefully performing each surgical step with enough time and achieving complete myocardial protection.
After verifying the mid-term results of SEIV, we doubt the well-known opinions regarding aortic valve surgery in BD patients. Until now, clinical reports by Jeong et al. [2] and Ando et al. [3] are the two largest-scale studies on aortic valve surgery in BD patients (19 and 10 patients, respectively), and the authors suggested ARR rather than AVR to prevent later prosthetic valve dehiscence. In the early 2000s, we anticipated the positive effect of ARR along with other researchers and conducted ARR in the first 6 cases regardless of the presence or absence of a Valsalva sinus aneurysm. However, as surgical experience was accumulated, we came to believe that the prosthesis implantation technique, not the range of operation, was the main factor preventing prosthetic valve dehiscence. Thus, only AVR was performed in the last SEIV case with a satisfactory outcome. However, as most of our cases were ARR and only one was AVR, it is difficult to make a definite conclusion as to what, among the extended range of operation (ARR) and the specific procedure (SEIV), was responsible for our superior results. On one hand, ARR can also lead to reoperation due to prosthetic valve dehiscence in a significant portion of patients (20–25%), as reported by Jeong et al. [2] and Ando et al. [3]. On the other hand, prosthetic valve dehiscence can be avoided by just AVR with a specific surgical technique such as subannular ring reinforcement reported by Azuma et al. [7], which indirectly supports our belief that SEIV is essential for avoiding prosthetic valve dehiscence, and so only AVR is enough. We think future studies with more cases of AVR by SEIV will be able to validate our hypothesis.
The need for postoperative immunosuppressive therapy, including steroid treatment, should be reconsidered. Ando et al. [3] emphasized the importance of steroid treatment for suppressing inflammatory reactions, which induces prosthetic valve dehiscence. Jeong et al. stressed the effectiveness of immunosuppressive therapy by demonstrating the inverse correlation of the postoperative ESR and CRP levels with an event-free duration [2]. Among the 7 patients in our study, only two began steroid therapy immediately postoperatively and maintained it without discontinuation, whereas the other patients received steroids only when symptoms that required steroid treatment occurred. Nevertheless, no prosthetic valve dehiscence occurred in our cases, presumably because SEIV is performed in the endomyocardium, which is unrelated to the disease activity of BD. Therefore, when SEIV is performed, postoperative steroid treatment does not seem associated with the outcome of prosthetic valve dehiscence, and is not mandatory.
Song et al. [9] reported cusp elongation and redundant motion, echocardiographic-free space and mass-like lesions as characteristic echocardiographic findings in 7 BD patients, and Jeong et al. [2] reported interventricular septal dissection as an additional characteristic finding. The echocardiographic findings above, except mass-like lesions and interventricular septal dissection, were identified in this study, and unlike previous studies, cusp perforation and motion limitation of the thickened cusp were frequently observed. As the most important prerequisite for preventing unnecessary reoperation is an accurate preoperative diagnosis, such characteristic echocardiographic findings of BD are considered useful for making a differential diagnosis of causes of AR. Additional large-scale studies are needed to establish diagnostic echocardiographic findings of BD.
Significant efforts have been made to prevent prosthetic valve dehiscence after aortic valve surgery in BD patients [4–8]. Homografts are known to be advantageous for less prosthetic valve dehiscence in aortitis patients [4, 5]. However, the supply of homografts is very limited. Additionally, considering that long-term outcomes are not validated, the use of homograft in young BD patients should be reconsidered. Kotsuka et al. [6] reported good mid-term results for AVR in aortitis patients using intravalvular implantation, in whom a normal aortic valve cusp is interposed between the felt pledget and the aortic annulus. However, they did not specify aortitis. Considering that the risk of postoperative prosthetic valve dehiscence is much higher in cases of BD than Takayasu’s arteritis [3], and the aortic valve cusps in BD patients are mostly inflamed or have myxoid changes [2], it seems inappropriate to use the technique in BD patients. Okada et al. [8] reported the translocated Bentall procedure using modified composite grafts, in which the prosthetic aortic valve is fixed 1 cm inside from the end of the vascular graft. However, the surgical results were not so satisfactory (1 early mortality and 2 prosthetic valve dehiscence among the 5 cases), so the usefulness of the procedure should be validated further.
Among the previously reported methods for preventing prosthetic valve dehiscence, we found subannular ring reinforcement presented by Azuma et al. to be interesting [7]. The authors successfully conducted AVR in 3 BD patients. A polyester tube graft of the same size as the aortic annulus was placed in the subannular position for reinforcement, and the aortic annulus was sandwiched between the subannular ring and valve prosthesis. No prosthetic valve dehiscence occurred during the mean 3 ± 1.8-year follow-up. The good mid-term results may be due to the subannular position of the reinforcement ring causing the prosthetic valve to become fixated to the endomyocardium of the left ventricular outflow tract, as in SEIV. The promise of this method seems worthy of further studies with more cases over a longer follow-up period.
Limitations
The limitations of this study include its retrospective design and small number of cases. However, since cardiovascular involvement in BD is rare, these limitations are unavoidable in a single centre study. A multi-centre collaborative study is needed to clarify the usefulness of SEIV.
Conclusion
In conclusion, SEIV seems useful for preventing prosthetic valve dehiscence after aortic valve surgery in BD patients. SEIV poses a risk of CAVB, but considering the high reoperation rate due to prosthetic valve dehiscence after conventional aortic valve surgery, this risk seems reasonable.
ACKNOWLEDGEMENTS
We thank Editage (www.editage.co.kr) for English language editing.
Funding
This work was supported by the budget of Department of Thoracic and Cardiovascular Surgery, Chonnam National University Hospital and received no external funding.
Conflict of interest: none declared.
REFERENCES
- complete atrioventricular block
- heart valve prosthesis
- echocardiography
- sudden cardiac death
- medical records
- behcet syndrome
- dehiscence
- aortic valve replacement
- follow-up
- objective (goal)
- repeat surgery
- surgical procedures, operative
- sutures
- mortality
- surgery specialty
- pacemaker, permanent
- anulus fibrosus of aorta
- aortic valve surgery
- aortic root replacement
- surgical mortality
- prostheses
- catheter cuffs




