-
PDF
- Split View
-
Views
-
Cite
Cite
Enrico Ferrari, Giorgio Franciosi, Sara Clivio, Francesco Faletra, Marco Moccetti, Tiziano Moccetti, Giovanni Pedrazzini, Stefanos Demertzis, Stent valve implantation in conventional redo aortic valve surgery to prevent patient–prosthesis mismatch, Interactive CardioVascular and Thoracic Surgery, Volume 24, Issue 3, March 2017, Pages 319–323, https://doi.org/10.1093/icvts/ivw397
Close - Share Icon Share
Abstract
OBJECTIVES: The goal was to show the technical details, feasibility and clinical results of balloon-expandable stent valve implantation in the aortic position during conventional redo open-heart surgery in selected obese patients with a small aortic prosthesis and severe patient–prosthesis mismatch.
METHODS: Two symptomatic overweight patients (body mass index of 31 and 38), each with a small aortic prosthesis (a 4-year-old, 21-mm Hancock II biological valve and a 29-year-old, 23-mm Duromedic mechanical valve), increased transvalvular gradients (59/31 and 74/44 mmHg) and a reduced indexed effective orifice area (0.50 and 0.43 cm2/m2) underwent implantation of two 26-mm balloon-expandable Sapien 3 valves during standard on-pump redo valve surgery.
RESULTS: Using full re-sternotomy, cardiopulmonary bypass and cardioplegic arrest, the two balloon-expandable stent valves were implanted under direct view using a standard aortotomy, after prosthesis removal and without annulus enlargement. Aortic cross-clamp times were 162 and 126 min; cardiopulmonary bypass times were 178 and 180 min; total surgical times were 360 and 318 min. At discharge, echocardiograms showed transvalvular peak and mean gradients of 13/9 and 23/13 mmHg and indexed effective orifice areas of 0.64 and 1.08 cm2/m2. The 3-month echocardiographic follow-up showed transvalvular peak and mean gradients of 18/9 and 19/11 mmHg and indexed effective orifice areas of 0.78 cm2/m2 and 0.84 cm2/m2, with improved symptoms (New York Heart Association class 1).
CONCLUSIONS: Implantation of a balloon-expandable stent valve during redo aortic valve surgery is feasible in selected cases and prevents patient–prosthesis mismatch in obese patients without need for aortic annulus enlargement. Moreover, in the case of stent valve degeneration, this approach permits additional valve-in-valve procedures with large stent valves and prevents re-redo surgery.
Introduction
Patient–prosthesis mismatch (PPM) (defined as moderate for an indexed aortic area of 0.85–0.65 cm2/m2 and severe when the area is <0.65 cm2/m2) occurs in symptomatic patients with biological or mechanical aortic valve prostheses who have small functional indexed effective orifice areas (iEOA) and high transvalvular gradients [1–2]. Small iEOA can be due either to a malfunctioning prosthesis or pannus (mechanical valves) or to degeneration (biological valves) or can be determined by a significant increase in the patient’s weight after surgical aortic valve implantation of a small valve in a narrow aortic annulus. Some patients can have both conditions.
The treatment of choice is valve replacement during on-pump redo open-heart surgery [3–5]. However, when the patient is overweight or obese, it is necessary to implant a large valve to minimize the risk of residual PPM. In redo valve surgery, the technical strategies used to enlarge the aortic annulus (to position a larger valve) present an important challenge for the surgeon and can significantly increase the bypass and the ischemic times, which increases surgical risk [6, 7].
The choice of the new prosthesis is also debatable because a mechanical valve still carries the risk of re-redo surgery and because the valve-in-valve technique is not applicable for a mechanical prosthesis. Therefore, in selected overweight or obese patients undergoing redo aortic valve surgery for PPM, the use of biological valves without annulus enlargement and allowing for additional transcatheter valve-in-valve placement in case of early degeneration seems the best surgical option with the lower surgical impact for the patient [8–10]. But a large valve is mandatory [11].
To the best of our knowledge, the use of balloon-expandable Sapien 3 transcatheter valves (Edwards Life Sciences, Irvine, CA) in open-heart redo aortic valve surgery has never been suggested before, and it carries the advantage of not requiring annulus enlargement despite the implantation of a large valve, thereby minimizing the risk of PPM in future valve-in-valve procedures. We report our experience with 2 consecutive symptomatic obese patients with small-aortic valve prostheses and PPM treated with surgical implantation of two 26-mm Sapien 3 valves in a standard on-pump redo setting.
Methods
Patient selection
Two overweight patients (101 and 105 kg, respectively, with a body mass index of 31 and 38, respectively), aged 73 and 55 years, already operated for aortic valve replacement with biological and mechanical prostheses (a 21-mm Medtronic Hancock II porcine valve implanted in 2012, at 69 years of age) and a 23-mm Baxter Duromedic mechanical valve implanted in 1987 (at 26 years of age) presented to our hospital with continuously increasing dyspnoea (New York Hospital Association [NYHA] class III). Echocardiograms showed good ventricular function with a normal valve prosthesis (except for a subvalvular pannus in the Duromedic valve, which slightly reduced the effective orifice area) and new moderate-to-severe mitral regurgitation in 1 patient. However, small functional iEOA (0.50 and 0.43 cm2/m2) and high transvalvular gradients were measured across the two aortic valve prostheses, indicating 2 cases of PPM in obese patients (body mass index of 31 and 38, respectively, with considerable weight increase during the past few years).
Patients’ baseline characteristics, valve haemodynamics and clinical outcomes are listed in Table 1. During the heart team meeting, standard redo open-heart surgery for aortic valve replacement with large valves was considered the best therapeutic option in both cases.
| . | Patient 1 . | Patient 2 . |
|---|---|---|
| Age, years | 73 | 55 |
| Gender | Male | Female |
| Height/weight/body surface area, cm/kg/m2 | 182/101/2.26 | 166/105/2.1 |
| Body mass index | 31 | 38 |
| Logistic EuroSCORE II, % | 4.9 | 2.5 |
| Concomitant coronary disease | Yes | No |
| Previous stroke | No | No |
| Hypertension | Yes | No |
| Pacemaker | No | No |
| Atrial fibrillation | Yes (paroxysmal) | No |
| Diabetes, type II | Yes | No |
| Date of previous aortic valve surgery | 2012 | 1987 |
| Type of prosthesis/size | 4-year-old biological Medtronic Hancock II, 21 mm | 29-year-old mechanical Baxter Duromedic, 23 mm |
| Functional preoperative EOA, cm2 | 1.156 | 0.89 |
| Preoperative iEOA, cm2/m2 | 0.50 | 0.43 |
| Preoperative peak/mean gradients, mmHg | 59/31 | 74/44 |
| Preoperative LVEF, % | 60 | 65 |
| Sapien 3 valve size, mm | 26 | 26 |
| Concomitant procedure | Mitral valve replacement | No |
| Functional postoperative EOA at discharge, cm2 | 1.46 | 2.24 |
| Postoperative iEOA at discharge, cm2/m2 | 0.64 | 1.08 |
| Postoperative peak/mean gradients, mmHg | 13/9 | 23/13 |
| Postoperative LVEF, % | 60 | 55 |
| Paravalvular leak | None | Trace |
| Cardiopulmonary bypass/aortic cross-clamp time, min | 178/162 | 180/126 |
| Complications | Respiratory failure-prolonged intubation | None |
| Postoperative electrocardiogram | SR | SR |
| Intensive + intermediate care unit stay, days | 26 | 4 |
| Hospital stay, days | 30 | 8 |
| . | Patient 1 . | Patient 2 . |
|---|---|---|
| Age, years | 73 | 55 |
| Gender | Male | Female |
| Height/weight/body surface area, cm/kg/m2 | 182/101/2.26 | 166/105/2.1 |
| Body mass index | 31 | 38 |
| Logistic EuroSCORE II, % | 4.9 | 2.5 |
| Concomitant coronary disease | Yes | No |
| Previous stroke | No | No |
| Hypertension | Yes | No |
| Pacemaker | No | No |
| Atrial fibrillation | Yes (paroxysmal) | No |
| Diabetes, type II | Yes | No |
| Date of previous aortic valve surgery | 2012 | 1987 |
| Type of prosthesis/size | 4-year-old biological Medtronic Hancock II, 21 mm | 29-year-old mechanical Baxter Duromedic, 23 mm |
| Functional preoperative EOA, cm2 | 1.156 | 0.89 |
| Preoperative iEOA, cm2/m2 | 0.50 | 0.43 |
| Preoperative peak/mean gradients, mmHg | 59/31 | 74/44 |
| Preoperative LVEF, % | 60 | 65 |
| Sapien 3 valve size, mm | 26 | 26 |
| Concomitant procedure | Mitral valve replacement | No |
| Functional postoperative EOA at discharge, cm2 | 1.46 | 2.24 |
| Postoperative iEOA at discharge, cm2/m2 | 0.64 | 1.08 |
| Postoperative peak/mean gradients, mmHg | 13/9 | 23/13 |
| Postoperative LVEF, % | 60 | 55 |
| Paravalvular leak | None | Trace |
| Cardiopulmonary bypass/aortic cross-clamp time, min | 178/162 | 180/126 |
| Complications | Respiratory failure-prolonged intubation | None |
| Postoperative electrocardiogram | SR | SR |
| Intensive + intermediate care unit stay, days | 26 | 4 |
| Hospital stay, days | 30 | 8 |
iEOA: indexed effective orifice area; LVEF: left ventricular ejection fraction; SR: sinus rhythm.
| . | Patient 1 . | Patient 2 . |
|---|---|---|
| Age, years | 73 | 55 |
| Gender | Male | Female |
| Height/weight/body surface area, cm/kg/m2 | 182/101/2.26 | 166/105/2.1 |
| Body mass index | 31 | 38 |
| Logistic EuroSCORE II, % | 4.9 | 2.5 |
| Concomitant coronary disease | Yes | No |
| Previous stroke | No | No |
| Hypertension | Yes | No |
| Pacemaker | No | No |
| Atrial fibrillation | Yes (paroxysmal) | No |
| Diabetes, type II | Yes | No |
| Date of previous aortic valve surgery | 2012 | 1987 |
| Type of prosthesis/size | 4-year-old biological Medtronic Hancock II, 21 mm | 29-year-old mechanical Baxter Duromedic, 23 mm |
| Functional preoperative EOA, cm2 | 1.156 | 0.89 |
| Preoperative iEOA, cm2/m2 | 0.50 | 0.43 |
| Preoperative peak/mean gradients, mmHg | 59/31 | 74/44 |
| Preoperative LVEF, % | 60 | 65 |
| Sapien 3 valve size, mm | 26 | 26 |
| Concomitant procedure | Mitral valve replacement | No |
| Functional postoperative EOA at discharge, cm2 | 1.46 | 2.24 |
| Postoperative iEOA at discharge, cm2/m2 | 0.64 | 1.08 |
| Postoperative peak/mean gradients, mmHg | 13/9 | 23/13 |
| Postoperative LVEF, % | 60 | 55 |
| Paravalvular leak | None | Trace |
| Cardiopulmonary bypass/aortic cross-clamp time, min | 178/162 | 180/126 |
| Complications | Respiratory failure-prolonged intubation | None |
| Postoperative electrocardiogram | SR | SR |
| Intensive + intermediate care unit stay, days | 26 | 4 |
| Hospital stay, days | 30 | 8 |
| . | Patient 1 . | Patient 2 . |
|---|---|---|
| Age, years | 73 | 55 |
| Gender | Male | Female |
| Height/weight/body surface area, cm/kg/m2 | 182/101/2.26 | 166/105/2.1 |
| Body mass index | 31 | 38 |
| Logistic EuroSCORE II, % | 4.9 | 2.5 |
| Concomitant coronary disease | Yes | No |
| Previous stroke | No | No |
| Hypertension | Yes | No |
| Pacemaker | No | No |
| Atrial fibrillation | Yes (paroxysmal) | No |
| Diabetes, type II | Yes | No |
| Date of previous aortic valve surgery | 2012 | 1987 |
| Type of prosthesis/size | 4-year-old biological Medtronic Hancock II, 21 mm | 29-year-old mechanical Baxter Duromedic, 23 mm |
| Functional preoperative EOA, cm2 | 1.156 | 0.89 |
| Preoperative iEOA, cm2/m2 | 0.50 | 0.43 |
| Preoperative peak/mean gradients, mmHg | 59/31 | 74/44 |
| Preoperative LVEF, % | 60 | 65 |
| Sapien 3 valve size, mm | 26 | 26 |
| Concomitant procedure | Mitral valve replacement | No |
| Functional postoperative EOA at discharge, cm2 | 1.46 | 2.24 |
| Postoperative iEOA at discharge, cm2/m2 | 0.64 | 1.08 |
| Postoperative peak/mean gradients, mmHg | 13/9 | 23/13 |
| Postoperative LVEF, % | 60 | 55 |
| Paravalvular leak | None | Trace |
| Cardiopulmonary bypass/aortic cross-clamp time, min | 178/162 | 180/126 |
| Complications | Respiratory failure-prolonged intubation | None |
| Postoperative electrocardiogram | SR | SR |
| Intensive + intermediate care unit stay, days | 26 | 4 |
| Hospital stay, days | 30 | 8 |
iEOA: indexed effective orifice area; LVEF: left ventricular ejection fraction; SR: sinus rhythm.
However, we discussed the option of implanting an open-heart Sapien 3 transcatheter heart valve to guarantee at first a larger valve size without concomitant annulus enlargement and then the use of valve-in-valve procedures with large transcatheter valves in case of premature Sapien 3 valve degeneration. The alternative option of a sutureless Perseval (LivaNova, London, UK) valve was discussed, but, at that moment, this valve was still not available in our unit. A mitral valve replacement with a bioprosthesis was also planned for 1 patient before the aortic valve replacement with the stent valve. We decided not to attempt to repair the mitral valve to avoid the risk of a residual intraoperative leak requiring mitral valve re-exploration with a Sapien 3 valve in place in the aortic position. Both patients agreed to have the procedure and signed informed consent forms.
Surgical strategy and technique
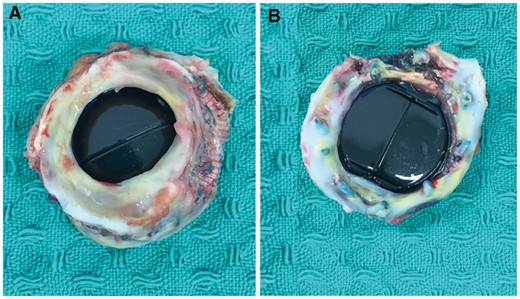
The 23-mm Duromedic mechanical valve with a subvalvular pannus that was removed from the patient.
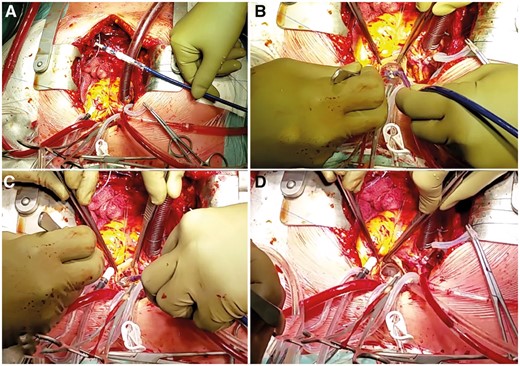
(A) The Sapien 3 valve (Edwards Lifesciences, Irvine, CA) is prepared and crimped in a transaortic-transcatheter orientation with the inflow close to the tip of the balloon catheter. (B) After the previously implanted valve is removed, the Sapien 3 is positioned in the aortic annulus such that the commissure corresponds with the anatomy of the native valve. (C) The balloon is slowly inflated to maintain the position. (D) The final result with the Sapien 3 valve in place.
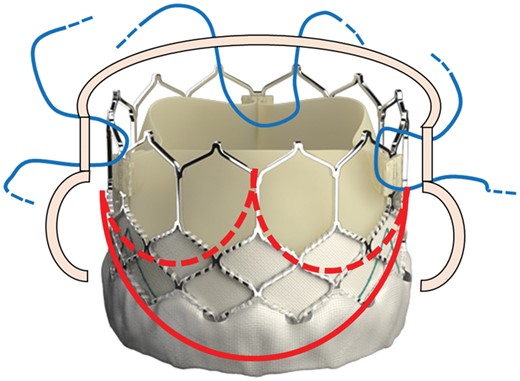
The Sapien 3 valve with the schematic view of the aortic valve annulus (red lines) and the location of the three 4-0 polypropylene U-shaped stitches (blue lines) placed across the metal frame at the top of the three valve commissures to fix the valve to the aortic wall.
Results
The procedure was completed successfully in the 2 patients without complications. Because the surgeon had a direct view of the standard surgical field during open-heart surgery, contrast injections and fluoroscopic guidance were not necessary for valve placement, positioning and deployment. Total procedural time was 360 and 318 min, respectively. The valve positioning and delivery in the landing zone were not demanding and were based on our previous experience positioning and implanting the Sapien valve during transaortic transcatheter aortic valve replacements [12].
Postprocedural transoesophageal echocardiograms showed perfect placement of the Sapien 3 valves with no signs of PPM or paravalvular leak. One patient was rapidly extubated and remained in the intensive and intermediate care units, respectively, for a total of 4 days. The second patient experienced postoperative respiratory failure complicated by pneumonia requiring a long period of intubation (10 days). Then, after a long stay in the intensive and intermediate care units, he was transferred to the ward on postoperative day 26 in good clinical and haemodynamic condition.
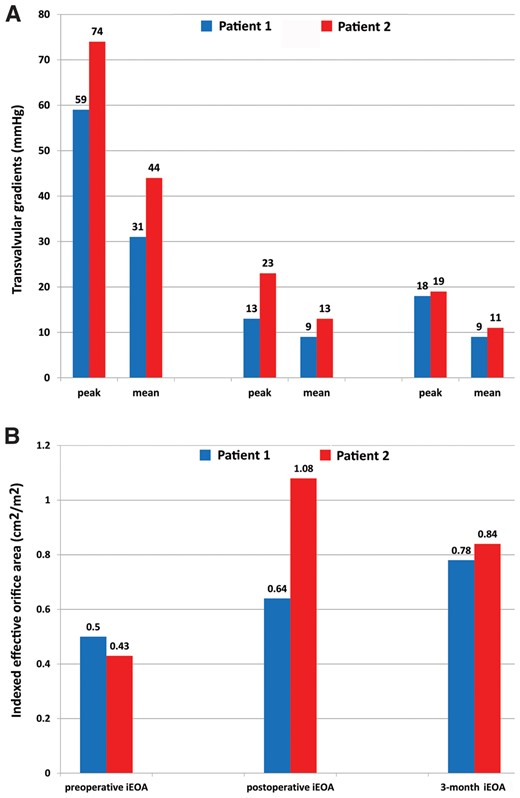
(A) Aortic transvalvular mean and peak gradients before and after the procedure. (B) iEOA before and after the procedure.
At the 3-month follow-up, the patients underwent both clinical and echocardiographic examinations: both were in stable condition with NYHA functional class I. The prosthesis showed good functioning with transvalvular peak and mean gradients of 18/9 and 19/11 mmHg, functional EOA of 1.7 and 1.7 cm2, iEOA of 0.78 and 0.84 cm2/m2, absence of paravalvular leak in 1 patient and a trace in the other one, and good left ventricle ejection fractions (55% and 56%, respectively) (Fig. 5). At the 6-month follow-up, both patients were still in stable clinical condition with NYHA functional class I.
Discussion
Aortic PPM is determined by several factors and can be due to the association of a small prosthesis implanted in an overweight or obese patient and requires redo open-heart aortic surgery to replace the small valve prosthesis with a larger one. This approach has some limitations and important considerations are required: (i) to prevent residual PPM, the new valve has to be larger; (ii) to implant a large prosthesis, a surgical technique for annulus enlargement is needed, which increases the surgical time and, sometimes, involves surgical risk associated with redo surgery; (iii) to prevent further re-redo surgery (in the case of valve failure), biological valves should be given priority because valve-in-valve techniques are easy to perform and they guarantee good haemodynamic results [9, 13].
In addition, the use of stent valves in open-heart redo surgery can be considered by the modern heart team because it involves implantation of a large valve (compared to similarly sized standard stented bioprostheses) without enlarging the surgical annulus and offers the advantage of the valve-in-valve technique instead of re-redo valve surgery in cases of premature failure. The procedure is not technically demanding, but it should be performed by experienced surgeons who are members of the heart team and are familiar with the transcatheter program, because they are familiar with the newest generation of transcatheter valves and delivery systems and with the necessary orientation and positioning of the valve during the procedure.
In our limited experience, the chosen balloon-expandable stent valve has the advantage of a short, simple delivery system, low valve profile and a symmetrical expansion during the ballooning that guarantees precise coaxial alignment, which happens during transaortic-transcatheter valve replacements [12]. Moreover, Sapien-in-Sapien strategies are already well defined with proven good results. Other available self-expandable stent valves were not considered for these redo cases because the height of the valve would have interfered with the aortotomy suture line.
In cases where the valve-in-valve options are not suitable, a standard surgical bioprosthesis has the advantages of valve-in-valve treatments but a 25-mm stented bioprosthesis, for example, has a small inner diameter compared to the 26-mm Sapien valve; therefore, it can only accept a 23-mm stent valve (with risk of new PPM), whereas the Sapien-in-Sapien can be performed with same-sized valves, at least during the first attempt [9–11, 13].
Another option that should always be considered in redo aortic valve surgery is the sutureless Perceval valve (LivaNova, London, UK). This valve is a self-expandable bioprosthesis available in four sizes that can easily be placed in the aortic position during redo aortic valve surgery after the removal of the failed prosthesis [14]. The technology used is similar to that used with Sapien valves (self-expandable nitinol stents) because it is an intra-annular implantation and uses radial forces to expand the annulus. However, this valve is not always available in all cardiac surgery units, and we should be afraid of performing further valve-in-valve procedures and of the risk of residual PPM because, so far, only 1 case has been reported of a valve-in-valve Perceval bioprosthesis. In contrast, the Sapien-in-Sapien option, as already mentioned, is an established technique that allows for same stent valve size implantation, at least during the first attempt [9–11, 13, 15].
The rapid-deployment, Edwards Intuity valve (Edwards Lifesciences, Irvine, CA) system is another valuable option but it is supra-annular and is more comparable to a standard stented aortic bioprosthesis. Moreover, implanting this valve after annulus enlargement is not recommended by the industry. Alternatively, surgical valves that can also be placed in small aortic annuli are the stentless valves (i.e. the Freedom Solo; LivaNova, London, UK): they feature larger orifice areas compared to a comparably sized stented bioprosthesis but, in redo aortic surgery, a Sapien valve can be placed much more easily and faster. In fact, after the prosthesis is removed, the annulus tissue can be partially destroyed, is not homogeneous and carries the risk of a bad haemodynamic result after implantation of the stentless valve. Moreover, not all hospitals are familiar with stentless valves.
At the time, we noted only the trace of a paravalvular leak in 1 patient who received the Sapien valve implant in non-calcified annuli, with no haemodynamic consequences or haemolysis. Patients will be followed up with transthoracic echocardiograms every year to identify new or worsening leaks.
In conclusion, this report shows that selected obese patients with aortic PPM can be safely treated with a biological large, low-profile transcatheter valve implant during standard open-heart on-pump redo surgery with the 2-fold advantage of no need for aortic annulus enlargement and an efficient valve-in-valve therapeutic option if needed.
Conflictofinterest: Enrico Ferrari is a consultant for Edwards Lifesciences.
REFERENCES
- aorta
- obesity
- open heart surgery
- stents
- cardiopulmonary bypass
- echocardiography
- body mass index procedure
- balloon dilatation
- follow-up
- objective (goal)
- surgical procedures, operative
- heart
- hypertrophy
- anulus fibrosus of aorta
- aortic valve surgery
- heart valve surgery
- overweight
- tissue degeneration
- prostheses
- aortotomy
- transcatheter heart valve prosthesis
- mismatch




