-
PDF
- Split View
-
Views
-
Cite
Cite
Shinji Ogawa, Yasuhide Okawa, Koshi Sawada, Yusuke Motoji, Yoshihiro Goto, Arishige Kimura, Mototsugu Tamaki, Yutaka Koyama, Masanori Yamamoto, Toshiaki Otsuka, Takayoshi Kato, Syunsuke Fukaya, Tomohiro Tsunekawa, Hideki Kitamura, Shinji Tomita, Takahiko Suzuki, Impact of glucose control on early vein graft failure after coronary artery bypass grafting: one-month angiographic results, Interactive CardioVascular and Thoracic Surgery, Volume 24, Issue 2, 1 February 2017, Pages 216–221, https://doi.org/10.1093/icvts/ivw343
Close - Share Icon Share
To identify factors that determine early saphenous vein graft failure (VGF) within 1 month after coronary artery bypass grafting (CABG).
Seven hundred forty-nine consecutive patients underwent primary isolated CABG with saphenous vein grafts at three Japanese centres from 1 January 2005 to 31 December 2014. According to angiographic findings within 1 month of CABG surgery, 63 patients (8.4%) developed early VGF. We examined the relationships between variables and early VGF by using multivariable logistic regression analysis.
The preoperative clinical characteristics were similar between patients with and without early VGF, except for median preoperative haemoglobin A1c levels, which were significantly higher among patients with early VGF (6.7 vs 6.4%, P = 0.046). Additionally, anastomosis to the vessel with chronic total obstruction was performed more frequently among patients with early VGF (22/63 [34.9%] vs 140/686 [20.4%], P = 0.007), and myocardial infarction during the hospital admission occurred more frequently among patients with early VGF (4/63 [6.3%] vs 2/686 [0.3%], P < 0.0001). Results of multivariable analysis showed that the preoperative haemoglobin A1c level was associated with early VGF (odds ratio per unit increase, 1.30; 95% confidence interval, 1.06–1.60; P = 0.013).
An increased preoperative haemoglobin A1c level was strongly associated with early VGF after CABG. Thus, VGF happened more frequently in patients with poorly controlled diabetes mellitus.
INTRODUCTION
Coronary artery bypass grafting (CABG) surgery remains the most common cardiac operation performed worldwide. In addition, it has recently been demonstrated that CABG should remain the standard of care for patients with complex lesions [1]. The long-term efficacy of CABG surgery remains limited because of vein graft failure (VGF). An important predictor of long-term success after CABG is graft patency [2]. Within 1 year of CABG surgery, 10–15% of vein grafts occlude, and almost half of the vein graft conduits fail by 10 years [3]. Patency rates are much higher in arterial grafts, especially when the internal mammary artery is used [3]. In patients with diabetes mellitus (DM), the rate of VGF has been reported to be as high as 19% at 1 year after CABG [4]. Despite a predisposition to failure, the saphenous vein graft (SVG) remains the most commonly used conduit for CABG even in patients with DM. Clinically, VGF leads to considerable morbidity through recurrent angina, myocardial infarction, repeat revascularization and increased mortality [5]. Understanding the factors associated with VGF may help improve patient outcomes. It has been reported that technical failure, thrombosis, smoking and problems intrinsic to the conduit, such as pre-existing vein pathology and a small diameter, are risk factors for early VGF [6], and preoperative glycaemic control may affect the preoperative vein pathology. It is important to evaluate whether VGF occurs more frequently in patients with poorly controlled DM. Therefore, the aim of the present study was to identify risk factors of early VGF.
METHODS
Patient population
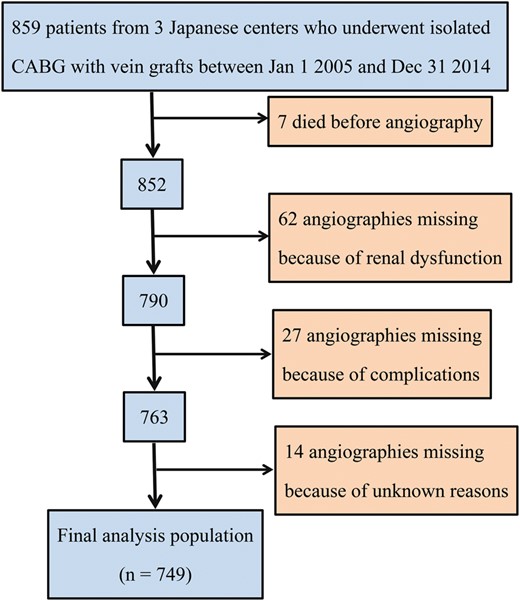
Flow chart detailing patients excluded from angiography and the reasons for exclusion.
Angiography and definition of vein graft failure
Postoperative angiography was performed using invasive radiography or computed tomography angiography before patients were discharged. In the present study, we excluded results from all angiograms performed later than 30 days after CABG. In the final cohort, 83 (11.1%) patients underwent computed tomography angiography postoperatively. All angiograms were read centrally.
Early VGF was defined as an occlusion detected on an angiogram within 1 month of CABG. For an SVG with multiple distal anastomoses, an occlusion of any component was considered VGF.
Anti-platelet therapy
Almost all patients received aspirin preoperatively except for on-pump cases of withdrawal. Postoperatively, all patients received aspirin within 12 h, and clopidogrel was administered according to the institution's preference.
Haemoglobin A1c value and perioperative glucose control
We extracted only preoperative haemoglobin A1c values obtained within 1 month preoperatively. We have previously reported our glucose control protocol [7]. In brief, we aimed to maintain blood glucose levels <200 mg/dl with insulin injections intraoperatively. A continuous insulin infusion started from the time of admission to the coronary care unit to maintain the blood glucose level between 120 and 180 mg/dl. When the patient was transferred to a ward, insulin infusion therapy was discontinued.
Statistical analysis
We used the Kolmogorov–Smirnov test for continuous variables to determine whether they were normally distributed. Only the body mass index showed a normal distribution. Thus, continuous variables are expressed as medians and interquartile ranges, except for the body mass index, whereas categorical variables are presented as frequencies and percentages. Comparisons of the perioperative data were performed using the Wilcoxon two-sample test for continuous variables and the χ2 test for categorical variables. P-values <0.05 were considered to indicate statistical significance.
To identify independent predictors of early VGF, we performed univariable and multivariable logistic regression analyses using selected variables. The following candidate variables were chosen based on clinical judgment and were considered for inclusion: age, male sex, body mass index, preoperative arrhythmia, current smoker status, preoperative serum creatinine level, haemodialysis use, HbA1c level, hyperlipidaemia, hypertension, DM, cerebrovascular disease, chronic obstructive pulmonary disease, peripheral vascular disease, New York Heart Association class III or IV, Canadian Cardiovascular Society class III or IV, left main trunk disease, three-vessel disease, syntax score, ejection fraction, elective surgery, endoscopic vein harvest technique, chronic total occlusion (CTO) of any target vessel, multiple distal anastomoses, use of cardiopulmonary bypass, cardiopulmonary bypass time, aortic cross-clamp time, surgical time, ventilator duration, use of an intra-aortic balloon pump postoperatively, new-onset atrial fibrillation, stroke and deep sternal wound infection. Variables with a likelihood ratio of P < 0.1 in univariable analysis were included in the multivariable logistic regression model, and final adjusted odds ratios (ORs) and 95% confidence intervals (CIs) were provided. A restricted cubic spline with four knots was used to test the non-linearity of the relationship between the HbA1c level and the risk of early VGF. The locations of the four knots were determined as the 5th, 35th, 65th and 95th percentiles of the distribution of the variables. All statistical analyses were performed using IBM SPSS, version 22 (IBM Japan, Tokyo, Japan) and Stata, version 14 (Stata Corp., College Station, TX, USA).
RESULTS
Patient and procedure characteristics
Among 749 patients included in our study, 63 (8.4%) had VGF within 1 month after CABG. However, occlusion of an internal mammary artery occurred in only 19 (2.5%) patients. The demographic characteristics and comorbid conditions were similar between patients with and without early VGF, with the exception of the preoperative HbA1c level, which was significantly higher among patients with early VGF (P = 0.046) (Table 1). Among the overall population, 692 (92.4%) underwent off-pump CABG. Additionally, anastomosis to the CTO vessel was performed more frequently among patients with early VGF (P = 0.007), and myocardial infarction during the hospital admission occurred significantly more often in patients with VGF (P < 0.0001). Three patients experienced operative mortality among those without early VGF (all due to low-output syndrome) (Table 2).
Patients' baseline characteristics according to the presence or absence of early VGF
| Characteristic . | With VGF (n = 63) . | Without VGF (n = 686) . | P-value . |
|---|---|---|---|
| Age, median (IQR), years | 70.0 (63.2–78.5) | 69.1 (63.5–75.7) | 0.24 |
| Male sex, n (%) | 47 (74.6) | 542 (79.0) | 0.41 |
| Body mass index, mean (SD), kg/m2 | 23.8 (3.8) | 23.9 (8.5) | 0.92a |
| Atrial fibrillation/flutter, n (%) | 2 (3.2) | 40 (5.8) | 0.54 |
| Current smoker, n (%) | 9 (14.3) | 148 (21.6) | 0.21 |
| Preoperative SCr, median (IQR), mg/dl | 0.86 (0.73–1.08) | 0.92 (0.78–1.22) | 0.56 |
| On haemodialysis, n (%) | 6 (9.5) | 77 (11.2) | 0.68 |
| Hyperlipidaemia, n (%) | 36 (57.1) | 389 (56.7) | 0.89 |
| Hypertension, n (%) | 44 (69.8) | 499 (72.7) | 0.62 |
| Diabetes mellitus, n (%) | 37 (58.7) | 353 (51.5) | 0.27 |
| With insulin use | 9 (14.3) | 82 (12.0) | 0.59 |
| Haemoglobin A1c, median (IQR), % | 6.7 (5.7–8.2) | 6.4 (5.8–7.1) | 0.046 |
| Cerebrovascular disease, n (%) | 9 (14.3) | 84 (12.2) | 0.58 |
| COPD, n (%) | 4 (6.3) | 52 (7.6) | 0.72 |
| Peripheral vascular disease, n (%) | 5 (7.9) | 44 (6.4) | 0.58 |
| NYHA class III or IV, n (%) | 10 (15.9) | 108 (15.7) | 0.93 |
| CCS class III or IV, n (%) | 17 (27.0) | 192 (28.0) | 0.82 |
| Left main trunk disease, n (%) | 22 (34.9) | 205 (29.9) | 0.41 |
| Three-vessel disease, n (%) | 53 (84.1) | 536 (78.1) | 0.27 |
| Syntax score, median (IQR) | 26.0 (22.0–31.8) | 24.0 (18.0–31.0) | 0.24 |
| Ejection fraction, median (IQR), % | 59.5 (49.6–65.6) | 58.0 (46.0–65.0) | 0.56 |
| Characteristic . | With VGF (n = 63) . | Without VGF (n = 686) . | P-value . |
|---|---|---|---|
| Age, median (IQR), years | 70.0 (63.2–78.5) | 69.1 (63.5–75.7) | 0.24 |
| Male sex, n (%) | 47 (74.6) | 542 (79.0) | 0.41 |
| Body mass index, mean (SD), kg/m2 | 23.8 (3.8) | 23.9 (8.5) | 0.92a |
| Atrial fibrillation/flutter, n (%) | 2 (3.2) | 40 (5.8) | 0.54 |
| Current smoker, n (%) | 9 (14.3) | 148 (21.6) | 0.21 |
| Preoperative SCr, median (IQR), mg/dl | 0.86 (0.73–1.08) | 0.92 (0.78–1.22) | 0.56 |
| On haemodialysis, n (%) | 6 (9.5) | 77 (11.2) | 0.68 |
| Hyperlipidaemia, n (%) | 36 (57.1) | 389 (56.7) | 0.89 |
| Hypertension, n (%) | 44 (69.8) | 499 (72.7) | 0.62 |
| Diabetes mellitus, n (%) | 37 (58.7) | 353 (51.5) | 0.27 |
| With insulin use | 9 (14.3) | 82 (12.0) | 0.59 |
| Haemoglobin A1c, median (IQR), % | 6.7 (5.7–8.2) | 6.4 (5.8–7.1) | 0.046 |
| Cerebrovascular disease, n (%) | 9 (14.3) | 84 (12.2) | 0.58 |
| COPD, n (%) | 4 (6.3) | 52 (7.6) | 0.72 |
| Peripheral vascular disease, n (%) | 5 (7.9) | 44 (6.4) | 0.58 |
| NYHA class III or IV, n (%) | 10 (15.9) | 108 (15.7) | 0.93 |
| CCS class III or IV, n (%) | 17 (27.0) | 192 (28.0) | 0.82 |
| Left main trunk disease, n (%) | 22 (34.9) | 205 (29.9) | 0.41 |
| Three-vessel disease, n (%) | 53 (84.1) | 536 (78.1) | 0.27 |
| Syntax score, median (IQR) | 26.0 (22.0–31.8) | 24.0 (18.0–31.0) | 0.24 |
| Ejection fraction, median (IQR), % | 59.5 (49.6–65.6) | 58.0 (46.0–65.0) | 0.56 |
CCS: Canadian Cardiovascular Society; COPD: chronic obstructive pulmonary disease; IQR: interquartile range; NYHA: New York Heart Association; SCr: serum creatinine; SD: standard deviation; VGF: vein graft failure.
aGroup comparisons were performed using the Student's t-test.
Patients' baseline characteristics according to the presence or absence of early VGF
| Characteristic . | With VGF (n = 63) . | Without VGF (n = 686) . | P-value . |
|---|---|---|---|
| Age, median (IQR), years | 70.0 (63.2–78.5) | 69.1 (63.5–75.7) | 0.24 |
| Male sex, n (%) | 47 (74.6) | 542 (79.0) | 0.41 |
| Body mass index, mean (SD), kg/m2 | 23.8 (3.8) | 23.9 (8.5) | 0.92a |
| Atrial fibrillation/flutter, n (%) | 2 (3.2) | 40 (5.8) | 0.54 |
| Current smoker, n (%) | 9 (14.3) | 148 (21.6) | 0.21 |
| Preoperative SCr, median (IQR), mg/dl | 0.86 (0.73–1.08) | 0.92 (0.78–1.22) | 0.56 |
| On haemodialysis, n (%) | 6 (9.5) | 77 (11.2) | 0.68 |
| Hyperlipidaemia, n (%) | 36 (57.1) | 389 (56.7) | 0.89 |
| Hypertension, n (%) | 44 (69.8) | 499 (72.7) | 0.62 |
| Diabetes mellitus, n (%) | 37 (58.7) | 353 (51.5) | 0.27 |
| With insulin use | 9 (14.3) | 82 (12.0) | 0.59 |
| Haemoglobin A1c, median (IQR), % | 6.7 (5.7–8.2) | 6.4 (5.8–7.1) | 0.046 |
| Cerebrovascular disease, n (%) | 9 (14.3) | 84 (12.2) | 0.58 |
| COPD, n (%) | 4 (6.3) | 52 (7.6) | 0.72 |
| Peripheral vascular disease, n (%) | 5 (7.9) | 44 (6.4) | 0.58 |
| NYHA class III or IV, n (%) | 10 (15.9) | 108 (15.7) | 0.93 |
| CCS class III or IV, n (%) | 17 (27.0) | 192 (28.0) | 0.82 |
| Left main trunk disease, n (%) | 22 (34.9) | 205 (29.9) | 0.41 |
| Three-vessel disease, n (%) | 53 (84.1) | 536 (78.1) | 0.27 |
| Syntax score, median (IQR) | 26.0 (22.0–31.8) | 24.0 (18.0–31.0) | 0.24 |
| Ejection fraction, median (IQR), % | 59.5 (49.6–65.6) | 58.0 (46.0–65.0) | 0.56 |
| Characteristic . | With VGF (n = 63) . | Without VGF (n = 686) . | P-value . |
|---|---|---|---|
| Age, median (IQR), years | 70.0 (63.2–78.5) | 69.1 (63.5–75.7) | 0.24 |
| Male sex, n (%) | 47 (74.6) | 542 (79.0) | 0.41 |
| Body mass index, mean (SD), kg/m2 | 23.8 (3.8) | 23.9 (8.5) | 0.92a |
| Atrial fibrillation/flutter, n (%) | 2 (3.2) | 40 (5.8) | 0.54 |
| Current smoker, n (%) | 9 (14.3) | 148 (21.6) | 0.21 |
| Preoperative SCr, median (IQR), mg/dl | 0.86 (0.73–1.08) | 0.92 (0.78–1.22) | 0.56 |
| On haemodialysis, n (%) | 6 (9.5) | 77 (11.2) | 0.68 |
| Hyperlipidaemia, n (%) | 36 (57.1) | 389 (56.7) | 0.89 |
| Hypertension, n (%) | 44 (69.8) | 499 (72.7) | 0.62 |
| Diabetes mellitus, n (%) | 37 (58.7) | 353 (51.5) | 0.27 |
| With insulin use | 9 (14.3) | 82 (12.0) | 0.59 |
| Haemoglobin A1c, median (IQR), % | 6.7 (5.7–8.2) | 6.4 (5.8–7.1) | 0.046 |
| Cerebrovascular disease, n (%) | 9 (14.3) | 84 (12.2) | 0.58 |
| COPD, n (%) | 4 (6.3) | 52 (7.6) | 0.72 |
| Peripheral vascular disease, n (%) | 5 (7.9) | 44 (6.4) | 0.58 |
| NYHA class III or IV, n (%) | 10 (15.9) | 108 (15.7) | 0.93 |
| CCS class III or IV, n (%) | 17 (27.0) | 192 (28.0) | 0.82 |
| Left main trunk disease, n (%) | 22 (34.9) | 205 (29.9) | 0.41 |
| Three-vessel disease, n (%) | 53 (84.1) | 536 (78.1) | 0.27 |
| Syntax score, median (IQR) | 26.0 (22.0–31.8) | 24.0 (18.0–31.0) | 0.24 |
| Ejection fraction, median (IQR), % | 59.5 (49.6–65.6) | 58.0 (46.0–65.0) | 0.56 |
CCS: Canadian Cardiovascular Society; COPD: chronic obstructive pulmonary disease; IQR: interquartile range; NYHA: New York Heart Association; SCr: serum creatinine; SD: standard deviation; VGF: vein graft failure.
aGroup comparisons were performed using the Student's t-test.
| Characteristic . | With VGF (n = 63) . | Without VGF (n = 686) . | P-value . |
|---|---|---|---|
| Elective surgery, n (%) | 50 (79.4) | 570 (83.1) | 0.45 |
| Off-pump CABG, n (%) | 61 (96.8) | 631 (92.0) | 0.17 |
| Endoscopic vein harvest technique, n (%) | 9 (14.3) | 84 (12.2) | 0.64 |
| Only SVG use, n (%) | 0 (0.0) | 12 (1.7) | 0.29 |
| Bilateral internal mammary artery use, n (%) | 45 (71.4) | 492 (71.7) | 0.90 |
| Radial artery use, n (%) | 0 (0.0) | 4 (0.6) | 0.54 |
| Gastroepiploic artery use, n (%) | 1 (1.6) | 9 (1.3) | 0.86 |
| Target artery of SVG | |||
| Left anterior descending artery, n (%) | 4 (6.3) | 47 (6.9) | 0.88 |
| Left circumflex artery, n (%) | 21 (33.3) | 216 (31.5) | 0.76 |
| Right coronary artery, n (%) | 53 (84.1) | 584 (85.1) | 0.83 |
| Worst target artery stenosis | |||
| CTO, n (%) | 22 (34.9) | 140 (20.4) | 0.007 |
| 91–99%, n (%) | 5 (7.9) | 81 (11.8) | 0.36 |
| 75–90%, n (%) | 34 (54.0) | 446 (65.0) | 0.08 |
| 50–74%, n (%) | 2 (3.2) | 19 (2.8) | 0.85 |
| Target artery of SVG endarterectomy, n (%) | 1 (1.6) | 5 (0.7) | 0.46 |
| Total number of distal anastomoses, mean (SD) | 3.8 (0.8) | 3.6 (0.9) | 0.12a |
| Multiple distal anastomoses with veins, n (%) | 27 (42.9) | 270 (39.4) | 0.59 |
| Surgical time, median (IQR), min | 285.5 (241.3–327.0) | 267.0 (235.0–305.0) | 0.089 |
| Ventilator duration, median (IQR), h | 8.0 (3.0–12.0) | 10.0 (4.0–15.0) | 0.22 |
| Use of postoperative IABP, n (%) | 8 (12.7) | 81 (11.8) | 0.77 |
| New-onset atrial fibrillation, n (%) | 15 (23.8) | 139 (20.3) | 0.48 |
| CT angiography, n (%) | 5 (7.9) | 78 (11.4) | 0.47 |
| Time of angiography after CABG, mean (SD), day | 10.4 (3.7) | 10.5 (5.8) | 0.89a |
| Operative mortality (<30 d) | 0 (0.0) | 3 (0.4) | 0.69 |
| MI during hospital admission, n (%) | 4 (6.3) | 2 (0.3) | <0.0001 |
| Stroke during hospital admission, n (%) | 1 (1.6) | 13 (1.9) | 0.95 |
| Deep sternal wound infection, n (%) | 0 (0.0) | 8 (1.2) | 0.46 |
| Characteristic . | With VGF (n = 63) . | Without VGF (n = 686) . | P-value . |
|---|---|---|---|
| Elective surgery, n (%) | 50 (79.4) | 570 (83.1) | 0.45 |
| Off-pump CABG, n (%) | 61 (96.8) | 631 (92.0) | 0.17 |
| Endoscopic vein harvest technique, n (%) | 9 (14.3) | 84 (12.2) | 0.64 |
| Only SVG use, n (%) | 0 (0.0) | 12 (1.7) | 0.29 |
| Bilateral internal mammary artery use, n (%) | 45 (71.4) | 492 (71.7) | 0.90 |
| Radial artery use, n (%) | 0 (0.0) | 4 (0.6) | 0.54 |
| Gastroepiploic artery use, n (%) | 1 (1.6) | 9 (1.3) | 0.86 |
| Target artery of SVG | |||
| Left anterior descending artery, n (%) | 4 (6.3) | 47 (6.9) | 0.88 |
| Left circumflex artery, n (%) | 21 (33.3) | 216 (31.5) | 0.76 |
| Right coronary artery, n (%) | 53 (84.1) | 584 (85.1) | 0.83 |
| Worst target artery stenosis | |||
| CTO, n (%) | 22 (34.9) | 140 (20.4) | 0.007 |
| 91–99%, n (%) | 5 (7.9) | 81 (11.8) | 0.36 |
| 75–90%, n (%) | 34 (54.0) | 446 (65.0) | 0.08 |
| 50–74%, n (%) | 2 (3.2) | 19 (2.8) | 0.85 |
| Target artery of SVG endarterectomy, n (%) | 1 (1.6) | 5 (0.7) | 0.46 |
| Total number of distal anastomoses, mean (SD) | 3.8 (0.8) | 3.6 (0.9) | 0.12a |
| Multiple distal anastomoses with veins, n (%) | 27 (42.9) | 270 (39.4) | 0.59 |
| Surgical time, median (IQR), min | 285.5 (241.3–327.0) | 267.0 (235.0–305.0) | 0.089 |
| Ventilator duration, median (IQR), h | 8.0 (3.0–12.0) | 10.0 (4.0–15.0) | 0.22 |
| Use of postoperative IABP, n (%) | 8 (12.7) | 81 (11.8) | 0.77 |
| New-onset atrial fibrillation, n (%) | 15 (23.8) | 139 (20.3) | 0.48 |
| CT angiography, n (%) | 5 (7.9) | 78 (11.4) | 0.47 |
| Time of angiography after CABG, mean (SD), day | 10.4 (3.7) | 10.5 (5.8) | 0.89a |
| Operative mortality (<30 d) | 0 (0.0) | 3 (0.4) | 0.69 |
| MI during hospital admission, n (%) | 4 (6.3) | 2 (0.3) | <0.0001 |
| Stroke during hospital admission, n (%) | 1 (1.6) | 13 (1.9) | 0.95 |
| Deep sternal wound infection, n (%) | 0 (0.0) | 8 (1.2) | 0.46 |
CABG: coronary artery bypass grafting; CT: computed tomography; CTO: chronic total occlusion; IABP: intra-aortic balloon pump; IQR: interquartile range; MI: myocardial infarction; SD: standard deviation; SVG: saphenous vein graft; VGF: vein graft failure.
aGroup comparisons were performed using Student's t-test.
| Characteristic . | With VGF (n = 63) . | Without VGF (n = 686) . | P-value . |
|---|---|---|---|
| Elective surgery, n (%) | 50 (79.4) | 570 (83.1) | 0.45 |
| Off-pump CABG, n (%) | 61 (96.8) | 631 (92.0) | 0.17 |
| Endoscopic vein harvest technique, n (%) | 9 (14.3) | 84 (12.2) | 0.64 |
| Only SVG use, n (%) | 0 (0.0) | 12 (1.7) | 0.29 |
| Bilateral internal mammary artery use, n (%) | 45 (71.4) | 492 (71.7) | 0.90 |
| Radial artery use, n (%) | 0 (0.0) | 4 (0.6) | 0.54 |
| Gastroepiploic artery use, n (%) | 1 (1.6) | 9 (1.3) | 0.86 |
| Target artery of SVG | |||
| Left anterior descending artery, n (%) | 4 (6.3) | 47 (6.9) | 0.88 |
| Left circumflex artery, n (%) | 21 (33.3) | 216 (31.5) | 0.76 |
| Right coronary artery, n (%) | 53 (84.1) | 584 (85.1) | 0.83 |
| Worst target artery stenosis | |||
| CTO, n (%) | 22 (34.9) | 140 (20.4) | 0.007 |
| 91–99%, n (%) | 5 (7.9) | 81 (11.8) | 0.36 |
| 75–90%, n (%) | 34 (54.0) | 446 (65.0) | 0.08 |
| 50–74%, n (%) | 2 (3.2) | 19 (2.8) | 0.85 |
| Target artery of SVG endarterectomy, n (%) | 1 (1.6) | 5 (0.7) | 0.46 |
| Total number of distal anastomoses, mean (SD) | 3.8 (0.8) | 3.6 (0.9) | 0.12a |
| Multiple distal anastomoses with veins, n (%) | 27 (42.9) | 270 (39.4) | 0.59 |
| Surgical time, median (IQR), min | 285.5 (241.3–327.0) | 267.0 (235.0–305.0) | 0.089 |
| Ventilator duration, median (IQR), h | 8.0 (3.0–12.0) | 10.0 (4.0–15.0) | 0.22 |
| Use of postoperative IABP, n (%) | 8 (12.7) | 81 (11.8) | 0.77 |
| New-onset atrial fibrillation, n (%) | 15 (23.8) | 139 (20.3) | 0.48 |
| CT angiography, n (%) | 5 (7.9) | 78 (11.4) | 0.47 |
| Time of angiography after CABG, mean (SD), day | 10.4 (3.7) | 10.5 (5.8) | 0.89a |
| Operative mortality (<30 d) | 0 (0.0) | 3 (0.4) | 0.69 |
| MI during hospital admission, n (%) | 4 (6.3) | 2 (0.3) | <0.0001 |
| Stroke during hospital admission, n (%) | 1 (1.6) | 13 (1.9) | 0.95 |
| Deep sternal wound infection, n (%) | 0 (0.0) | 8 (1.2) | 0.46 |
| Characteristic . | With VGF (n = 63) . | Without VGF (n = 686) . | P-value . |
|---|---|---|---|
| Elective surgery, n (%) | 50 (79.4) | 570 (83.1) | 0.45 |
| Off-pump CABG, n (%) | 61 (96.8) | 631 (92.0) | 0.17 |
| Endoscopic vein harvest technique, n (%) | 9 (14.3) | 84 (12.2) | 0.64 |
| Only SVG use, n (%) | 0 (0.0) | 12 (1.7) | 0.29 |
| Bilateral internal mammary artery use, n (%) | 45 (71.4) | 492 (71.7) | 0.90 |
| Radial artery use, n (%) | 0 (0.0) | 4 (0.6) | 0.54 |
| Gastroepiploic artery use, n (%) | 1 (1.6) | 9 (1.3) | 0.86 |
| Target artery of SVG | |||
| Left anterior descending artery, n (%) | 4 (6.3) | 47 (6.9) | 0.88 |
| Left circumflex artery, n (%) | 21 (33.3) | 216 (31.5) | 0.76 |
| Right coronary artery, n (%) | 53 (84.1) | 584 (85.1) | 0.83 |
| Worst target artery stenosis | |||
| CTO, n (%) | 22 (34.9) | 140 (20.4) | 0.007 |
| 91–99%, n (%) | 5 (7.9) | 81 (11.8) | 0.36 |
| 75–90%, n (%) | 34 (54.0) | 446 (65.0) | 0.08 |
| 50–74%, n (%) | 2 (3.2) | 19 (2.8) | 0.85 |
| Target artery of SVG endarterectomy, n (%) | 1 (1.6) | 5 (0.7) | 0.46 |
| Total number of distal anastomoses, mean (SD) | 3.8 (0.8) | 3.6 (0.9) | 0.12a |
| Multiple distal anastomoses with veins, n (%) | 27 (42.9) | 270 (39.4) | 0.59 |
| Surgical time, median (IQR), min | 285.5 (241.3–327.0) | 267.0 (235.0–305.0) | 0.089 |
| Ventilator duration, median (IQR), h | 8.0 (3.0–12.0) | 10.0 (4.0–15.0) | 0.22 |
| Use of postoperative IABP, n (%) | 8 (12.7) | 81 (11.8) | 0.77 |
| New-onset atrial fibrillation, n (%) | 15 (23.8) | 139 (20.3) | 0.48 |
| CT angiography, n (%) | 5 (7.9) | 78 (11.4) | 0.47 |
| Time of angiography after CABG, mean (SD), day | 10.4 (3.7) | 10.5 (5.8) | 0.89a |
| Operative mortality (<30 d) | 0 (0.0) | 3 (0.4) | 0.69 |
| MI during hospital admission, n (%) | 4 (6.3) | 2 (0.3) | <0.0001 |
| Stroke during hospital admission, n (%) | 1 (1.6) | 13 (1.9) | 0.95 |
| Deep sternal wound infection, n (%) | 0 (0.0) | 8 (1.2) | 0.46 |
CABG: coronary artery bypass grafting; CT: computed tomography; CTO: chronic total occlusion; IABP: intra-aortic balloon pump; IQR: interquartile range; MI: myocardial infarction; SD: standard deviation; SVG: saphenous vein graft; VGF: vein graft failure.
aGroup comparisons were performed using Student's t-test.
Factors associated with vein graft failure
| Variable . | Odds ratio (95% CI) . | P-value . |
|---|---|---|
| HbA1c level (per unit increase) | 1.30 (1.06–1.60) | 0.013 |
| CTO of any target vessel | 1.83 (0.95–3.53) | 0.073 |
| Surgical time (per minute increase) | 1.00 (1.00–1.01) | 0.073 |
| Variable . | Odds ratio (95% CI) . | P-value . |
|---|---|---|
| HbA1c level (per unit increase) | 1.30 (1.06–1.60) | 0.013 |
| CTO of any target vessel | 1.83 (0.95–3.53) | 0.073 |
| Surgical time (per minute increase) | 1.00 (1.00–1.01) | 0.073 |
CI: confidence interval; CTO: chronic total occlusion; HbA1c: haemoglobin A1c; VGF: vein graft failure.
| Variable . | Odds ratio (95% CI) . | P-value . |
|---|---|---|
| HbA1c level (per unit increase) | 1.30 (1.06–1.60) | 0.013 |
| CTO of any target vessel | 1.83 (0.95–3.53) | 0.073 |
| Surgical time (per minute increase) | 1.00 (1.00–1.01) | 0.073 |
| Variable . | Odds ratio (95% CI) . | P-value . |
|---|---|---|
| HbA1c level (per unit increase) | 1.30 (1.06–1.60) | 0.013 |
| CTO of any target vessel | 1.83 (0.95–3.53) | 0.073 |
| Surgical time (per minute increase) | 1.00 (1.00–1.01) | 0.073 |
CI: confidence interval; CTO: chronic total occlusion; HbA1c: haemoglobin A1c; VGF: vein graft failure.
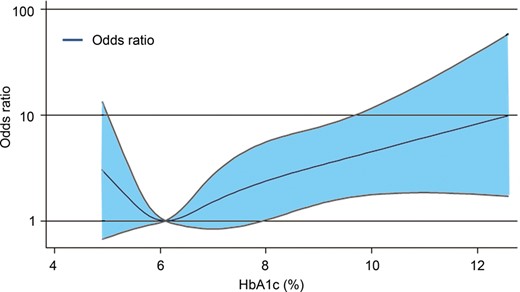
Non-linear relationship between the haemoglobin (Hb) A1c level and risk of early vein graft failure according to restricted cubic spline with four knots (5.2, 5.8, 6.7 and 9.2%). The odds ratio was adjusted for the surgical time and chronic total occlusion of any target vessel. The reference was set at HbA1c = 6.1%, which corresponded to the lowest odds ratio.
A sensitivity analysis showed that the HbA1c levels maintained a significantly increased OR for early VGF in multivariable logistic regression analysis, even after filling missing variables by using multiple imputations (Supplementary Table 1).
DISCUSSION
The findings of our study suggest that VGF occurs more frequently in patients with poorly controlled DM. The prevalence of DM in patients undergoing CABG has increased annually [8], so more patients with DM are expected to require CABG surgery in the future. However, these patients often have a more complicated postoperative course than do those without DM [8]. One of the causes is the low patency rate of vein grafts in patients with DM after CABG. In these patients, the use of two arterial conduits is clearly preferable to one [4, 9]. Thus, CABG surgery without SVG can be suitable for patients with uncontrolled DM.
Interest in understanding the relationship between the preoperative HbA1c value and adverse events after CABG has been longstanding [10]. Previous studies have reported that an increased preoperative HbA1c value is associated with mortality, myocardial infarction, wound infection and acute kidney injury [11, 12]. However, previous reports have not described the relationship between the preoperative HbA1c level and graft patency after CABG.
Conversely, numerous studies have reported many factors associated with VGF [2, 6, 13]. VGF is an independent predictor of adverse outcome with a higher risk of death, myocardial infarction and need for repeat revascularization [13]. Recent data suggest that early VGF may be of more prognostic importance than the angiographic detection of late graft failure [13]. In patients with DM, VGF occurs more frequently than it does in those without DM, and the use of venous grafts is associated with poor survival [4]. However, some studies have reported that DM is not associated with the rate of VGF, except in patients with DM using insulin [14, 15].
Our study differs from previous studies in several ways. First, we used preoperative HbA1c levels as a parameter of DM severity. Second, we defined VGF as only an occlusion of the vein graft, and we included the period within 1 month of CABG, which is an acute phase, so we judged VGF more strictly.
We found a number of factors that were associated with VGF. Pathological studies have demonstrated that atherosclerosis is the main aetiology of late (>12 months) VGF, whereas early (<1 month) and subacute (<12 months) VGF is due to thrombosis, surgical technical errors and intimal hyperplasia [16]. Some previous studies have reported that SVG dysfunctions are associated with diabetic conditions such as hyperglycaemia-induced venous endothelial dysfunction [17], gene expression disorder [18] and venous smooth muscle cell dysfunction [19]. These studies have suggested that dysfunction is associated with SVGs harvested from patients who have an increased preoperative HbA1c value.
Another factor associated with VGF in our analysis was the CTO of any target vessel. The element of a smaller vessel diameter may indicate poor distal runoff, and this has been previously associated with VGF [20]. Furthermore, it has been reported that the HbA1c level is associated with the severity of coronary artery disease [21], and this condition indicates abnormal myocardial blood flow reserve in patients with DM. Thus, the severity of coronary artery disease may correlate with an increased level of HbA1c, meaning that the increased HbA1c level and CTO of the target vessel are similar.
Additionally, off-pump CABG may be a risk factor of VGF. One meta-analysis reported that off-pump CABG increases the incidence of VGF [22]. Interestingly, occlusion of the internal mammary artery was not associated with either on-pump or off-pump CABG [22]. It has also been reported that the total heparin dose affects that difference [22].
Finally, various studies have shown that damage to vessels during surgical preparation affects vein graft patency [23]. It has been reported that harvesting the SVG with the no-touch technique rather than the conventional technique resulted in a significantly higher patency [23].
To our knowledge, the present study is one of the few studies that has assessed whether the HbA1c level is a potential risk factor for early VGF in patients undergoing CABG, especially in those with DM. Compared to a patient's diabetic status alone, a preoperative HbA1c level may provide highly accurate prognostic information about outcomes after CABG. Moreover, in patients with a high HbA1c level who do not require urgent or emergency CABG, consideration should be given to maximizing blood glucose control preoperatively to minimize early VGF. Alternatively, an SVG should not be used in such situations, and total arterial revascularization should be considered. Regarding deep sternal wound infections with the use of bilateral internal mammary arteries, we have previously reported that strict glyacemic control perioperatively reduces this kind of infection [7].
Study limitations
This study was limited by the relatively small number of subjects, and its retrospective and observational nature. Additionally, we only included patients who underwent CABG with SVG, thus selection bias exists. We were not able to assess VGF in patients who died before angiography or in those who did not undergo angiography for various reasons such as renal dysfunction or other complications. Our study population may have differed from those of other studies with regard to the surgical approach. Approximately 76% of patients underwent CABG without the use of cardiopulmonary bypass. Furthermore, not all patients in our analysis had a preoperative HbA1c level measurement.
CONCLUSIONS
The current study showed that the preoperative HbA1c level is a powerful predictor of early VGF. This simple laboratory test, which can be performed preoperatively, may provide clinicians with a more accurate risk profile, and it may facilitate a new way of thinking about graft selection especially in patients with DM. However, further studies with larger samples are needed to confirm our findings.
SUPPLEMENTARY MATERIAL
Supplementary material is available at ICVTS online.
Conflict of interest: none declared.




