-
PDF
- Split View
-
Views
-
Cite
Cite
Marco Russo, Paolo Nardi, Guglielmo Saitto, Emanuele Bovio, Antonio Pellegrino, Antonio Scafuri, Giovanni Ruvolo, Single versus double antiplatelet therapy in patients undergoing coronary artery bypass grafting with coronary endarterectomy: mid-term results and clinical implications, Interactive CardioVascular and Thoracic Surgery, Volume 24, Issue 2, 1 February 2017, Pages 203–208, https://doi.org/10.1093/icvts/ivw351
Close - Share Icon Share
Coronary endarterectomy (CE) represents a useful adjunctive technique to coronary artery bypass grafting (CABG) in the presence of diffuse coronary artery disease. Nevertheless, the long-term patency of the graft remains unclear, and no standard anticoagulation and antiplatelet protocols exist for use after CE. The aim of this retrospective study was to evaluate and possibly to clarify the role of single (SAT) versus dual antiplatelet therapy (DAT) at mid-term follow-up.
Between January 2006 and December 2013, CE was performed in 90 patients (mean age 67 ± 8.2 years) who also underwent isolated CABG. After surgery, 20 patients received aspirin 100 mg daily (SAT group), and 52 patients received aspirin plus clopidogrel 75 mg daily (DAT group). Clopidogrel was discontinued in the DAT group 12 months after the operation.
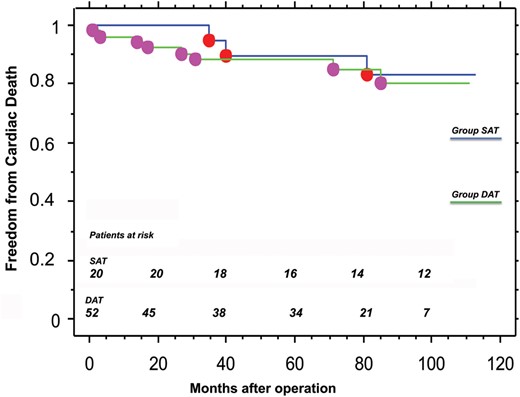
The overall in-hospital mortality rate was 2.7% (SAT 0% vs DAT 3.8%; P = ns). Perioperative myocardial infarction was 12.3% (SAT 15.0% vs DAT 11.5%; P = ns), and major bleeding requiring surgical re-exploration was 4.1% (SAT 10.0% vs DAT 1.9%; P = ns). Mean follow-up duration was 71.3 ± 32.7 months (median 79 months), and was 100% complete (5208/5208 pt-months). At 7 years of follow-up, freedom from cardiac death was 84 ± 9% in group SAT versus 85 ± 5% in group DAT (P = ns); freedom from new percutaneous coronary intervention was 93 ± 6% versus 100% (P = ns), and freedom from major adverse cardiac and cerebrovascular events was 73 ± 10% versus 75 ± 6% (P = ns).
In patients with diffuse coronary disease, CE is a safe and feasible technique with acceptable mid-term results. No differences were observed in terms of major clinical outcomes between patients treated with single versus dual antiplatelet therapy at least in a mid-term period of follow-up.
INTRODUCTION
Coronary endarterectomy (CE) was introduced in the 1950s to decrease angina in patients affected by diffuse coronary artery occlusive disease. Later, it was combined with coronary artery bypass grafting (CABG/CE) techniques to treat patients with severe advanced coronary atherosclerosis. Nevertheless, long-term results and graft patency remain controversial, and surgeons still have doubts regarding the real usefulness of this procedure [1–3].
During the last decade, percutaneous coronary interventions (PCIs) showed good results, and current international guidelines encourage percutaneous coronary revascularization [4]. Therefore, the patient having the operation is older, diabetic, has more complex coronary lesions, and has a higher risk of incomplete revascularization. This change in the surgical scenario led to CE being reconsidered as a treatment strategy in selected cases; thus, reassessment of the technique and its indications and evaluation of its outcomes are now necessary.
Several reports have shown the importance of different anticoagulation protocols to increase graft patency. Nevertheless, no standard protocols exist for patients treated with CABG/CE. After CE, the lack of endothelium leads to activation of the coagulation cascade, because the subendothelial material is exposed to blood flow [5].
Many authors have proposed the use of an infusion of heparin followed by warfarin for 3 months with an international normalized ratio between 2.0 and 2.5; other groups have suggested that double antiplatelet therapy (DAT) be started immediately after the operation [6].
In a recent meta-analysis of the outcome of CE, 155 articles were identified by a systematic search; 10 of these, comprising a total of 1203 patients, best answered the clinical question. A different anticoagulation or antiplatelet aggregation protocol was proposed in each of the papers [7].
We retrospectively evaluated the mid-term clinical results of two different anticoagulation protocols adopted during a 7-year period in our centre for patients treated by concomitant CABG and CE.
MATERIALS AND METHODS
Patients and antiplatelet protocol
Between January 2006 and December 2013, 90 consecutive patients (67 ± 8.2 years) at the Cardiac Surgery Unit of Tor Vergata, University of Rome, underwent isolated CABG and adjunct CE. In all patients, all antiplatelet therapies were discontinued before the operation and switched to twice-daily subcutaneous administration of heparin (100 IU/kg).
Different postoperative anticoagulation protocols were used based on the patient's characteristics (e.g. complexity of coronary lesions, diffuse occlusive disease, permanent atrial fibrillation, aspirin allergy). Only patients treated postoperatively with 100 mg aspirin daily and aspirin plus 75 mg clopidogrel daily were included in the study. Our cohort comprised 72 patients, divided into two groups according to antiplatelet therapy: 20 patients (mean age 66.8 ± 9.4 years) received aspirin (the single antiplatelet [SAT] group) and 52 (mean age 66.8 ± 7.8 years) received aspirin plus clopidogrel (the DAT group). Clopidogrel was discontinued in the DAT group 12 months after the operation. The preoperative characteristics of the patients are summarized in Table 1.
| Characteristics . | SAT group (n = 20) . | DAT group (n = 52) . | P-value . |
|---|---|---|---|
| Age (years), mean ± SD | 66.8 ± 9.3 | 66.6 ± 7.8 | ns |
| Male gender, n (%) | 17 (85) | 35 (67) | ns |
| EuroSCORE II, mean ± SD | 3.6 ± 2.0 | 3.2 ± 1.6 | ns |
| BMI (Kg/m2) | 28 ± 3.4 | 27.2 ± 4.3 | ns |
| Obesity, n (%) | 5 (25) | 10 (19) | ns |
| Hypertension, n (%) | 19 (95) | 47 (90) | ns |
| Diabetes mellitus, n (%) | 6 (30) | 8 (15) | ns |
| Dyslipidaemia, n (%) | 10 (50) | 22 (42) | ns |
| Active smoker, n (%) | 5 (25) | 13 (25) | ns |
| Chronic renal dysfunction, n (%) | 3 (15) | 4 (7) | ns |
| Chronic obstructive pulmonary disease, n (%) | 2 (10) | 4 (7) | ns |
| Peripheral vascular disease, n (%) | 6 (30) | 9 (17) | ns |
| Recent MI, <60 days, n (%) | 4 (20) | 15 (28) | ns |
| Left main stenosis >50%, n (%) | 4 (20) | 15 (28) | ns |
| LVEF (%), mean ± SD | 45.6 ± 10.1 | 51.1 ± 9.87 | 0.037 |
| CCS class, mean ± SD | 2 ± 1.6 | 2.4 ± 1.3 | ns |
| Angina at rest, n (%) | 6 (30) | 13 (25) | ns |
| Mean number of diseased vessels | 2.7 ± 0.4 | 2.8 ± 0.3 | ns |
| Urgency, n (%) | 4 (20) | 12 (23) | ns |
| Characteristics . | SAT group (n = 20) . | DAT group (n = 52) . | P-value . |
|---|---|---|---|
| Age (years), mean ± SD | 66.8 ± 9.3 | 66.6 ± 7.8 | ns |
| Male gender, n (%) | 17 (85) | 35 (67) | ns |
| EuroSCORE II, mean ± SD | 3.6 ± 2.0 | 3.2 ± 1.6 | ns |
| BMI (Kg/m2) | 28 ± 3.4 | 27.2 ± 4.3 | ns |
| Obesity, n (%) | 5 (25) | 10 (19) | ns |
| Hypertension, n (%) | 19 (95) | 47 (90) | ns |
| Diabetes mellitus, n (%) | 6 (30) | 8 (15) | ns |
| Dyslipidaemia, n (%) | 10 (50) | 22 (42) | ns |
| Active smoker, n (%) | 5 (25) | 13 (25) | ns |
| Chronic renal dysfunction, n (%) | 3 (15) | 4 (7) | ns |
| Chronic obstructive pulmonary disease, n (%) | 2 (10) | 4 (7) | ns |
| Peripheral vascular disease, n (%) | 6 (30) | 9 (17) | ns |
| Recent MI, <60 days, n (%) | 4 (20) | 15 (28) | ns |
| Left main stenosis >50%, n (%) | 4 (20) | 15 (28) | ns |
| LVEF (%), mean ± SD | 45.6 ± 10.1 | 51.1 ± 9.87 | 0.037 |
| CCS class, mean ± SD | 2 ± 1.6 | 2.4 ± 1.3 | ns |
| Angina at rest, n (%) | 6 (30) | 13 (25) | ns |
| Mean number of diseased vessels | 2.7 ± 0.4 | 2.8 ± 0.3 | ns |
| Urgency, n (%) | 4 (20) | 12 (23) | ns |
BMI: body mass index; CCS: Canadian Cardiovascular Society; DAT: dual antiplatelet therapy; LVEF: left ventricular ejection fraction; MI: myocardial infarction; SAT: single antiplatelet therapy; SD: standard deviation.
| Characteristics . | SAT group (n = 20) . | DAT group (n = 52) . | P-value . |
|---|---|---|---|
| Age (years), mean ± SD | 66.8 ± 9.3 | 66.6 ± 7.8 | ns |
| Male gender, n (%) | 17 (85) | 35 (67) | ns |
| EuroSCORE II, mean ± SD | 3.6 ± 2.0 | 3.2 ± 1.6 | ns |
| BMI (Kg/m2) | 28 ± 3.4 | 27.2 ± 4.3 | ns |
| Obesity, n (%) | 5 (25) | 10 (19) | ns |
| Hypertension, n (%) | 19 (95) | 47 (90) | ns |
| Diabetes mellitus, n (%) | 6 (30) | 8 (15) | ns |
| Dyslipidaemia, n (%) | 10 (50) | 22 (42) | ns |
| Active smoker, n (%) | 5 (25) | 13 (25) | ns |
| Chronic renal dysfunction, n (%) | 3 (15) | 4 (7) | ns |
| Chronic obstructive pulmonary disease, n (%) | 2 (10) | 4 (7) | ns |
| Peripheral vascular disease, n (%) | 6 (30) | 9 (17) | ns |
| Recent MI, <60 days, n (%) | 4 (20) | 15 (28) | ns |
| Left main stenosis >50%, n (%) | 4 (20) | 15 (28) | ns |
| LVEF (%), mean ± SD | 45.6 ± 10.1 | 51.1 ± 9.87 | 0.037 |
| CCS class, mean ± SD | 2 ± 1.6 | 2.4 ± 1.3 | ns |
| Angina at rest, n (%) | 6 (30) | 13 (25) | ns |
| Mean number of diseased vessels | 2.7 ± 0.4 | 2.8 ± 0.3 | ns |
| Urgency, n (%) | 4 (20) | 12 (23) | ns |
| Characteristics . | SAT group (n = 20) . | DAT group (n = 52) . | P-value . |
|---|---|---|---|
| Age (years), mean ± SD | 66.8 ± 9.3 | 66.6 ± 7.8 | ns |
| Male gender, n (%) | 17 (85) | 35 (67) | ns |
| EuroSCORE II, mean ± SD | 3.6 ± 2.0 | 3.2 ± 1.6 | ns |
| BMI (Kg/m2) | 28 ± 3.4 | 27.2 ± 4.3 | ns |
| Obesity, n (%) | 5 (25) | 10 (19) | ns |
| Hypertension, n (%) | 19 (95) | 47 (90) | ns |
| Diabetes mellitus, n (%) | 6 (30) | 8 (15) | ns |
| Dyslipidaemia, n (%) | 10 (50) | 22 (42) | ns |
| Active smoker, n (%) | 5 (25) | 13 (25) | ns |
| Chronic renal dysfunction, n (%) | 3 (15) | 4 (7) | ns |
| Chronic obstructive pulmonary disease, n (%) | 2 (10) | 4 (7) | ns |
| Peripheral vascular disease, n (%) | 6 (30) | 9 (17) | ns |
| Recent MI, <60 days, n (%) | 4 (20) | 15 (28) | ns |
| Left main stenosis >50%, n (%) | 4 (20) | 15 (28) | ns |
| LVEF (%), mean ± SD | 45.6 ± 10.1 | 51.1 ± 9.87 | 0.037 |
| CCS class, mean ± SD | 2 ± 1.6 | 2.4 ± 1.3 | ns |
| Angina at rest, n (%) | 6 (30) | 13 (25) | ns |
| Mean number of diseased vessels | 2.7 ± 0.4 | 2.8 ± 0.3 | ns |
| Urgency, n (%) | 4 (20) | 12 (23) | ns |
BMI: body mass index; CCS: Canadian Cardiovascular Society; DAT: dual antiplatelet therapy; LVEF: left ventricular ejection fraction; MI: myocardial infarction; SAT: single antiplatelet therapy; SD: standard deviation.
The study was approved by our local institutional review board that waived the need for patient consent.
Surgical technique
Access to the heart was obtained through a complete median sternotomy in all patients. Myocardial revascularization was performed on-pump or off-pump according to the surgeon's preference. When cardiopulmonary bypass (CPB) was used, it was established by direct cannulation of the ascending aorta and right atrium for venous drainage. Cardiac arrest was achieved using tepid blood cardioplegia.
A left internal thoracic artery (LITA) or right internal thoracic artery (RITA) graft was used in all patients to revascularize the left anterior descending artery (LADA) system. The RITA graft was harvested from patients 60 years old or younger and anastomosed to the LADA, whereas the LITA was used for the left circumflex artery (LCA) system. The other grafts were performed using the saphenous vein, harvested under direct vision.
Adjunct CE was established intraoperatively on the basis of the diffuse occlusive disease and the target coronary artery. It was performed by means of a closed technique (the coronary artery was longitudinally incised 10–15 mm, and the sclerotic intima was stripped with fine forceps before the anastomosis was performed) or an open technique (the arteriotomy was prolonged proximally and distally for several centimetres, and a longer anastomosis was performed).
Data collection and follow-up
Data from our institutional database regarding preoperative features, intraoperative characteristics and postoperative outcome were analysed retrospectively.
All patients were followed for at least 12 months. The global clinical follow-up included 5208 patient-months, and was 100% complete. Clinical follow-up (mean 71.3 ± 32.7 months, median 79 months) was obtained by personal interview of the patient or of his/her cardiologist and by recording the results of the noninvasive stress tests; death was verified on the national registry for deceased people provided by the Italian Ministry of Health. The clinical follow-up was usually performed every 12 months in our outpatient control unit, for patients living far from our hospital who could not be included in a regular follow-up in our department, clinical information was obtained by telephone interview.
Definitions
Perioperative myocardial infarction was defined as an increase in the level of postoperative troponin I greater than 20 ng/ml, associated with an increase in the level of serum creatine kinase-MB above normal values and more than 10% of total creatine kinase, and the onset of electrocardiographic anomalies.
Pulmonary complication was defined as an episode of primary respiratory failure requiring mechanical ventilation for more than 48 h, reintubation, or intermittent application of noninvasive positive pressure ventilation.
A permanent neurological complication attributable to a focal or general cerebral lesion was defined as a stroke, whereas a transient ischaemic attack was defined as neurological symptoms lasting less than 24 h before disappearing.
Acute kidney injury was defined as a two-fold increase in the preoperative serum creatinine level or oliguria necessitating continuous venovenous haemofiltration.
The operative mortality rate included death in hospital after the operation at any time or within 30 days after discharge.
Statistical analysis
Statistical analysis was performed with Stat View 4.5 (SAS Institute Inc., Abacus Concepts, Berkeley, CA, USA). All continuous values were expressed as mean plus or minus 1 standard deviation of the mean. We used Student's t-test for continuous data and the χ2 or Fisher's exact test for categorical data. Risk factor analysis to detect independent predictors of late survival was performed using the Cox analysis. Freedom from all-cause death, cardiac death, need of new revascularization and occurrence of major adverse cardiac and cerebrovascular events during the follow-up period were expressed as mean values plus or minus 1 standard deviation and computed using the Kaplan–Meier method. Differences between the two groups were tested with the Mantel–Cox method. All P-values less than 0.05 were considered statistically significant.
RESULTS
Early results
In the SAT group, all patients underwent on-pump CABG surgery, whereas in the DAT group, 46 patients had on-pump CABG and 6 had the operation off-pump.
LITA was used in all patients except for one patient in the DAT group. In almost all of these patients, the LITA was anastomosed to the LADA (19/20 in the SAT group and 47/51 in the DAT group). In the other cases, the RITA was used. Bilateral internal thoracic arteries were used in four patients in the SAT group and in six patients in the DAT group. The other grafts were performed using the saphenous vein harvested under direct vision.
Mean CPB (101 ± 24 in the SAT group vs 96 ± 21 in the DAT group, P = ns) and cross-clamp times (63 ± 18 in the SAT group vs 58 ± 14 in the DAT group, P = ns) were similar even if patients in the SAT group required a higher number of distal anastomoses (3.5 ± 0.9 vs 2.8 ± 0.7, P = 0.007).
In 55% of cases, the CE was performed by means of a closed technique. The target vessel for CE was the LADA in most cases (55% in the SAT group vs 75% in the DAT group). Multivessel CE was performed in one patient in the SAT group and in two patients in the DAT group (P = 0.04). The mean length of the CE procedure was 38 ± 14 mm with no differences between the two groups. Intraoperative characteristics are reported in Table 2.
| Variables . | SAT group (n = 20) . | DAT group (n = 52) . | P-value . |
|---|---|---|---|
| Cardiopulmonary bypass time (min), mean ± SD | 101 ± 24 | 96 ± 21 | ns |
| Aortic cross-clamp time (min), mean ± SD | 63 ± 18 | 58 ± 14 | ns |
| Distal anastomoses (n), mean ± SD | 3.5 ± 0.9 | 2.9 ± 0.7 | 0.007 |
| Left internal thoracic artery on LADA, n (%) | 20 (100) | 51 (98.1) | ns |
| Anterior wall | 19 (95) | 47 (92.2) | ns |
| Lateral wall | 1 (5) | 4 (7.8) | ns |
| Bilateral internal thoracic artery, n (%) | 4 (20) | 6 (11) | ns |
| Saphenous vein graft, n (%) | 19 (95) | 48 (92) | ns |
| Anterior wall | 2 (10.5) | 4 (8.3) | ns |
| Lateral wall | 8 (42.1) | 20 (41.7) | ns |
| Posterior wall | 9 (47.4) | 24 (50) | ns |
| Off-pump surgery, n (%) | 0 (0) | 6 (11) | ns |
| CE on LADA, n (%) | 11 (55) | 40 (76) | ns |
| CE on diagonal branches/Int, n (%) | 2 (10) | 1 (2) | ns |
| CE on RCA/RPD, n (%) | 4 (20) | 10 (19) | ns |
| CE on OM, n (%) | 4 (20) | 3 (5) | ns |
| Multivessel CE, n (%) | 1 (5) | 2 (4) | ns |
| Variables . | SAT group (n = 20) . | DAT group (n = 52) . | P-value . |
|---|---|---|---|
| Cardiopulmonary bypass time (min), mean ± SD | 101 ± 24 | 96 ± 21 | ns |
| Aortic cross-clamp time (min), mean ± SD | 63 ± 18 | 58 ± 14 | ns |
| Distal anastomoses (n), mean ± SD | 3.5 ± 0.9 | 2.9 ± 0.7 | 0.007 |
| Left internal thoracic artery on LADA, n (%) | 20 (100) | 51 (98.1) | ns |
| Anterior wall | 19 (95) | 47 (92.2) | ns |
| Lateral wall | 1 (5) | 4 (7.8) | ns |
| Bilateral internal thoracic artery, n (%) | 4 (20) | 6 (11) | ns |
| Saphenous vein graft, n (%) | 19 (95) | 48 (92) | ns |
| Anterior wall | 2 (10.5) | 4 (8.3) | ns |
| Lateral wall | 8 (42.1) | 20 (41.7) | ns |
| Posterior wall | 9 (47.4) | 24 (50) | ns |
| Off-pump surgery, n (%) | 0 (0) | 6 (11) | ns |
| CE on LADA, n (%) | 11 (55) | 40 (76) | ns |
| CE on diagonal branches/Int, n (%) | 2 (10) | 1 (2) | ns |
| CE on RCA/RPD, n (%) | 4 (20) | 10 (19) | ns |
| CE on OM, n (%) | 4 (20) | 3 (5) | ns |
| Multivessel CE, n (%) | 1 (5) | 2 (4) | ns |
CE: coronary endarterectomy; DAT: double antiplatelet therapy; LADA: left anterior descending artery; OM: obtuse marginal branch; RCA: right coronary artery; RPD: right posterior descending branch; SAT: single antiplatelet therapy; SD: standard deviation.
| Variables . | SAT group (n = 20) . | DAT group (n = 52) . | P-value . |
|---|---|---|---|
| Cardiopulmonary bypass time (min), mean ± SD | 101 ± 24 | 96 ± 21 | ns |
| Aortic cross-clamp time (min), mean ± SD | 63 ± 18 | 58 ± 14 | ns |
| Distal anastomoses (n), mean ± SD | 3.5 ± 0.9 | 2.9 ± 0.7 | 0.007 |
| Left internal thoracic artery on LADA, n (%) | 20 (100) | 51 (98.1) | ns |
| Anterior wall | 19 (95) | 47 (92.2) | ns |
| Lateral wall | 1 (5) | 4 (7.8) | ns |
| Bilateral internal thoracic artery, n (%) | 4 (20) | 6 (11) | ns |
| Saphenous vein graft, n (%) | 19 (95) | 48 (92) | ns |
| Anterior wall | 2 (10.5) | 4 (8.3) | ns |
| Lateral wall | 8 (42.1) | 20 (41.7) | ns |
| Posterior wall | 9 (47.4) | 24 (50) | ns |
| Off-pump surgery, n (%) | 0 (0) | 6 (11) | ns |
| CE on LADA, n (%) | 11 (55) | 40 (76) | ns |
| CE on diagonal branches/Int, n (%) | 2 (10) | 1 (2) | ns |
| CE on RCA/RPD, n (%) | 4 (20) | 10 (19) | ns |
| CE on OM, n (%) | 4 (20) | 3 (5) | ns |
| Multivessel CE, n (%) | 1 (5) | 2 (4) | ns |
| Variables . | SAT group (n = 20) . | DAT group (n = 52) . | P-value . |
|---|---|---|---|
| Cardiopulmonary bypass time (min), mean ± SD | 101 ± 24 | 96 ± 21 | ns |
| Aortic cross-clamp time (min), mean ± SD | 63 ± 18 | 58 ± 14 | ns |
| Distal anastomoses (n), mean ± SD | 3.5 ± 0.9 | 2.9 ± 0.7 | 0.007 |
| Left internal thoracic artery on LADA, n (%) | 20 (100) | 51 (98.1) | ns |
| Anterior wall | 19 (95) | 47 (92.2) | ns |
| Lateral wall | 1 (5) | 4 (7.8) | ns |
| Bilateral internal thoracic artery, n (%) | 4 (20) | 6 (11) | ns |
| Saphenous vein graft, n (%) | 19 (95) | 48 (92) | ns |
| Anterior wall | 2 (10.5) | 4 (8.3) | ns |
| Lateral wall | 8 (42.1) | 20 (41.7) | ns |
| Posterior wall | 9 (47.4) | 24 (50) | ns |
| Off-pump surgery, n (%) | 0 (0) | 6 (11) | ns |
| CE on LADA, n (%) | 11 (55) | 40 (76) | ns |
| CE on diagonal branches/Int, n (%) | 2 (10) | 1 (2) | ns |
| CE on RCA/RPD, n (%) | 4 (20) | 10 (19) | ns |
| CE on OM, n (%) | 4 (20) | 3 (5) | ns |
| Multivessel CE, n (%) | 1 (5) | 2 (4) | ns |
CE: coronary endarterectomy; DAT: double antiplatelet therapy; LADA: left anterior descending artery; OM: obtuse marginal branch; RCA: right coronary artery; RPD: right posterior descending branch; SAT: single antiplatelet therapy; SD: standard deviation.
The overall in-hospital mortality rate was 2.7% (0% for the SAT group vs 3.8% for the DAT group, P = ns). In the DAT group, one patient died of low cardiac output syndrome due to postoperative myocardial infarction and the other one, never discharged at home, died three months after the surgery of mediastinitis.
We did not observe significant differences between the groups regarding the incidence of major postoperative complications: postoperative myocardial infarction (the SAT group 15.0% vs the DAT group 11.5%; P = ns), acute kidney injury (the SAT group 5.0% vs the DAT group 2.0%; P = ns) and neurological events (the SAT group 5% vs the DAT group 0%; P = ns). Furthermore, DAT was not associated with an increase in major bleeding (the SAT group 10.0% vs the DAT group 2.0%) or a major need for transfusion. Postoperative outcomes are summarized in Table 3.
| Variables . | SAT group (n = 20) . | DAT group (n = 52) . | P-value . |
|---|---|---|---|
| In-hospital mortality, n (%) | 0 (0) | 2 (4) | ns |
| Postoperative LVEF (%), mean ± SD | 50 ± 6.2 | 51 ± 8.7 | ns |
| Atrial fibrillation, n (%) | 3 (15) | 14 (26) | ns |
| Acute kidney injury, n (%) | 1 (5) | 1 (2) | ns |
| Major bleeding, n (%) | 2 (10) | 1 (2) | ns |
| Neurological injury, n (%) | 1 (5) | 0 (0) | ns |
| Respiratory failure, n (%) | 1 (5) | 1 (2) | ns |
| Myocardial Infarction, n (%) | 3 (15) | 6 (11) | ns |
| Postoperative length of stay (days), mean ± SD | 10.1 ± 11.2 | 6.5 ± 2.6 | 0.03 |
| Variables . | SAT group (n = 20) . | DAT group (n = 52) . | P-value . |
|---|---|---|---|
| In-hospital mortality, n (%) | 0 (0) | 2 (4) | ns |
| Postoperative LVEF (%), mean ± SD | 50 ± 6.2 | 51 ± 8.7 | ns |
| Atrial fibrillation, n (%) | 3 (15) | 14 (26) | ns |
| Acute kidney injury, n (%) | 1 (5) | 1 (2) | ns |
| Major bleeding, n (%) | 2 (10) | 1 (2) | ns |
| Neurological injury, n (%) | 1 (5) | 0 (0) | ns |
| Respiratory failure, n (%) | 1 (5) | 1 (2) | ns |
| Myocardial Infarction, n (%) | 3 (15) | 6 (11) | ns |
| Postoperative length of stay (days), mean ± SD | 10.1 ± 11.2 | 6.5 ± 2.6 | 0.03 |
DAT: double antiplatelet therapy; LVEF: left ventricular ejection fraction; SAT: single antiplatelet therapy; SD: standard deviation.
| Variables . | SAT group (n = 20) . | DAT group (n = 52) . | P-value . |
|---|---|---|---|
| In-hospital mortality, n (%) | 0 (0) | 2 (4) | ns |
| Postoperative LVEF (%), mean ± SD | 50 ± 6.2 | 51 ± 8.7 | ns |
| Atrial fibrillation, n (%) | 3 (15) | 14 (26) | ns |
| Acute kidney injury, n (%) | 1 (5) | 1 (2) | ns |
| Major bleeding, n (%) | 2 (10) | 1 (2) | ns |
| Neurological injury, n (%) | 1 (5) | 0 (0) | ns |
| Respiratory failure, n (%) | 1 (5) | 1 (2) | ns |
| Myocardial Infarction, n (%) | 3 (15) | 6 (11) | ns |
| Postoperative length of stay (days), mean ± SD | 10.1 ± 11.2 | 6.5 ± 2.6 | 0.03 |
| Variables . | SAT group (n = 20) . | DAT group (n = 52) . | P-value . |
|---|---|---|---|
| In-hospital mortality, n (%) | 0 (0) | 2 (4) | ns |
| Postoperative LVEF (%), mean ± SD | 50 ± 6.2 | 51 ± 8.7 | ns |
| Atrial fibrillation, n (%) | 3 (15) | 14 (26) | ns |
| Acute kidney injury, n (%) | 1 (5) | 1 (2) | ns |
| Major bleeding, n (%) | 2 (10) | 1 (2) | ns |
| Neurological injury, n (%) | 1 (5) | 0 (0) | ns |
| Respiratory failure, n (%) | 1 (5) | 1 (2) | ns |
| Myocardial Infarction, n (%) | 3 (15) | 6 (11) | ns |
| Postoperative length of stay (days), mean ± SD | 10.1 ± 11.2 | 6.5 ± 2.6 | 0.03 |
DAT: double antiplatelet therapy; LVEF: left ventricular ejection fraction; SAT: single antiplatelet therapy; SD: standard deviation.
Follow-up results
During follow-up, 14 (20%) new deaths occurred (5 in the SAT group and 9 in the DAT group): 8 cardiac deaths, 2 of neoplasms, 2 of cerebrovascular events, and 2 for unknown reasons that were considered cardiac for the statistical analysis.
The overall 7-year survival rate was 75 ± 5%. Advanced age (P = 0.003), chronic pulmonary disease (P = 0.033) and CPB time (P = 0.01) were identified as independent predictors of death for any cause, including operative mortality. Several factors, including left ventricular ejection fraction, chronic renal disease, postoperative stroke and postoperative myocardial infarction, were considered significant by univariate analysis, but were not confirmed by multivariate computation (Table 4).
| Characteristics . | Odds ratio . | 95% CI . | P-value . |
|---|---|---|---|
| Age | 1.29 | 1.09–1.53 | 0.003 |
| Chronic obstructive pulmonary disease | 23.8 | 1.29–439 | 0.033 |
| NYHA class III/IV | 1.12 | 0.14–8.6 | 0.9 |
| Chronic renal disease | 2.13 | 0.000 | 0.99 |
| Perioperative MI | 14 | 0.7–256 | 0.72 |
| Postoperative LVEF | 0.9 | 0.85–1.059 | 0.81 |
| Cardiopulmonary bypass time | 0.92 | 0.86–0.987 | 0.01 |
| Postoperative stroke/TIA | 23 | 0.000 | 0.99 |
| Characteristics . | Odds ratio . | 95% CI . | P-value . |
|---|---|---|---|
| Age | 1.29 | 1.09–1.53 | 0.003 |
| Chronic obstructive pulmonary disease | 23.8 | 1.29–439 | 0.033 |
| NYHA class III/IV | 1.12 | 0.14–8.6 | 0.9 |
| Chronic renal disease | 2.13 | 0.000 | 0.99 |
| Perioperative MI | 14 | 0.7–256 | 0.72 |
| Postoperative LVEF | 0.9 | 0.85–1.059 | 0.81 |
| Cardiopulmonary bypass time | 0.92 | 0.86–0.987 | 0.01 |
| Postoperative stroke/TIA | 23 | 0.000 | 0.99 |
LVEF: left ventricular ejection fraction; MI: myocardial infarction; NYHA: New York Heart Association; TIA: transient ischaemic attack.
| Characteristics . | Odds ratio . | 95% CI . | P-value . |
|---|---|---|---|
| Age | 1.29 | 1.09–1.53 | 0.003 |
| Chronic obstructive pulmonary disease | 23.8 | 1.29–439 | 0.033 |
| NYHA class III/IV | 1.12 | 0.14–8.6 | 0.9 |
| Chronic renal disease | 2.13 | 0.000 | 0.99 |
| Perioperative MI | 14 | 0.7–256 | 0.72 |
| Postoperative LVEF | 0.9 | 0.85–1.059 | 0.81 |
| Cardiopulmonary bypass time | 0.92 | 0.86–0.987 | 0.01 |
| Postoperative stroke/TIA | 23 | 0.000 | 0.99 |
| Characteristics . | Odds ratio . | 95% CI . | P-value . |
|---|---|---|---|
| Age | 1.29 | 1.09–1.53 | 0.003 |
| Chronic obstructive pulmonary disease | 23.8 | 1.29–439 | 0.033 |
| NYHA class III/IV | 1.12 | 0.14–8.6 | 0.9 |
| Chronic renal disease | 2.13 | 0.000 | 0.99 |
| Perioperative MI | 14 | 0.7–256 | 0.72 |
| Postoperative LVEF | 0.9 | 0.85–1.059 | 0.81 |
| Cardiopulmonary bypass time | 0.92 | 0.86–0.987 | 0.01 |
| Postoperative stroke/TIA | 23 | 0.000 | 0.99 |
LVEF: left ventricular ejection fraction; MI: myocardial infarction; NYHA: New York Heart Association; TIA: transient ischaemic attack.
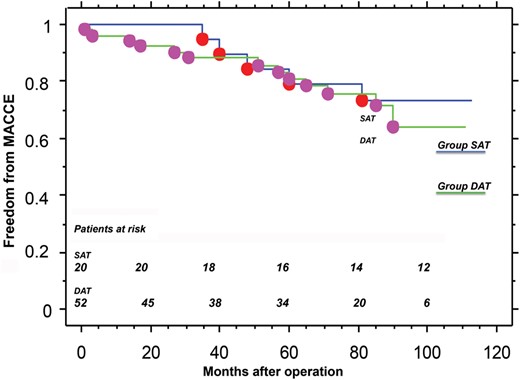
Freedom from major adverse cardiac and cerebrovascular events.
At multivariate analysis, only chronic pulmonary disease was a significant independent predictor of cardiac death during the follow-up period (P = 0.022), whereas advanced age at operation reached the limit of statistical significance (P = 0.055) (Table 5).
| Characteristics . | Odds ratio . | 95% CI . | P-value . |
|---|---|---|---|
| Advanced age | 1.123 | 0.99–1.264 | 0.055 |
| Chronic obstructive pulmonary disease | 1.14 | 1.41–102.3 | 0.022 |
| Perioperative MI | 5.8 | 0.69–49.1 | 0.103 |
| Postoperative LVEF | 0.9 | 0.83–1.109 | 0.111 |
| Characteristics . | Odds ratio . | 95% CI . | P-value . |
|---|---|---|---|
| Advanced age | 1.123 | 0.99–1.264 | 0.055 |
| Chronic obstructive pulmonary disease | 1.14 | 1.41–102.3 | 0.022 |
| Perioperative MI | 5.8 | 0.69–49.1 | 0.103 |
| Postoperative LVEF | 0.9 | 0.83–1.109 | 0.111 |
LVEF: left ventricular ejection fraction; MI: myocardial infarction.
| Characteristics . | Odds ratio . | 95% CI . | P-value . |
|---|---|---|---|
| Advanced age | 1.123 | 0.99–1.264 | 0.055 |
| Chronic obstructive pulmonary disease | 1.14 | 1.41–102.3 | 0.022 |
| Perioperative MI | 5.8 | 0.69–49.1 | 0.103 |
| Postoperative LVEF | 0.9 | 0.83–1.109 | 0.111 |
| Characteristics . | Odds ratio . | 95% CI . | P-value . |
|---|---|---|---|
| Advanced age | 1.123 | 0.99–1.264 | 0.055 |
| Chronic obstructive pulmonary disease | 1.14 | 1.41–102.3 | 0.022 |
| Perioperative MI | 5.8 | 0.69–49.1 | 0.103 |
| Postoperative LVEF | 0.9 | 0.83–1.109 | 0.111 |
LVEF: left ventricular ejection fraction; MI: myocardial infarction.
During follow-up, two patients (10%) in the SAT group and four patients (8%) in the DAT group experienced new myocardial infarctions. Furthermore, two patients needed new PCIs: one from the SAT group after 60 months for non-ST segment elevation myocardial infarction due to saphenous vein graft stenosis that was successfully treated by stenting, and one from the DAT group after 90 months for chronic angina related to marginal branch stenosis, treated by medical therapy. In both patients, the vessel treated by CE was still patent on the coronary angiograph. No patients needed redo CABG.
Freedom from a new PCI at 7 years was 93 ± 6% in the SAT group versus 100% in the DAT group (P = ns).
Two patients experienced a fatal stroke: one patient from the SAT group after 48 months and one patient from the DAT group after 51 months. Freedom from neurological events at 7 years was 100% in the SAT group and 97 ± 4% in the DAT group (P = ns). No major bleeding was observed in any of the patients.
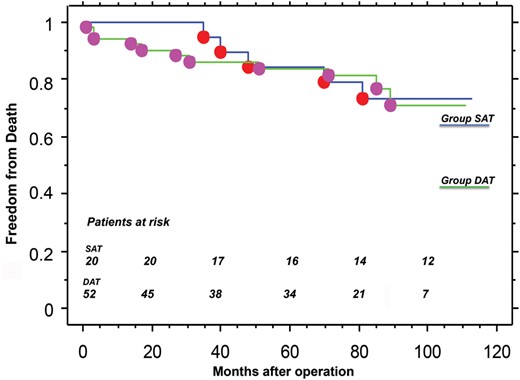
For all survivors, clinical status was established by clinical examination. No patients in either group had angina at rest. There was a significant reduction in angina and dyspnoea in the entire cohort postoperatively compared to the preoperative period (P < 0.001).
DISCUSSION
Patients with diffuse coronary atherosclerotic disease represent a challenge in the current era of cardiac surgery. Even though PCI showed good results during the last decade and the current international guidelines encourage percutaneous coronary revascularization, CABG remains the best treatment for patients with diffuse coronary disease [4].
Furthermore, many studies indicate that complete revascularization is one of the main achievements of coronary surgery [8, 9]: Untreated residual coronary artery disease increases the risk of perioperative myocardial infarction and in-hospital mortality, and it is associated with a late recurrence of symptoms and the need for revascularization [5]. In this complex scenario, CE seems to be a useful and sometimes mandatory surgical option for these patients.
CE was introduced by Baeily in 1957 for the treatment of coronary occlusion. Since then, its role in cardiac surgery has remained unclear, and the results are still debated.
Operative mortality following CABG/CE ranges from 2.0% to 6.5%, and it is higher compared to CABG without CE [9–12]. Despite the presence of diffuse coronary artery disease, CABG with endarterectomy on LAD offers favourable long-term survival [13].
Furthermore, late graft patency rates after CE range from 40% to 81.5%. Schwann et al. analysed the results of 288 patients operated with CABG/CE. In this series, the graft failure rate, determined from angiographic studies, was 33% of endarterectomised vessels during a mean follow-up of 400 days; the graft failure rate following CE was not statistically different from that of non-endarterectomized patients [1].
Nishi et al. treated 127 patients with diffuse CAD by CABG/CE between January 1994 and April 2003. At mid-term follow-up (16–22 months), 61% of patients underwent new coronary angiographic examinations that indicated that 85% of the vessels treated by CE were still patent [14].
The role of antiplatelet therapy in improving long-term graft patency after coronary operations is well documented. Although aspirin after CABG is designated as a Class I medication [15], the benefit of taking clopidogrel concurrently is controversial. Some studies showed that DAT increases saphenous vein graft patency and plays an important role in keeping the graft’s patent after off-pump bypass surgery or after CABG performed to treat acute coronary syndrome disease [16–18]. On the other hand, these results are limited to specific subgroups of patients, and the majority of clinical trials have failed to demonstrate an improvement in graft patency with DAT [19]. Nevertheless, there are no specific anticoagulation/antiplatelet therapy protocols for the postoperative management of patients treated with CABG/CE.
In our series, we analysed the mid-term clinical results of two homogeneous groups of patients who underwent CABG and CE during a 7-year period and were treated with two different antiplatelet protocols according to the patient's characteristics (e.g. complexity of coronary lesions, diffuse occlusive disease, permanent atrial fibrillation). The SAT group (n = 20) was treated postoperatively with aspirin 100 mg daily, whereas patients in the DAT group had aspirin plus 75 mg clopidogrel daily. In the absence of bleeding, all patients received 250 mg of acetylsalicylic acid intravenously 6 h after surgery. During the follow-up period, coronary risk factors were carefully monitored, and all patients were treated with statins to decrease the levels of low-density lipoproteins.
In our population, freedom from cardiac death at 7 years was 84 ± 9% in the SAT group compared with 85 ± 5% in the DAT group, whereas freedom from new PCIs was 93 ± 6% versus 100%, respectively (P = ns).
During follow-up, two patients needed new PCIs: one in the SAT group after 60 months and one in the DAT group after 90 months. After 90 months, just 9 patients in the DAT group were at risk, so we could not consider these data as statistically powerful. During the follow-up period, no patient required revascularization. We also analysed freedom from major adverse cardiac and cerebrovascular events at 7 years: 73 ± 10% in the SAT group and 75 ± 6% in the DAT group (P = ns). Moreover, at follow-up, no patient presented with angina at rest. The significant reduction of postoperative angina compared to preoperative angina in the entire cohort was remarkable.
Our results compare favourably with the results reported by other authors. Shapira and colleagues reported a 5-year survival rate of 70% (including 2% in-hospital mortality) in a series of 151 patients [17]. Schwan and colleagues reported a 77% 5-year survival rate for 270 patients who had CABG/CE who were discharged from the hospital [1].
In our report, there were no statistically significant differences between the two different antiplatelet protocols, even if the DAT group showed a better trend in terms of early and late outcomes.
It is interesting to note that, as reported elsewhere in the literature [20, 21], in this series, chronic obstructive pulmonary disease represents a continuing detrimental risk factor for long-term survival: Patients with diffuse coronary artery disease represent a complex group in which both cardiac and not-cardiac factors play a crucial role during the follow-up period.
Our study has several limitations: First, it is retrospective and not randomized. Moreover, we analysed only clinical outcomes and not graft patency by imaging techniques. Therefore, many events considered in the statistical analyses could not be related to the vessel treated with CE. In addition, convincing apparently healthy patients to submit to a follow-up angiographic study is difficult. Thus, it is not possible from our data to provide evidence of the superiority of SAT or DAT. Another study with more patients and angiographic follow-up is necessary to evaluate the effect of antiplatelet aggregation protocol on early and late outcomes.
In conclusion, CE represents a safe, feasible technique for the treatment of patients with diffuse coronary artery disease; it results in acceptable mid-term morbidity and mortality rates and in a significant reduction of symptoms. Different postoperative antiplatelet or anticoagulation regimens could play a key role in improving clinical results and graft patency. We found no differences in terms of major clinical outcomes between patients treated with a single compared with a dual antiplatelet regimen during a mid-term follow-up period, even if the DAT administered for a 12-month period seems to guarantee a better outcome.
Conflict of interest: none declared.
REFERENCES
- anticoagulation
- antiplatelet agents
- aspirin
- clopidogrel
- percutaneous coronary intervention
- coronary artery bypass surgery
- coronary arteriosclerosis
- hemorrhage
- follow-up
- objective (goal)
- hospital mortality
- surgical procedures, operative
- tissue transplants
- heart
- treatment outcome
- coronary heart disease
- perioperative myocardial infarction
- endarterectomy of coronary artery
- dual anti-platelet therapy




