-
PDF
- Split View
-
Views
-
Cite
Cite
Silje Ekroll Jahren, Bernhard Michael Winkler, Paul Philipp Heinisch, Jessica Wirz, Thierry Carrel, Dominik Obrist, Aortic root stiffness affects the kinematics of bioprosthetic aortic valves, Interactive CardioVascular and Thoracic Surgery, Volume 24, Issue 2, 1 February 2017, Pages 173–180, https://doi.org/10.1093/icvts/ivw284
Close - Share Icon Share
In this study, the influence of aortic root distensibility on the haemodynamic parameters and valve kinematics of a bioprosthetic aortic valve was investigated in a controlled in vitro experiment.
An Edwards INTUITY Elite 21 mm sutureless aortic valve (Edwards Lifesciences, Irvine, CA, USA) was inserted in three transparent aortic root phantoms with different wall thicknesses (0.55, 0.85 and 1.50 mm) mimicking different physiological distensibilities. Haemodynamic measurements were performed in an in vitro flow loop at heart rates of 60, 80 and 100 bpm with corresponding cardiac outputs of 3.5, 4.0 and 5.0 l/min and aortic pressures of 100/60, 120/90 and 145/110 mmHg, respectively. Aortic valve kinematics were assessed using a high-speed camera. The geometric orifice area (GOA) was measured by counting pixels in the lumen of the open aortic valve. The effective orifice area (EOA) was calculated from the root-mean-square value of the systolic aortic valve flow rate and the mean systolic trans-valvular pressure gradient.
The tested aortic root phantoms reproduce physiological distensibilities of healthy individuals in age groups ranging from 40 to 70 years (±10 years). The haemodynamic results show only minor differences between the aortic root phantoms: the trans-valvular pressure gradient tends to increase for stiffer aortic roots, whereas the systolic aortic valve flow rate remains constant. As a consequence, the EOA decreased slightly for less distensible aortic roots. The GOA and the aortic valve opening and closing velocities increase significantly with reduced distensibility for all haemodynamic measurements. The resulting mean systolic flow velocity in the aortic valve orifice is lower for the stiffer aortic root.
Aortic root distensibility may influence GOA and aortic valve kinematics, which affects the mechanical load on the aortic valve cusps. Whether these changes have a significant effect on the onset of structural valve deterioration of bioprosthetic heart valves needs to be further investigated.
INTRODUCTION
The native aortic valve shows competent function without early structural failure for challenging physiological demands. The aortic root including the sinus portions has long been known to be important for the correct functioning of the aortic valve cusps and therefore also for the competence of the aortic valve [1–3]. During systole, vortices form in the sinus portions and affect the position of the cusps. It has been suggested that they contribute during end-systole to the prevention of jet formation and competent closure of the valve [4, 5]. In 1999, a study by Yacoub et al. [6] indicated the importance of further studies of the haemodynamic influence of the aortic root on aortic valve performance.
The distensibility of the aortic root is related to the opening and closing kinematics of the native aortic valve and to an increase of the valve orifice area during exercise [7]. These physiological mechanisms may be altered in stiff roots, e.g. heavily calcified roots. The distensibility of the aortic root also has an effect on the blood flow patterns in the aortic root and on the related mechanical stresses, which may affect aortic valve cusp tissue remodelling or pathological changes [3]. Degeneration and subsequent calcification of the aortic valve have been connected to zones with high mechanical stress [8]. Valvular diseases such as aortic stenosis are associated with aortic valve tissue and annulus thickening, reduction in elasticity and the ability to adapt to changes during the cardiac cycle, reduction in distensibility, and increasing stiffness of the aortic valve cusps and root [8, 9]. In patients with bicuspid aortic valves, the reduction in aortic wall elasticity was associated with aneurysms of the ascending aorta, left ventricular (LV) hypertrophy and aortic valve regurgitation [10]. In humans older than 50–60 years of age, it has been reported that decreased distensibility and changes in the aortic root geometry can lead to different biomechanical and haemodynamic characteristics of the aortic valve [8, 11].
In the light of the general trend towards bioprosthetic and transcatheter aortic valves, the topic of aortic root distensibility is of growing interest because bioprosthetic structural valve deterioration (SVD) and anatomical changes are important prognostic factors. The ideal design of artificial heart valves has not been identified [12]. Serious problems remain with respect to the long-term performance of bioprosthetic valves and patient outcomes [13–16] related to SVD, thrombosis, tissue overgrowth, paravalvular leaks and calcification. Different independent factors have been suggested to influence SVD [8]. In recent studies [17, 18], the sinus portions and the distensibility of aortic root grafts have been shown to affect aortic valve kinematics after aortic valve sparing procedures. The objective of this study is to investigate the effect of aortic root distensibility in the context of bioprosthetic aortic valves. For this purpose, we studied haemodynamic parameters and kinematics of a bioprosthetic aortic valve in a controlled in vitro experiment with systematically varied aortic root distensibilities.
MATERIALS AND METHODS
Aortic root phantoms
![Overview of the left heart simulator set-up [20]. (A) Sideway camera view of the aortic root phantom with the aortic valve; (B) retrograde axial camera view of the aortic valve.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/icvts/24/2/10.1093_icvts_ivw284/2/m_ivw28401.jpeg?Expires=1749851544&Signature=hfNPYTb4OW4eLwA99gsUhUF9WlikBoQYeGNYcOtw0A4VapFLTwX5ytera37cmays2Q0j~ppfkw6ZY82TH0~f42GtjpBPZBfE~WQxYvlhhLXrwbABvRPsS3D5LSBxCuq5NC1TmoVtXf3Ei5KQ5Y-Y7JwbTRE9aoxJBmJfaJODfdPkoAq9YdH2jCoXEbWqAClCuPv~1OazPU6XCnI5CfAdo3mbKXFnjr1-jIlgp2PgAKAn91IELC3Pn3EmxKKoYQGsSCgv6TlLa1Wsdfjxo8wFL~hQeGmhKpYOACu24B5hh69hJ4NTPSxS1QEi1tECXafXzaKipMjpKy2GiQ5bqUscfw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Overview of the left heart simulator set-up [20]. (A) Sideway camera view of the aortic root phantom with the aortic valve; (B) retrograde axial camera view of the aortic valve.
Left heart simulator
The aortic root phantoms with the inserted aortic valve were tested in a left heart simulator (Fig. 1) described previously [20]. The left heart simulator consisted of four polymethyl-methacrylate chambers representing the left atrium (LA) and the left ventricle (LV), a water-filled chamber enclosing the aortic root phantom to allow undistorted optical access and a chamber representing the systemic arterial tree compliance. A bileaflet mechanical valve separated the LA and LV chambers, mimicking the mitral valve (MV), and a gated valve between the compliance chamber and the LA chamber representing the systemic resistance. A mixture of 40/60% glycerine and water at room temperature was used to mimic the viscosity of blood.
Haemodynamic measurements
Haemodynamic measurements for each aortic root phantom were performed for three different haemodynamic configurations:
At 60 bpm, with a cardiac output of ∼3.5 l/min and an aortic pressure of 100/60 mmHg.
At 80 bpm, with a cardiac output of ∼4.0 l/min and an aortic pressure of 120/90 mmHg.
At 100 bpm, with a cardiac output of ∼5.0 l/min and an aortic pressure of 145/110 mmHg.
The LV and aortic pressures (measured in the LV and the compliance chamber, respectively), the pump flow and the MV flow were recorded. All haemodynamic measurements were performed for 25 s with a sampling rate of 200 Hz.
Aortic valve kinematics assessment
A high-speed camera (Basler piA640-210gc GigE, Basler AG, Ahrensburg, Germany) captured the aortic valve movements during the haemodynamic measurements described above at a frame rate of 200 Hz for 20 s. The camera was mounted either sideways to visualize cusp motion in the axial plane of the aortic root phantoms as well as the dilatation of the phantom walls (Fig. 1A) or axially (retrograde axial view) to visualize the cusp motion in the valvular plane (Fig. 1B). Experiments for each haemodynamic configuration were performed twice with the camera in either the sideways or axial position, yielding a total of six performed measurements for each aortic root phantom. The haemodynamic measurements and the camera frames were synchronized using a synthetic trigger signal.
Data analysis
The camera frames taken in the axial view were used to measure the geometric orifice area (GOA) by counting (frame by frame) the pixels in the lumen of the open aortic valve, which was identified by an edge detection algorithm using MATLAB. The conversion from pixels to centimetres was calibrated using the distance (∼100 pixels) between two well-defined reference points on the stent ring. The uncertainty in the reference length measurement was ±2 pixels, which led to an uncertainty of ±4% in the resulting area measurements. The robustness of the method for measuring the GOA was tested by comparing the results obtained with slight variations of the method (e.g. different parameter settings for the edge detection method, different choice of reference points for calibration). It was found that the results were robust and accurate within the given range of uncertainty.
Analysis of variance (ANOVA) was performed to test if there was a statistically significant difference between the means of the averaged systolic GOAs of the different aortic root phantoms, as well as between the means of the EOAs and the aortic valve opening and closing velocities. The ANOVA was performed separately for each haemodynamic configuration. Pairwise t-tests were performed to determine between which aortic root phantoms these parameters were significantly different. The differences were considered significant for P-values less than 0.05.
RESULTS
Aortic root phantom distensibility
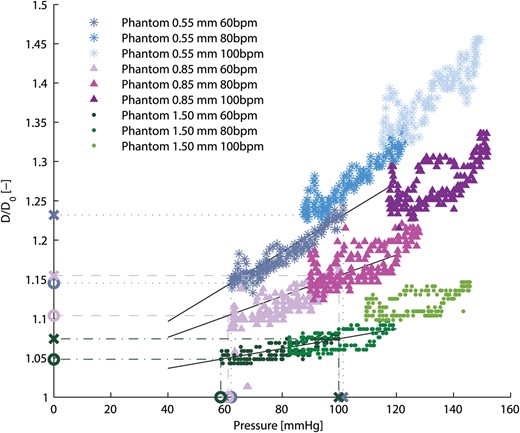
Relative aortic root phantom diameter (D/D0) at the ascending aorta as a function of aortic pressure for all aortic root phantoms and haemodynamic measurements. The values used to calculate the aortic root phantom distensibilities are marked on the axis with X for the systolic values and O for the diastolic values. The solid black lines indicate the slopes between the corresponding systolic and diastolic values.
Haemodynamic results
| Phantom thickness (mm) . | HR (bpm) . | Aortic systolic pressure (mmHg) . | Aortic diastolic pressure (mmHg) . | Mean pressure gradient (mmHg) . | Mean systolic aortic valve rms flow (ml/s) . | Cardiac flow output (l/min) . | Camera position . |
|---|---|---|---|---|---|---|---|
| 0.55 | 59.8 (0.3) | 101.8 (0.2) | 62.2 (0.2) | 9.8 (0.2) | 264.0 (1.7) | 3.5 (0.03) | Sideways |
| 59.9 (0.2) | 102.2 (0.4) | 63.8 (0.3) | 9.9 (0.2) | 266.6 (2.2) | 3.5 (0.03) | Axial | |
| 78.6 (0.2) | 123.4 (0.2) | 87.6 (0.4) | 11.3 (0.2) | 294.5 (2.6) | 4.2 (0.03) | Sideways | |
| 78.6 (0.3) | 123.4 (0.2) | 86.8 (0.2) | 11.4 (0.3) | 296.1 (2.2) | 4.2 (0.03) | Axial | |
| 94.7 (2.8) | 145.5 (2.8) | 110.6 (3.3) | 12.6 (0.4) | 315.8 (5.0) | 4.7 (0.09) | Sideways | |
| 98.3 (0.5) | 148.7 (0.4) | 113.9 (0.4) | 12.3 (0.3) | 319.9 (3.7) | 4.7 (0.04) | Axial | |
| 0.85 | 56.5 (0.2) | 101.2 (0.7) | 62.3 (0.6) | 9.6 (0.2) | 248.0 (2.9) | 3.5 (0.03) | Sideways |
| 58.4 (0.2) | 105.7 (0.3) | 65.1 (0.4) | 9.6 (0.1) | 261.7 (1.9) | 3.6 (0.03) | Axial | |
| 78.5 (0.3) | 128.2 (0.2) | 89.8 (0.2) | 12.3 (0.2) | 287.3 (3.4) | 4.3 (0.03) | Sideways | |
| 78.1(0.3) | 128.3 (0.7) | 91.0 (0.8) | 12.1 (0.3) | 291.0 (3.0) | 4.4 (0.04) | Axial | |
| 97.3 (0.5) | 153.9 (0.7) | 118.2 (0.4) | 12.9 (0.3) | 313.4 (4.9) | 5.0 (0.03) | Sideways | |
| 95.3 (0.3) | 150.3 (0.4) | 115.6 (0.2) | 13.5 (0.3) | 305.5 (3.9) | 5.0 (0.03) | Axial | |
| 1.50 | 56.0 (0.3) | 100.1 (0.2) | 58.4 (0.2) | 8.5 (0.2) | 264.7 (2.3) | 3.6 (0.03) | Sideways |
| 58.5 (0.3) | 101.3 (0.4) | 60.1 (0.3) | 10.0 (0.1) | 267.8 (1.5) | 3.7 (0.03) | Axial | |
| 78.1 (0.3) | 119.5 (0.3) | 81.9 (0.1) | 12.2 (0.3) | 297.8 (3.3) | 4.4 (0.03) | Sideways | |
| 78.2 (0.4) | 121.5 (0.2) | 82.7 (0.3) | 12.4 (0.2) | 302.0 (2.6) | 4.4 (0.03) | Axial | |
| 94.7 (1.0) | 145.5 (0.8) | 108.3 (1.2) | 14.9 (0.4) | 327.4 (3.3) | 5.1 (0.05) | Sideways | |
| 98.3 (0.4) | 146.3 (0.3) | 110.1 (0.5) | 14.6 (0.4) | 321.7 (3.7) | 5.1 (0.05) | Axial |
| Phantom thickness (mm) . | HR (bpm) . | Aortic systolic pressure (mmHg) . | Aortic diastolic pressure (mmHg) . | Mean pressure gradient (mmHg) . | Mean systolic aortic valve rms flow (ml/s) . | Cardiac flow output (l/min) . | Camera position . |
|---|---|---|---|---|---|---|---|
| 0.55 | 59.8 (0.3) | 101.8 (0.2) | 62.2 (0.2) | 9.8 (0.2) | 264.0 (1.7) | 3.5 (0.03) | Sideways |
| 59.9 (0.2) | 102.2 (0.4) | 63.8 (0.3) | 9.9 (0.2) | 266.6 (2.2) | 3.5 (0.03) | Axial | |
| 78.6 (0.2) | 123.4 (0.2) | 87.6 (0.4) | 11.3 (0.2) | 294.5 (2.6) | 4.2 (0.03) | Sideways | |
| 78.6 (0.3) | 123.4 (0.2) | 86.8 (0.2) | 11.4 (0.3) | 296.1 (2.2) | 4.2 (0.03) | Axial | |
| 94.7 (2.8) | 145.5 (2.8) | 110.6 (3.3) | 12.6 (0.4) | 315.8 (5.0) | 4.7 (0.09) | Sideways | |
| 98.3 (0.5) | 148.7 (0.4) | 113.9 (0.4) | 12.3 (0.3) | 319.9 (3.7) | 4.7 (0.04) | Axial | |
| 0.85 | 56.5 (0.2) | 101.2 (0.7) | 62.3 (0.6) | 9.6 (0.2) | 248.0 (2.9) | 3.5 (0.03) | Sideways |
| 58.4 (0.2) | 105.7 (0.3) | 65.1 (0.4) | 9.6 (0.1) | 261.7 (1.9) | 3.6 (0.03) | Axial | |
| 78.5 (0.3) | 128.2 (0.2) | 89.8 (0.2) | 12.3 (0.2) | 287.3 (3.4) | 4.3 (0.03) | Sideways | |
| 78.1(0.3) | 128.3 (0.7) | 91.0 (0.8) | 12.1 (0.3) | 291.0 (3.0) | 4.4 (0.04) | Axial | |
| 97.3 (0.5) | 153.9 (0.7) | 118.2 (0.4) | 12.9 (0.3) | 313.4 (4.9) | 5.0 (0.03) | Sideways | |
| 95.3 (0.3) | 150.3 (0.4) | 115.6 (0.2) | 13.5 (0.3) | 305.5 (3.9) | 5.0 (0.03) | Axial | |
| 1.50 | 56.0 (0.3) | 100.1 (0.2) | 58.4 (0.2) | 8.5 (0.2) | 264.7 (2.3) | 3.6 (0.03) | Sideways |
| 58.5 (0.3) | 101.3 (0.4) | 60.1 (0.3) | 10.0 (0.1) | 267.8 (1.5) | 3.7 (0.03) | Axial | |
| 78.1 (0.3) | 119.5 (0.3) | 81.9 (0.1) | 12.2 (0.3) | 297.8 (3.3) | 4.4 (0.03) | Sideways | |
| 78.2 (0.4) | 121.5 (0.2) | 82.7 (0.3) | 12.4 (0.2) | 302.0 (2.6) | 4.4 (0.03) | Axial | |
| 94.7 (1.0) | 145.5 (0.8) | 108.3 (1.2) | 14.9 (0.4) | 327.4 (3.3) | 5.1 (0.05) | Sideways | |
| 98.3 (0.4) | 146.3 (0.3) | 110.1 (0.5) | 14.6 (0.4) | 321.7 (3.7) | 5.1 (0.05) | Axial |
The standard deviation is given in parentheses.
HR: heart rate; rms: root-mean-square.
| Phantom thickness (mm) . | HR (bpm) . | Aortic systolic pressure (mmHg) . | Aortic diastolic pressure (mmHg) . | Mean pressure gradient (mmHg) . | Mean systolic aortic valve rms flow (ml/s) . | Cardiac flow output (l/min) . | Camera position . |
|---|---|---|---|---|---|---|---|
| 0.55 | 59.8 (0.3) | 101.8 (0.2) | 62.2 (0.2) | 9.8 (0.2) | 264.0 (1.7) | 3.5 (0.03) | Sideways |
| 59.9 (0.2) | 102.2 (0.4) | 63.8 (0.3) | 9.9 (0.2) | 266.6 (2.2) | 3.5 (0.03) | Axial | |
| 78.6 (0.2) | 123.4 (0.2) | 87.6 (0.4) | 11.3 (0.2) | 294.5 (2.6) | 4.2 (0.03) | Sideways | |
| 78.6 (0.3) | 123.4 (0.2) | 86.8 (0.2) | 11.4 (0.3) | 296.1 (2.2) | 4.2 (0.03) | Axial | |
| 94.7 (2.8) | 145.5 (2.8) | 110.6 (3.3) | 12.6 (0.4) | 315.8 (5.0) | 4.7 (0.09) | Sideways | |
| 98.3 (0.5) | 148.7 (0.4) | 113.9 (0.4) | 12.3 (0.3) | 319.9 (3.7) | 4.7 (0.04) | Axial | |
| 0.85 | 56.5 (0.2) | 101.2 (0.7) | 62.3 (0.6) | 9.6 (0.2) | 248.0 (2.9) | 3.5 (0.03) | Sideways |
| 58.4 (0.2) | 105.7 (0.3) | 65.1 (0.4) | 9.6 (0.1) | 261.7 (1.9) | 3.6 (0.03) | Axial | |
| 78.5 (0.3) | 128.2 (0.2) | 89.8 (0.2) | 12.3 (0.2) | 287.3 (3.4) | 4.3 (0.03) | Sideways | |
| 78.1(0.3) | 128.3 (0.7) | 91.0 (0.8) | 12.1 (0.3) | 291.0 (3.0) | 4.4 (0.04) | Axial | |
| 97.3 (0.5) | 153.9 (0.7) | 118.2 (0.4) | 12.9 (0.3) | 313.4 (4.9) | 5.0 (0.03) | Sideways | |
| 95.3 (0.3) | 150.3 (0.4) | 115.6 (0.2) | 13.5 (0.3) | 305.5 (3.9) | 5.0 (0.03) | Axial | |
| 1.50 | 56.0 (0.3) | 100.1 (0.2) | 58.4 (0.2) | 8.5 (0.2) | 264.7 (2.3) | 3.6 (0.03) | Sideways |
| 58.5 (0.3) | 101.3 (0.4) | 60.1 (0.3) | 10.0 (0.1) | 267.8 (1.5) | 3.7 (0.03) | Axial | |
| 78.1 (0.3) | 119.5 (0.3) | 81.9 (0.1) | 12.2 (0.3) | 297.8 (3.3) | 4.4 (0.03) | Sideways | |
| 78.2 (0.4) | 121.5 (0.2) | 82.7 (0.3) | 12.4 (0.2) | 302.0 (2.6) | 4.4 (0.03) | Axial | |
| 94.7 (1.0) | 145.5 (0.8) | 108.3 (1.2) | 14.9 (0.4) | 327.4 (3.3) | 5.1 (0.05) | Sideways | |
| 98.3 (0.4) | 146.3 (0.3) | 110.1 (0.5) | 14.6 (0.4) | 321.7 (3.7) | 5.1 (0.05) | Axial |
| Phantom thickness (mm) . | HR (bpm) . | Aortic systolic pressure (mmHg) . | Aortic diastolic pressure (mmHg) . | Mean pressure gradient (mmHg) . | Mean systolic aortic valve rms flow (ml/s) . | Cardiac flow output (l/min) . | Camera position . |
|---|---|---|---|---|---|---|---|
| 0.55 | 59.8 (0.3) | 101.8 (0.2) | 62.2 (0.2) | 9.8 (0.2) | 264.0 (1.7) | 3.5 (0.03) | Sideways |
| 59.9 (0.2) | 102.2 (0.4) | 63.8 (0.3) | 9.9 (0.2) | 266.6 (2.2) | 3.5 (0.03) | Axial | |
| 78.6 (0.2) | 123.4 (0.2) | 87.6 (0.4) | 11.3 (0.2) | 294.5 (2.6) | 4.2 (0.03) | Sideways | |
| 78.6 (0.3) | 123.4 (0.2) | 86.8 (0.2) | 11.4 (0.3) | 296.1 (2.2) | 4.2 (0.03) | Axial | |
| 94.7 (2.8) | 145.5 (2.8) | 110.6 (3.3) | 12.6 (0.4) | 315.8 (5.0) | 4.7 (0.09) | Sideways | |
| 98.3 (0.5) | 148.7 (0.4) | 113.9 (0.4) | 12.3 (0.3) | 319.9 (3.7) | 4.7 (0.04) | Axial | |
| 0.85 | 56.5 (0.2) | 101.2 (0.7) | 62.3 (0.6) | 9.6 (0.2) | 248.0 (2.9) | 3.5 (0.03) | Sideways |
| 58.4 (0.2) | 105.7 (0.3) | 65.1 (0.4) | 9.6 (0.1) | 261.7 (1.9) | 3.6 (0.03) | Axial | |
| 78.5 (0.3) | 128.2 (0.2) | 89.8 (0.2) | 12.3 (0.2) | 287.3 (3.4) | 4.3 (0.03) | Sideways | |
| 78.1(0.3) | 128.3 (0.7) | 91.0 (0.8) | 12.1 (0.3) | 291.0 (3.0) | 4.4 (0.04) | Axial | |
| 97.3 (0.5) | 153.9 (0.7) | 118.2 (0.4) | 12.9 (0.3) | 313.4 (4.9) | 5.0 (0.03) | Sideways | |
| 95.3 (0.3) | 150.3 (0.4) | 115.6 (0.2) | 13.5 (0.3) | 305.5 (3.9) | 5.0 (0.03) | Axial | |
| 1.50 | 56.0 (0.3) | 100.1 (0.2) | 58.4 (0.2) | 8.5 (0.2) | 264.7 (2.3) | 3.6 (0.03) | Sideways |
| 58.5 (0.3) | 101.3 (0.4) | 60.1 (0.3) | 10.0 (0.1) | 267.8 (1.5) | 3.7 (0.03) | Axial | |
| 78.1 (0.3) | 119.5 (0.3) | 81.9 (0.1) | 12.2 (0.3) | 297.8 (3.3) | 4.4 (0.03) | Sideways | |
| 78.2 (0.4) | 121.5 (0.2) | 82.7 (0.3) | 12.4 (0.2) | 302.0 (2.6) | 4.4 (0.03) | Axial | |
| 94.7 (1.0) | 145.5 (0.8) | 108.3 (1.2) | 14.9 (0.4) | 327.4 (3.3) | 5.1 (0.05) | Sideways | |
| 98.3 (0.4) | 146.3 (0.3) | 110.1 (0.5) | 14.6 (0.4) | 321.7 (3.7) | 5.1 (0.05) | Axial |
The standard deviation is given in parentheses.
HR: heart rate; rms: root-mean-square.
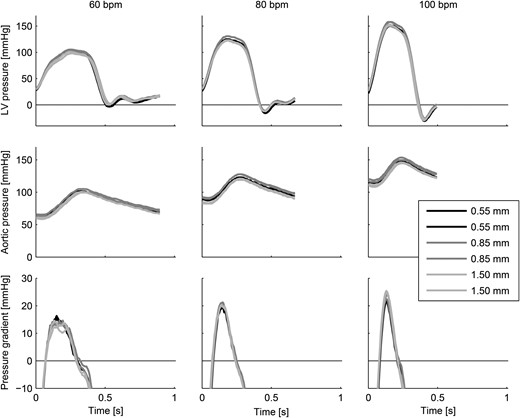
Aortic and left ventricular pressures and trans-valvular pressure gradients for all measurements showing the ensemble-averaged heartbeats.
Aortic valve kinematics
Video sequence with axial and side views of the bioprosthetic aortic valve inserted in the three different aortic root phantoms (from left to right: 0.55, 0.85 and 1.50 mm wall thickness) at 100 bpm and 5 l/min cardiac output. In the first part of the video, four heartbeats are shown in real time (200 frames/s). The second part shows two heartbeats in slow motion (10 frames/s).
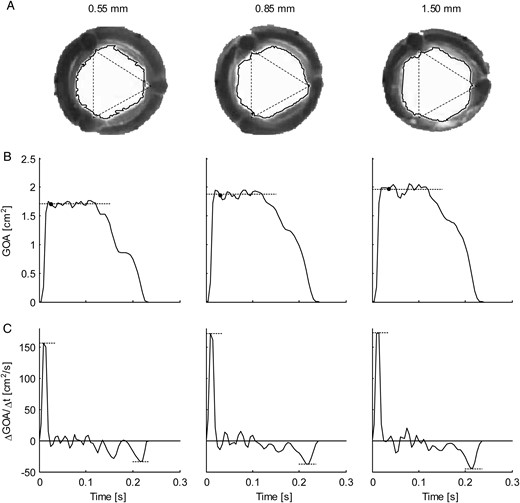
(A) Representative frames (retrograde axial camera view) taken of the aortic valve during systole for the three aortic root phantoms (from left to right: 0.55, 0.85 and 1.50 mm wall thickness) at 100 bpm. The solid lines show the borders of the geometric orifice area (GOA); the corners of the triangles (dotted lines, equal size) indicate the locations of the valve commissures. (B) Ensemble-averaged GOA plotted over time. The dotted lines indicate the mean mid-systolic GOA, and the black dots indicate the timing of the frames in (A). (C) Ensemble-averaged rate of change in GOA per time unit (ΔGOA/Δt). The dotted lines indicate the maximum and minimum rates of change (aortic valve opening and closing velocities, respectively).
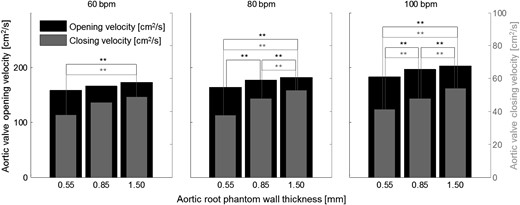
Mean aortic valve opening (black) and closing (grey) velocities for all measurements. **Significantly different aortic valve opening (black) or closing (grey) velocities.
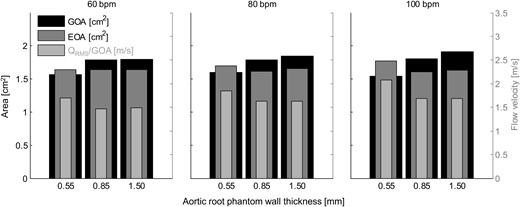
The mean values of the geometric orifice area (GOA), the effective orifice area (EOA) and the mean systolic flow velocity through the aortic valve orifice for all measurements.
DISCUSSION
The aim of this study was to investigate the effect of aortic root distensibility on the haemodynamic parameters and valve kinematics of a sutureless bioprosthetic aortic valve. The measured distensibilities of the aortic root phantoms were found to be in the physiological range reported for healthy individuals [22]. The distensibility of the aortic root phantom with 0.55 mm wall thickness corresponded to values found for healthy individuals in the age group 40 ± 10 years; the phantom with lowest distensibility (1.50 mm wall thickness) corresponded to the age group 70 ± 10 years for healthy individuals. The stiffest phantom may also be a good model for younger patients with cardiovascular diseases that may impact the stiffness of the aortic root (e.g. calcifications).
The haemodynamic performance of the Edwards INTUITY Elite 21 mm aortic valve was found to be in accordance with values given in the literature [23, 24], indicating that the left heart simulator reproduces appropriate physiological flow conditions in the aortic valve and the aortic root. The haemodynamic results showed only minor variations between the different aortic root phantoms. This finding implies that aortic root distensibility has no significant effect on the overall haemodynamic performance of a bioprosthetic aortic valve.
Aortic root distensibility affected the GOA and the aortic valve opening and closing velocities significantly (Fig. 5). We found that the aortic valve opened more (higher GOA) for stiffer aortic root phantoms. This phenomenon was more pronounced for higher heart rates. Most significantly, the GOA for 100 bpm was almost 20% larger for the least distensible aortic root than for the most distensible root. At the same time, the systolic trans-valvular pressure gradients dPmean tended to increase for less distensible roots and, consequently, the EOA decreased slightly. This result can be attributed to the smaller systolic lumen of the less distensible aortic root phantom, which led to higher viscous pressure losses (see Fig. 2: ascending aortic diameter for 100 bpm at peak systole for the thick-walled phantom was ∼20% smaller than for the thin-walled phantom).
The reason why the aortic valve opened more in a stiff aortic root is not well understood. We suspect that it is related to the flow conditions in the sinus portions, which may depend on the distension of the sinus portion. Most likely, the vortices that formed in the sinus portions of the stiff aortic roots had a different character from the vortices in the soft roots, which offered a larger volume for vortex formation. More detailed investigations of the flow in the sinus portions are required to understand the complex dynamics of the aortic root and to explain the increase of GOA in stiffer roots.
The increased GOA in stiffer aortic roots led to a lower mean systolic flow velocity (QRMS/GOA) in the aortic valve orifice. This result implies that the systolic wall-shear-stress acting on the aortic valve cusps was probably lower for these roots. It also suggests that the Reynolds number of the systolic blood stream was lower, which is expected to lead to lower levels of turbulence in the aortic root. At the same time, the stiffer aortic root phantoms showed higher aortic valve opening and closing velocities, which might lead to higher mechanical load on the aortic valve cusps during opening and closure.
One limitation of this study is that only one type of bioprosthetic aortic valve was tested. The Edwards INTUITY Elite sutureless aortic valve is a stented surgical valve similar to the well-studied Edwards PERIMOUNT Magna valve when it comes to cusp design [24, 25]. It is expected that other stented bioprosthetic valves with similar cusp designs will exhibit the same effects related to aortic root stiffness. However, for different bioprosthetic aortic valve design concepts, e.g. stentless or transcatheter designs, the effect of aortic root distensibility can be different and needs further investigation. A second limitation is that only one aortic valve size and aortic root size were tested. The distensibility of the aortic root also depends on the chosen prosthetic valve size and type [26]. Aortic valve size versus aortic root size is expected to influence the flow field behind the valve and thereby also the aortic valve kinematics. Similarly, aortic root morphology and aortic valve positioning in the annulus are expected to have an influence. In this study, only one position of the aortic valve and only one aortic root morphological type were investigated.
Additional research is necessary to clarify the changes in distensibility in the long-term. In particular, scar tissue formation after surgical valve replacement may influence aortic root distensibility [26]. With a better understanding of aortic root physiology, future research on aortic valve replacement should take into account the integrated structural and functional asymmetry of aortic root dynamics to minimize stress on the aortic cusps to mitigate premature SVD.
In conclusion, we have shown that the GOA increases for less distensible aortic roots, and with it also the aortic valve opening and closing velocities. The larger GOA is expected to decrease the systolic wall-shear-stress, acting on the aortic valve cusps due to the lower mean flow velocity and to reduce turbulence levels in the aortic root. The higher opening and closing speeds of the aortic valve lead to higher mechanical load on the cusps for stiffer aortic roots. Aortic root stiffness might influence the onset of SVD, due to the alteration in GOA, cusp kinematics and wall-shear-stress. Whether or not these factors have a significant influence on the lifetime of the bioprosthetic valve needs further investigation.
FUNDING
This work was supported through basic research funds of the University of Bern.
Conflict of interest: none declared.
REFERENCES
Author notes
Presented at the Swiss Cardio Annual Meeting, Lausanne, Switzerland, 15–17 June 2016.
The first two authors contributed equally to this study.




