-
PDF
- Split View
-
Views
-
Cite
Cite
Arudo Hiraoka, Kota Suzuki, Genta Chikazawa, Shinsaku Nogami, Taichi Sakaguchi, Hidenori Yoshitaka, Adaptive servo-ventilation suppresses elevation of C-reactive protein and sympathetic activity in acute uncomplicated type B aortic dissection, Interactive CardioVascular and Thoracic Surgery, Volume 24, Issue 1, January 2017, Pages 27–33, https://doi.org/10.1093/icvts/ivw286
Close - Share Icon Share
The aim of this prospective, randomized study was to investigate the effects of adaptive servo-ventilation (ASV), based on haemodynamic parameters, sympathetic status and respiratory conditions in patients with acute uncomplicated type B aortic dissection.
We enrolled 28 patients with acute uncomplicated type B aortic dissection requiring antihypertensive therapies, who had been admitted within 24 h from onset. Study subjects were randomly assigned either to the ASV group (n = 14) or to the non-ASV group (n = 14).
Antihypertensive therapy at an acute phase led to significant reduction in blood pressure in both groups. Heart rate significantly dropped in the ASV group. In the non-ASV group, noradrenaline (746 ± 343 to 912 ± 402 pg/ml, P = 0.033) and dopamine (30 ± 21 to 42 ± 28 pg/ml, P = 0.015) significantly increased at 1 h after admission. Low frequency/high frequency ratios significantly decreased in the ASV group (2.1 ± 1.6 to 1.7 ± 1.1, P = 0.045). During follow-up at the subacute period, pleural effusion significantly increased in the non-ASV group (649 ± 611 vs 190 ± 292%, P = 0.033). Peak C-reactive protein (CRP) had a significant positive correlation with pleural effusion volume (P = 0.039) and was significantly greater in the non-ASV group (15.5 ± 6.3 vs 8.5 ± 6.1 mg/dl, P= 0.009).
In acute type B aortic dissection, ASV was considered to have suppressed the development of sympathetic nervous activity, pleural effusion and elevation of peak CRP.
INTRODUCTION
With the advent of thoracic endovascular aortic repair, the optimal strategy for treating uncomplicated type B aortic dissection is currently under debate. In addition, the optimal timing of performing thoracic endovascular aneurysm repair (TEVAR) is still controversial [1, 2]. However, with regard to the initial therapeutic approach for uncomplicated type B aortic dissection at an acute phase, conventional treatments of antihypertensive drugs are routinely administered. In the process of administering optimal medical treatment, pleural effusion and oxygenation impairment are frequently observed, and respiratory distress can lead to delayed rehabilitation [3–5]. Although the mechanisms of pleural effusion and oxygenation impairment are not fully known, they should be recognized as serious complications of aortic dissection. Administration of high-dose oxygen can lead to risk of damage to the lungs. In patients with aortic dissection, appropriate management for oxygenation impairment remains an undetermined issue.
Adaptive servo-ventilation (ASV) is a compact ventilator support device designed to achieve synchronized support and comfortable respiration. The efficacy of ASV in patients with heart failure and sleep apnoea has been reported in several studies [6–8], and sleep apnoea is recognized as a significant risk factor for aortic dissection [9]. However, the influence of ASV therapy in patients with acute aortic dissection has not yet been evaluated. The aim of this study was to investigate the prompt effects of ASV based on haemodynamic parameters, sympathetic status and the respiratory condition in patients with acute uncomplicated type B aortic dissection.
MATERIALS AND METHODS
Study design and patient selection
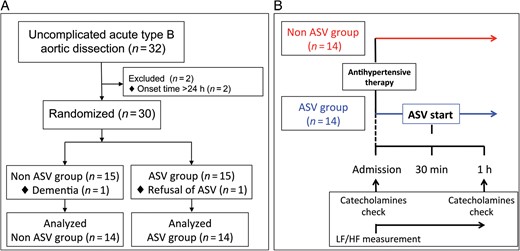
(A) Flow diagram showing the patient selection process. (B) Flow chart of the present study. ASV: adaptive servo-ventilation; LF/HF: low frequency/high frequency.
Patient demographics
The average age was 70.9 ± 15.0 years. Eleven females (39%) were included in the cohort. The mean duration from onset to admission was 4.8 ± 3.2 h, and there were no significant differences between the non-ASV group and the ASV group (5.2 ± 3.1 vs 4.4 ± 3.4 h, P = 0.492). The morphological features of a false lumen were categorized in patients as follows: fully open false lumen in 3 (11%), ulcer-like projections in 3 (11%), complete thrombosis in 17 (61%) and partial thrombosis in 5 (18%). There were no significant differences in baseline data for backgrounds, cardiac function, morphological features of aortic dissection and laboratory findings between patients with and without ASV therapy (Table 1). There was no patient with atrial fibrillation in either group.
Baseline characteristics between patients with and without adaptive servo-ventilation therapy
| Variables . | All (n = 28) . | ASV (−) (n = 14) . | ASV (+) (n = 14) . | P-value . |
|---|---|---|---|---|
| Age (years) | 70.9 ± 15.0 | 71.1 ± 12.1 | 70.7 ± 18.0 | 0.942 |
| Female gender | 11 (39%) | 6 (43%) | 5 (36%) | 1.000 |
| Body surface area (m2) | 1.64 ± 0.26 | 1.61 ± 0.25 | 1.67 ± 0.27 | 0.544 |
| Hypertension | 24 (86%) | 12 (86%) | 12 (86%) | 1.000 |
| Hyperlipidaemia | 7 (25%) | 3 (21%) | 4 (29%) | 1.000 |
| Diabetes mellitus | 7 (25%) | 3 (21%) | 4 (29%) | 1.000 |
| Smoking history | 16 (57%) | 7 (50%) | 9 (64%) | 0.704 |
| COPD | 2 (7%) | 2 (14%) | 0 (0%) | 0.482 |
| LVEF (%) | 66.4 ± 5.6 | 67.7 ± 4.9 | 65.1 ± 6.0 | 0.227 |
| Onset time (h) | 4.8 ± 3.2 | 5.2 ± 3.1 | 4.4 ± 3.4 | 0.492 |
| Aortic diameter (mm) | 39.5 ± 6.9 | 39.1 ± 7.4 | 39.9 ± 6.6 | 0.781 |
| False lumen size (mm) | 11.4 ± 5.4 | 10.8 ± 4.1 | 12.3 ± 7.0 | 0.504 |
| Lumen status | 0.158 | |||
| Fully open | 3 (11%) | 1 (7%) | 2 (14%) | |
| Ulcer-like projections | 3 (11%) | 0 (0%) | 3 (21%) | |
| Complete thrombosis | 17 (61%) | 9 (64%) | 8 (57%) | |
| Partial thrombosis | 5 (18%) | 4 (29%) | 1 (7%) | |
| Laboratory tests | ||||
| WBC (102/μl) | 103 ± 38 | 109 ± 43 | 96 ± 33 | 0.351 |
| Haemoglobin (g/dl) | 12.9 ± 1.9 | 13.3 ± 1.8 | 12.5 ± 2.0 | 0.346 |
| Platelet (104/μl) | 17.9 ± 6.0 | 17.7 ± 6.8 | 18.1 ± 5.4 | 0.872 |
| Creatinine (mg/dl) | 0.8 ± 0.3 | 0.8 ± 0.3 | 0.9 ± 0.3 | 0.417 |
| Albumin (g/dl) | 3.9 ± 0.3 | 3.9 ± 0.3 | 3.9 ± 0.3 | 0.985 |
| CRP (mg/dl) | 0.9 ± 2.6 | 0.4 ± 0.7 | 1.5 ± 3.6 | 0.305 |
| d-dimer (μg/ml) | 12.5 ± 19.7 | 18.5 ± 25.9 | 6.6 ± 7.4 | 0.110 |
| BNP (pg/ml) | 132.7 ± 249.1 | 59.5 ± 55.7 | 205.9 ± 338.1 | 0.122 |
| Variables . | All (n = 28) . | ASV (−) (n = 14) . | ASV (+) (n = 14) . | P-value . |
|---|---|---|---|---|
| Age (years) | 70.9 ± 15.0 | 71.1 ± 12.1 | 70.7 ± 18.0 | 0.942 |
| Female gender | 11 (39%) | 6 (43%) | 5 (36%) | 1.000 |
| Body surface area (m2) | 1.64 ± 0.26 | 1.61 ± 0.25 | 1.67 ± 0.27 | 0.544 |
| Hypertension | 24 (86%) | 12 (86%) | 12 (86%) | 1.000 |
| Hyperlipidaemia | 7 (25%) | 3 (21%) | 4 (29%) | 1.000 |
| Diabetes mellitus | 7 (25%) | 3 (21%) | 4 (29%) | 1.000 |
| Smoking history | 16 (57%) | 7 (50%) | 9 (64%) | 0.704 |
| COPD | 2 (7%) | 2 (14%) | 0 (0%) | 0.482 |
| LVEF (%) | 66.4 ± 5.6 | 67.7 ± 4.9 | 65.1 ± 6.0 | 0.227 |
| Onset time (h) | 4.8 ± 3.2 | 5.2 ± 3.1 | 4.4 ± 3.4 | 0.492 |
| Aortic diameter (mm) | 39.5 ± 6.9 | 39.1 ± 7.4 | 39.9 ± 6.6 | 0.781 |
| False lumen size (mm) | 11.4 ± 5.4 | 10.8 ± 4.1 | 12.3 ± 7.0 | 0.504 |
| Lumen status | 0.158 | |||
| Fully open | 3 (11%) | 1 (7%) | 2 (14%) | |
| Ulcer-like projections | 3 (11%) | 0 (0%) | 3 (21%) | |
| Complete thrombosis | 17 (61%) | 9 (64%) | 8 (57%) | |
| Partial thrombosis | 5 (18%) | 4 (29%) | 1 (7%) | |
| Laboratory tests | ||||
| WBC (102/μl) | 103 ± 38 | 109 ± 43 | 96 ± 33 | 0.351 |
| Haemoglobin (g/dl) | 12.9 ± 1.9 | 13.3 ± 1.8 | 12.5 ± 2.0 | 0.346 |
| Platelet (104/μl) | 17.9 ± 6.0 | 17.7 ± 6.8 | 18.1 ± 5.4 | 0.872 |
| Creatinine (mg/dl) | 0.8 ± 0.3 | 0.8 ± 0.3 | 0.9 ± 0.3 | 0.417 |
| Albumin (g/dl) | 3.9 ± 0.3 | 3.9 ± 0.3 | 3.9 ± 0.3 | 0.985 |
| CRP (mg/dl) | 0.9 ± 2.6 | 0.4 ± 0.7 | 1.5 ± 3.6 | 0.305 |
| d-dimer (μg/ml) | 12.5 ± 19.7 | 18.5 ± 25.9 | 6.6 ± 7.4 | 0.110 |
| BNP (pg/ml) | 132.7 ± 249.1 | 59.5 ± 55.7 | 205.9 ± 338.1 | 0.122 |
ASV: adaptive servo-ventilation; COPD: chronic obstructive pulmonary disease; LVEF: left ventricular ejection fraction; WBC: white blood cell; CRP: C-reactive protein; BNP: B-type natriuretic peptide.
Baseline characteristics between patients with and without adaptive servo-ventilation therapy
| Variables . | All (n = 28) . | ASV (−) (n = 14) . | ASV (+) (n = 14) . | P-value . |
|---|---|---|---|---|
| Age (years) | 70.9 ± 15.0 | 71.1 ± 12.1 | 70.7 ± 18.0 | 0.942 |
| Female gender | 11 (39%) | 6 (43%) | 5 (36%) | 1.000 |
| Body surface area (m2) | 1.64 ± 0.26 | 1.61 ± 0.25 | 1.67 ± 0.27 | 0.544 |
| Hypertension | 24 (86%) | 12 (86%) | 12 (86%) | 1.000 |
| Hyperlipidaemia | 7 (25%) | 3 (21%) | 4 (29%) | 1.000 |
| Diabetes mellitus | 7 (25%) | 3 (21%) | 4 (29%) | 1.000 |
| Smoking history | 16 (57%) | 7 (50%) | 9 (64%) | 0.704 |
| COPD | 2 (7%) | 2 (14%) | 0 (0%) | 0.482 |
| LVEF (%) | 66.4 ± 5.6 | 67.7 ± 4.9 | 65.1 ± 6.0 | 0.227 |
| Onset time (h) | 4.8 ± 3.2 | 5.2 ± 3.1 | 4.4 ± 3.4 | 0.492 |
| Aortic diameter (mm) | 39.5 ± 6.9 | 39.1 ± 7.4 | 39.9 ± 6.6 | 0.781 |
| False lumen size (mm) | 11.4 ± 5.4 | 10.8 ± 4.1 | 12.3 ± 7.0 | 0.504 |
| Lumen status | 0.158 | |||
| Fully open | 3 (11%) | 1 (7%) | 2 (14%) | |
| Ulcer-like projections | 3 (11%) | 0 (0%) | 3 (21%) | |
| Complete thrombosis | 17 (61%) | 9 (64%) | 8 (57%) | |
| Partial thrombosis | 5 (18%) | 4 (29%) | 1 (7%) | |
| Laboratory tests | ||||
| WBC (102/μl) | 103 ± 38 | 109 ± 43 | 96 ± 33 | 0.351 |
| Haemoglobin (g/dl) | 12.9 ± 1.9 | 13.3 ± 1.8 | 12.5 ± 2.0 | 0.346 |
| Platelet (104/μl) | 17.9 ± 6.0 | 17.7 ± 6.8 | 18.1 ± 5.4 | 0.872 |
| Creatinine (mg/dl) | 0.8 ± 0.3 | 0.8 ± 0.3 | 0.9 ± 0.3 | 0.417 |
| Albumin (g/dl) | 3.9 ± 0.3 | 3.9 ± 0.3 | 3.9 ± 0.3 | 0.985 |
| CRP (mg/dl) | 0.9 ± 2.6 | 0.4 ± 0.7 | 1.5 ± 3.6 | 0.305 |
| d-dimer (μg/ml) | 12.5 ± 19.7 | 18.5 ± 25.9 | 6.6 ± 7.4 | 0.110 |
| BNP (pg/ml) | 132.7 ± 249.1 | 59.5 ± 55.7 | 205.9 ± 338.1 | 0.122 |
| Variables . | All (n = 28) . | ASV (−) (n = 14) . | ASV (+) (n = 14) . | P-value . |
|---|---|---|---|---|
| Age (years) | 70.9 ± 15.0 | 71.1 ± 12.1 | 70.7 ± 18.0 | 0.942 |
| Female gender | 11 (39%) | 6 (43%) | 5 (36%) | 1.000 |
| Body surface area (m2) | 1.64 ± 0.26 | 1.61 ± 0.25 | 1.67 ± 0.27 | 0.544 |
| Hypertension | 24 (86%) | 12 (86%) | 12 (86%) | 1.000 |
| Hyperlipidaemia | 7 (25%) | 3 (21%) | 4 (29%) | 1.000 |
| Diabetes mellitus | 7 (25%) | 3 (21%) | 4 (29%) | 1.000 |
| Smoking history | 16 (57%) | 7 (50%) | 9 (64%) | 0.704 |
| COPD | 2 (7%) | 2 (14%) | 0 (0%) | 0.482 |
| LVEF (%) | 66.4 ± 5.6 | 67.7 ± 4.9 | 65.1 ± 6.0 | 0.227 |
| Onset time (h) | 4.8 ± 3.2 | 5.2 ± 3.1 | 4.4 ± 3.4 | 0.492 |
| Aortic diameter (mm) | 39.5 ± 6.9 | 39.1 ± 7.4 | 39.9 ± 6.6 | 0.781 |
| False lumen size (mm) | 11.4 ± 5.4 | 10.8 ± 4.1 | 12.3 ± 7.0 | 0.504 |
| Lumen status | 0.158 | |||
| Fully open | 3 (11%) | 1 (7%) | 2 (14%) | |
| Ulcer-like projections | 3 (11%) | 0 (0%) | 3 (21%) | |
| Complete thrombosis | 17 (61%) | 9 (64%) | 8 (57%) | |
| Partial thrombosis | 5 (18%) | 4 (29%) | 1 (7%) | |
| Laboratory tests | ||||
| WBC (102/μl) | 103 ± 38 | 109 ± 43 | 96 ± 33 | 0.351 |
| Haemoglobin (g/dl) | 12.9 ± 1.9 | 13.3 ± 1.8 | 12.5 ± 2.0 | 0.346 |
| Platelet (104/μl) | 17.9 ± 6.0 | 17.7 ± 6.8 | 18.1 ± 5.4 | 0.872 |
| Creatinine (mg/dl) | 0.8 ± 0.3 | 0.8 ± 0.3 | 0.9 ± 0.3 | 0.417 |
| Albumin (g/dl) | 3.9 ± 0.3 | 3.9 ± 0.3 | 3.9 ± 0.3 | 0.985 |
| CRP (mg/dl) | 0.9 ± 2.6 | 0.4 ± 0.7 | 1.5 ± 3.6 | 0.305 |
| d-dimer (μg/ml) | 12.5 ± 19.7 | 18.5 ± 25.9 | 6.6 ± 7.4 | 0.110 |
| BNP (pg/ml) | 132.7 ± 249.1 | 59.5 ± 55.7 | 205.9 ± 338.1 | 0.122 |
ASV: adaptive servo-ventilation; COPD: chronic obstructive pulmonary disease; LVEF: left ventricular ejection fraction; WBC: white blood cell; CRP: C-reactive protein; BNP: B-type natriuretic peptide.
Adaptive servo-ventilation introduction, antihypertensive therapy and evaluation of plasma catecholamines
On admission, laboratory work, including serum concentration of adrenaline, noradrenaline and dopamine, was initially evaluated. Antihypertensive therapy (calcium antagonists, β-blocker, analgesics and sedatives) was initiated as promptly as possible under intra-arterial and electrocardiographic monitoring for strict maintenance of systemic blood pressure <120 mmHg. At 30 min after administering antihypertensive therapy, ASV (AutoSet CS, ResMed, Sydney, NSW, Australia) support was introduced from the default setting [positive end-expiratory pressure (PEEP): 5 cmH2O; minimum and maximum pressure support (PS): 3 and 10 cmH2O] in the ASV group. PEEP and minimum PS were adjusted according to patients' symptom and could be reduced to 4 and 0 cmH2O, respectively. In the ASV group, ASV was continuously used for no less than 30 min on the day of admission by referring to a previous report [10]. On the next day after admission, ASV was intermittently used for no less than 30 min and continued for no less than 1 week. To obtain patients' cooperation, a multidisciplinary team consisting of a doctor, nurse, public health nurse and physiotherapist intervened. The statuses of ASV usage and rehabilitation progression were discussed in the team conference to continue reasonable use of ASV. The average time of daily ASV usage was 287 ± 167 min. Plasma catecholamines were investigated at 1 h after starting antihypertensive therapy to evaluate the effects of continuous usage of ASV on the sympathetic status. A flow chart of the present study is shown in Fig. 1B.
Spectral analysis of heart rate variability
Changes in heart rate and blood pressure were analysed under electrocardiographic monitoring, and low frequency (LF) and high frequency (HF) were calculated by using MemCalc/Tonam2C (GMS, Tokyo, Japan) to evaluate sympathetic nervous activity [11–13]. LF and HF were measured under electrocardiographic monitoring at intervals of 5 s. The mean LF/HF ratios at 30 min from admission and those between 30 min and 1 h after admission were respectively calculated. The differences were evaluated in both groups (Fig. 1B).
Evaluation of pleural effusion and oxygenation impairment
Follow-up enhanced computed tomography (CT) was examined within 1 week (3–6 days) after admission, depending on patient condition. Pleural effusion was estimated by a previously validated technique [5, 14], using the following equations: left-side pleural effusion volume (ml) = [0.108 × area of pleural effusion (mm2)] + 20.972 ml; right-side pleural effusion volume (ml) = [0.107 × area of pleural effusion (mm2)] + 2.33 ml. Total volume of pleural effusion was defined as the summation of the left- and right-side effusion volume. The area of pleural effusion was calculated from an enhanced 5 mm slice CT using ZIOSTATION 2 PLUS ZWS-2000 (Zio Software Inc., Tokyo, Japan). Oxygenation impairment in patients with aortic dissection was defined as a ratio of the partial pressure arterial oxygen/fractional inspired oxygen (PaO2/FIO2) less than or equal to 200 mmHg. Since measurements of FIO2 under ASV support were not considered to be accurate, the PaO2/FIO2 ratio was alternatively evaluated on admission and followed under non-ASV support at 1 and 24 h after admission.
Statistical analysis
Continuous data are presented as mean ± standard deviation and were analysed using two-tailed t-tests or compared with a Mann–Whitney U-test for independent data, as appropriate. Categorical variables are given as a count and percentage of patients and compared using χ2 or Fisher's exact test. The correlation between parameters was assessed using Pearson's correlation coefficient. Changes in sympathetic nervous activities, arterial blood gas data and haemodynamic parameters between patients with and without ASV therapy were analysed by multivariate analysis of variance (MANOVA). A P-value <0.05 was considered statistically significant. All data were analysed using the Statistical Analysis Systems software JMP 9.0 (SAS Institute Inc., Cary, NC, USA).
RESULTS
Changes of haemodynamic parameters under antihypertensive therapy
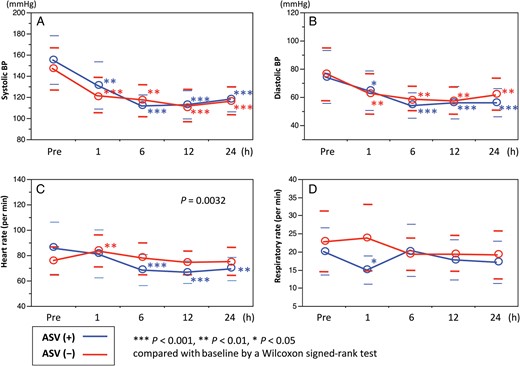
(A and B) In both groups, significant reduction of systolic and diastolic blood pressures (BPs) was achieved. (C) However, heart rate significantly decreased in the adaptive servo-ventilation (ASV) group; no significant change was found in the non-ASV group. (D) There was no significant difference in respiratory rate between patients with and without ASV support.
Pain was measured with the use of the numeric rating scale (0–10). Pentazocine was equally used for patients with a pain score ≥3 (4.3 ± 7.0 mg in the non-ASV group and 5.4 ± 6.9 mg in the ASV group, P = 0.686) as a first-line, anti-pain therapy. Compared with baseline, the pain score similarly decreased at 1, 6, 12 and 24 h after admission in both groups (from 2.4 ± 2.3 to 1.3 ± 1.7, 1.0 ± 1.4, 0.3 ± 0.8 and 0.2 ± 0.6, P = 0.008 in the non-ASV group and from 2.5 ± 1.9 to 1.3 ± 1.3, 0.9 ± 1.8, 0.5 ± 1.3 and 0.4 ± 0.9, P = 0.002 in the ASV group).
In the ASV group, the average of the PaO2/FIO2 ratio at 1 h after admission tended to be higher under the condition without ASV support. The mean of the PaO2/FIO2 ratio and the overall incidence rate of oxygenation impairment at 24 h after admission were 317 ± 92 and 14.3% (4/28), respectively. There were no significant differences in the PaO2/FIO2 ratio (309 ± 91 vs 325 ± 95, P = 0.652) between the non-ASV and ASV groups. Oxygenation impairment at 24 h after admission was found more frequently in the non-ASV group (21% [3/14]) compared with the ASV group (7% [1/14]), but the differences were not significant (P = 0.596).
Changes of status of sympathetic nervous parameters
Heart rate was an independent parameter correlated with the value of noradrenaline and dopamine at the baseline (r = 0.551, P = 0.004 and r = 0.619, P = 0.001) and 30 min after introducing ASV (r = 0.412, P = 0.033 and r = 0.418, P = 0.030). In the ASV group, significant changes in plasma catecholamine concentration were not observed between baseline and 30 min after initiating ASV support (noradrenaline: 794 ± 350 to 839 ± 426 pg/ml, P = 0.502; adrenaline: 178 ± 141 to 171 ± 166 pg/ml, P = 0.764; dopamine: 35 ± 30 to 35 ± 24 pg/ml, P = 0.960). In patients without ASV support, noradrenaline and dopamine significantly increased at 1 h after admission, compared with baseline (noradrenaline: 746 ± 343 to 912 ± 402 pg/ml, P = 0.033; adrenaline: 216 ± 205 to 231 ± 178 pg/ml, P = 0.923; dopamine: 30 ± 21 to 42 ± 28 pg/ml, P = 0.015). Serum dopamine elevation was insignificantly greater in the non-ASV group (P = 0.061).
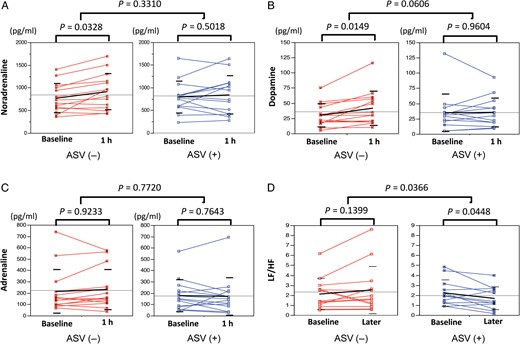
(A–C) In patients without adaptive servo-ventilation (ASV) support, noradrenaline and dopamine significantly increased at 1 h after admission compared with baseline. (D) A significant decrease in the low frequency/high frequency (LF/HF) ratio was found in patients with ASV therapy. MANOVA analysis showed that the change of LF/HF was significant between the non-ASV and the ASV groups.
Pleural effusion and serum C-reactive protein during follow-up at subacute phase
At baseline, the overall mean estimated volume of right- and left-side pleural effusion was 24.0 ± 71.6 and 42.9 ± 72.3 ml, respectively. A follow-up CT within several days after onset revealed significantly greater left-side pleural effusion volumes, compared with those of the right side (39.0 ± 57.3 vs 131.0 ± 101.2 ml, P < 0.001). Between the non-ASV and ASV groups, there were no significant differences in total volume of bilateral pleural effusion at baseline (75.3 ± 186.2 vs 57.1 ± 77.0 ml, P = 0.755) and follow-up (197.5 ± 135.2 vs 137.6 ± 140.4 ml, P = 0.300); however, a significantly lower rate of increase in the total volume of bilateral pleural effusion was observed in the ASV group (649 ± 611 vs 190 ± 292%, P = 0.033) (Table 2).
Comparison of change of pleural effusion between patients with and without adaptive servo-ventilation therapy
| Variables . | All (n = 28) . | ASV (−) (n = 14) . | ASV (+) (n = 14) . | P-value . |
|---|---|---|---|---|
| Right-side PE volume on admission (ml) | 24.0 ± 71.6 | 28.2 ± 92.7 | 19.1 ± 38.3 | 0.754 |
| Left-side PE volume on admission (ml) | 42.9 ± 72.3 | 47.1 ± 93.5 | 37.9 ± 38.7 | 0.754 |
| Right-side PE volume at follow-up (ml) | 39.0 ± 57.3 | 49.9 ± 60.6 | 26.0 ± 53.0 | 0.320 |
| Left-side PE volume at follow-up (ml) | 131.0 ± 101.2 | 147.6 ± 101.5 | 111.5 ± 102.1 | 0.397 |
| Change rate of PE volume (%) | 439 ± 535 | 649 ± 611 | 190 ± 292 | 0.033 |
| Variables . | All (n = 28) . | ASV (−) (n = 14) . | ASV (+) (n = 14) . | P-value . |
|---|---|---|---|---|
| Right-side PE volume on admission (ml) | 24.0 ± 71.6 | 28.2 ± 92.7 | 19.1 ± 38.3 | 0.754 |
| Left-side PE volume on admission (ml) | 42.9 ± 72.3 | 47.1 ± 93.5 | 37.9 ± 38.7 | 0.754 |
| Right-side PE volume at follow-up (ml) | 39.0 ± 57.3 | 49.9 ± 60.6 | 26.0 ± 53.0 | 0.320 |
| Left-side PE volume at follow-up (ml) | 131.0 ± 101.2 | 147.6 ± 101.5 | 111.5 ± 102.1 | 0.397 |
| Change rate of PE volume (%) | 439 ± 535 | 649 ± 611 | 190 ± 292 | 0.033 |
ASV: adaptive servo-ventilation; PE: pleural effusion.
Comparison of change of pleural effusion between patients with and without adaptive servo-ventilation therapy
| Variables . | All (n = 28) . | ASV (−) (n = 14) . | ASV (+) (n = 14) . | P-value . |
|---|---|---|---|---|
| Right-side PE volume on admission (ml) | 24.0 ± 71.6 | 28.2 ± 92.7 | 19.1 ± 38.3 | 0.754 |
| Left-side PE volume on admission (ml) | 42.9 ± 72.3 | 47.1 ± 93.5 | 37.9 ± 38.7 | 0.754 |
| Right-side PE volume at follow-up (ml) | 39.0 ± 57.3 | 49.9 ± 60.6 | 26.0 ± 53.0 | 0.320 |
| Left-side PE volume at follow-up (ml) | 131.0 ± 101.2 | 147.6 ± 101.5 | 111.5 ± 102.1 | 0.397 |
| Change rate of PE volume (%) | 439 ± 535 | 649 ± 611 | 190 ± 292 | 0.033 |
| Variables . | All (n = 28) . | ASV (−) (n = 14) . | ASV (+) (n = 14) . | P-value . |
|---|---|---|---|---|
| Right-side PE volume on admission (ml) | 24.0 ± 71.6 | 28.2 ± 92.7 | 19.1 ± 38.3 | 0.754 |
| Left-side PE volume on admission (ml) | 42.9 ± 72.3 | 47.1 ± 93.5 | 37.9 ± 38.7 | 0.754 |
| Right-side PE volume at follow-up (ml) | 39.0 ± 57.3 | 49.9 ± 60.6 | 26.0 ± 53.0 | 0.320 |
| Left-side PE volume at follow-up (ml) | 131.0 ± 101.2 | 147.6 ± 101.5 | 111.5 ± 102.1 | 0.397 |
| Change rate of PE volume (%) | 439 ± 535 | 649 ± 611 | 190 ± 292 | 0.033 |
ASV: adaptive servo-ventilation; PE: pleural effusion.
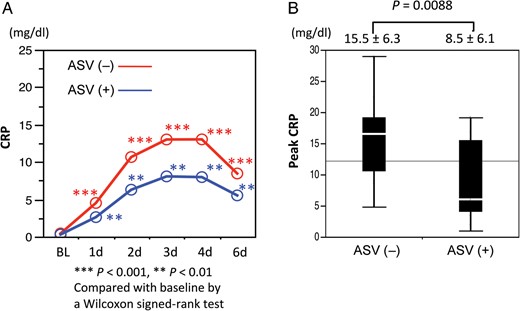
(A) Serum C-reactive protein (CRP) significantly elevated in both the non-adaptive servo-ventilation (ASV) and ASV groups. (B) Peak CRP level was significantly higher in the non-ASV group than in the ASV group.
Clinical states in follow-up
Rehabilitation was planned and performed as scheduled for patients in both groups. Bed rest was continued from admission to the next day (initial 24 h). On the third day after admission, independent standing was begun; walking around the bed was initiated on the fourth day. Follow-up CT at 2 weeks after admission showed no significant difference in the change of the aortic diameter and morphological features between the non-ASV and ASV groups. There were no significant differences in intensive care unit and hospital stay between the non-ASV and ASV groups (3.1 ± 1.1 vs 3.1 ± 1.3 days, P = 1.000; and 14.4 ± 4.4 vs 18.4 ± 7.8 days, P = 0.120). During 6 months of follow-up, TEVAR was performed for 2 patients in the non-ASV group and for 1 patient in the ASV group. Two patients in the ASV group underwent surgical repair (P = 0.305).
DISCUSSION
In general, acute aortic dissection is accompanied by severe and unbearable back pain which can increase sympathetic nervous activity. Moreover, respiratory problems due to pleural effusion and oxygenation impairment are additional factors that activate catecholamines and elevate blood pressure [3–5, 15, 16]. Inflammation and aortic dilatation were reported as risk factors for pleural effusion in patients with acute type B aortic dissection [5]. Incidence of oxygenation impairment in aortic dissection was reported to be 49–51%, and body mass index ≥22 kg/m2, maximum body temperature ≥36.5°C, patent false lumen and a lower PaO2/FIO2 ratio were reported as risk factors [3, 4]. In addition, respiratory impairment can be induced by other causes such as hypoxic pulmonary vasoconstriction due to vasodilators and sleep apnoea in patients requiring antihypertensive therapy for acute aortic dissection [17, 18]. Based on these diverse backgrounds, positive pressure ventilation was thought to be helpful to prevent respiratory impairment and suppress increased sympathetic nervous activity. ASV is compact and simple to apply, and synchronized support of ocean waveform type in AutoSet CS was expected to be more suitable for patients with acute aortic dissection.
In the present study, ASV therapy can partially suppress the increases in serum catecholamines and significantly control the elevation of LF/HF. While blood pressures significantly decreased in both groups by equivalent antihypertensive therapy, only in the ASV group significantly decreased heart rates were achieved. Used dosage of Landiolol and Precedex did not correlate with haemodynamic and sympathetic parameters. Considering that vasodilators generally increase heart rate in return for antihypertensive effects, our results may reflect the efficacy of ASV. Regarding oxygenation impairment, the influence of ASV on oxygenation impairment could not be precisely evaluated because of difficulty in the accurate measurement of FIO2 under ASV support. Considering that oxygenation impairment frequently occurred between 24 h and 3 days after onset, evaluation of oxygenation impairment within 24 h after admission was likely to be inadequate [15]. At 1 h after admission, continuous ASV support tended to improve the PaO2/FIO2 ratio. However, significant prevention of oxygenation impairment at 24 h was not achieved. After 30 min of continuous use of ASV, it was used intermittently. Time differences of use may have an influence on results.
On the other hand, ASV was considered to help reduce development of pleural effusion. Peak CRP had a significant positive correlation with pleural effusion volume, and peak CRP was significantly lower in patients with ASV therapy during early follow-up. CRP was reported to be a good marker for risk of oxygenation impairment and long-term outcomes in type B acute aortic dissection [3, 19, 20]. Peak CRP level ≥15 mg/dl was detected as an independent determinant in the development of oxygenation impairment, and the long-term survival rate was significantly lower in patients with higher peak CRP level (14.90–32.60 mg/dl) [3, 20]. Therefore, maintaining peak CRP level below 15 mg/dl is thought to be meaningful in the treatment of acute uncomplicated type B aortic dissection. In the present study, the peak CRP level was significantly lower in patients with ASV therapy, and the mean peak CRP level was 8.5 ± 6.1. On the other hand, the mean peak CRP level was 15.5 ± 6.3 in the non-ASV group, and ASV therapy achieved the inhibition of elevation of CRP ≥15 mg/dl. However, CRP is not a specific marker and is influenced by many factors. Therefore, the results in the present study could not markedly demonstrate the efficacy of ASV. Based on the obtained results, non-invasive positive pressure ventilation by ASV helped to maintain respiratory conditions at the hyper-acute phase in uncomplicated type B dissection. Decreases in pleural effusion may be caused by optimized lung inflations with positive pressure, and they helped to suppress the excessive sympathetic nervous activity. Consequently, all these actions might induce the suppression of elevation in peak CRP level in multifactorial ways. Although optimal positive PS is reported to be required to resolve pleural effusion increases, the mechanism is not completely determined, and several trials are ongoing [21, 22]. We, on the other hand, could present positive impacts of ASV on uncomplicated type B aortic dissection at hyper-acute phases. However, the clinical benefits of ASV at follow-up periods were not fully obtained by the present protocol. Therefore, further evaluation is required to reveal whether the introduction of ASV, in addition to cardiovascular rehabilitation and antihypertensive therapy, can truly contribute to improve late outcomes of acute uncomplicated type B aortic dissection.
Study limitations
Our study was a prospective, randomized investigation; however, it has several limitations. First, it does not have a high statistical power because of the small sample size. It has been difficult to obtain a sufficient number of enrolled patients with uncomplicated type B dissection in a single institute for a certain time. Second, different onset times, the timing of follow-up CT and lumen type may influence the data on haemodynamics, sympathetic nervous activity and pleural effusion volume. Plasma catecholamines were not measured several times. Deciding the severity of uncomplicated type B dissection was difficult. Although the usage of Landiolol and Precedex were equal in both groups, these agents influenced sympathetic nervous activity. Third, oxygen therapy was begun in several patients before admission and these different conditions affected the results. Additionally, the doses of oxygen administration could not be evaluated uniformly between non-ASV and ASV groups, and FIO2 in ASV support was not accurately evaluated. Different markers and cytokines of inflammation, such as interleukin, were not evaluated. Finally, a comparison between continuous, positive airway pressure and ASV was not performed. Therefore, the advantages of ASV may have been influenced by confounding variables.
CONCLUSIONS
In patients with uncomplicated acute type B aortic dissection, excessive sympathetic nervous activity, the development of pleural effusion volume and elevation of peak CRP level were possibly suppressed under ASV therapy. The results suggest that ASV introduction may have the potential to contribute to a positive impact on early clinical states of uncomplicated type B acute aortic dissection.
Conflict of interest: none declared.




