-
PDF
- Split View
-
Views
-
Cite
Cite
Ai Sugimoto, Noritaka Ota, Masaya Murata, Kisaburo Sakamoto, Pulmonary root translocation for borderline complex coarctation of aorta and multiple ventricular septal defects, Interactive CardioVascular and Thoracic Surgery, Volume 24, Issue 1, January 2017, Pages 129–131, https://doi.org/10.1093/icvts/ivw294
Close - Share Icon Share
We describe a case of complex left ventricular outflow tract obstruction, multiple muscular ventricular septal defects, aortic coarctation and a hypoplastic aortic arch, where staged biventricular repair was performed successfully using pulmonary root translocation.
INTRODUCTION
The best treatment option for neonates and small infants with complex left ventricular outflow tract (LVOT) obstruction, ventricular septal defects (VSDs) and aortic arch obstruction is controversial.
CASE REPORT
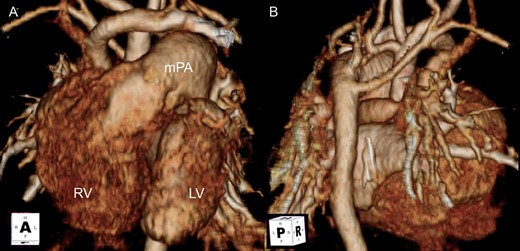
Preoperative CT. (A) Anterior view. (B) Posterior view. Five individual cervical branches arise separately. The transverse arch is severely hypoplastic. Left and aberrant right subclavian arteries arise from the descending aorta. RV: right ventricle; LV: left ventricle; mPA: main pulmonary artery.
We initially chose bilateral pulmonary artery banding and maintained PDA with continuous prostaglandin E1 infusion, leaving time to decide on further surgical options. Definite biventricular repair was scheduled when she presented with a drop in saturation of around 75%, at 4 months of age, weighing 4 kg. Her LVOT had not showed any apparent increase in size, and PR remained trivial. Because of the operative demands to deal with multiple muscular VSDs, we decided against conventional repair with LVOT resection and aortic valvotomy.
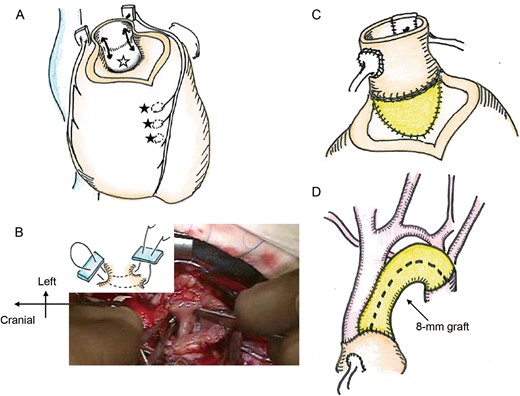
(A) After pulmonary autograft removal, the subaortic valvular muscle protrusion was resected (black arrows). Muscular ventricular septal defect (VSD) (black stars) and a perimembranous VSD (white star) are indicated. (B) The right angle clamp was inserted from muscular VSDs on right ventricule side to the perimembranous VSD through LV. The sandwich technique was applied. (C) A pulmonary autograft was implanted in the left ventricular outflow tract, and the VSD was rerouted using patch. (D) The aortic arch was bridged with an 8-mm expanded polytetrafluoroethylene graft.
Cardiac arrest lasted 180 min. She was extubated on the 10th postoperative day and discharged 25 days after surgery. Postoperative CT showed a straight LVOT and sufficient retrosternal space for RVOT reconstruction. Echocardiography, 47 months after the operation, revealed mild neo-aortic valve regurgitation and mild LVOT obstruction (PG: 36 mmHg). Moderate pulmonary valve stenosis (PG: 61 mmHg) was detected and successfully reduced to 20 mmHg by balloon dilatation.
DISCUSSION
This definite biventricular repair involved LVOT reconstruction, multiple muscular VSD closure and aortic arch repair.
Nakano et al. [1] proposed a minimum LVOT diameter of body weight plus 1 mm as an indication for the Yasui operation. We decided against conventional repair, because of total surgical demands and her small LVOT.
To establish an LV-to-aorta connection, VSDs need to have some relation to the aorta. Generally, inlet VSD or apically located muscular VSDs are not suitable for intraventricular rerouting. Hazekamp et al. [2] described a surgical procedure for transposition of the great arteries and LVOT obstruction, with detachment of the aortic root from the right ventricle and resection of the outlet septum, facilitating exposure for ventricular septation. In cases with normally related great arteries and LVOT obstruction, detachment of the pulmonary root from the right ventricle and resection of the conal septum have a similar effect. In the present case, the choice of pulmonary root translocation was made during the surgery, when the surgeon inspected VSDs. Harvesting the pulmonary autograft and removing the bridge of the conal septum made it easier to detect the perimembranous and muscular VSDs, giving a better surgical view for rerouting and closure. Despite its greater invasiveness, biventricular repair, using pulmonary root translocation, is worth employing in selected cases with multiple muscular VSDs, and complex LVOT lesions.
Conflict of interest: none declared.




