-
PDF
- Split View
-
Views
-
Cite
Cite
Ahmad Mahir Shamsuddin, Yen Chuan Chen, Abdul Rahim Wong, Trong-Phi Le, Robert H. Anderson, Antonio F. Corno, Surgery for doubly committed ventricular septal defects, Interactive CardioVascular and Thoracic Surgery, Volume 23, Issue 2, August 2016, Pages 231–234, https://doi.org/10.1093/icvts/ivw129
Close - Share Icon Share
Abstract
Doubly committed ventricular septal defects (VSDs) account for up to almost one-third of isolated ventricular septal defects in Asian countries, compared with only 1/20th in western populations. In our surgical experience, this type of defect accounted for almost three-quarters of our practice. To date, patch closure has been considered the gold standard for surgical treatment of these lesions. Our objectives are to evaluate the indications and examine the outcomes of surgery for doubly committed VSDs.
Between October 2013, when our service of paediatric cardiac surgery was opened, and December 2014, 24 patients were referred for surgical closure of VSDs. Among them, 17 patients (71%), with the median age of 6 years, ranging from 2 to 9 years, and with a median body weight of 19 kg, ranging from 11 to 56 kg, underwent surgical repair for doubly committed defects. In terms of size, the defect was considered moderate in 4 and large in 13. Aortic valvular regurgitation (AoVR) was present in 11 patients (65%) preoperatively, with associated malformations found in 14 (82%), with 5 patients (29%) having two or more associated defects.
After surgery, there was trivial residual shunting in 2 patients (12%). AoVR persisted in 6 (35%), reducing to trivial in 5 (29%) and mild in 1 (6%). Mean stays in the intensive care unit and hospital were 2.6 ± 1.2 days, ranging from 2 to 7 days, and 6.8 ± 0.8 days, ranging from 6 to 9 days, respectively. The mean follow-up was 14 ± 4 months, ranging from 6 to 20 months, with no early or late deaths and without clinical deterioration.
The incidence of doubly committed lesions is high in our experience, frequently associated with AoVR and other associated malformation. Early detection is crucial to prevent further progression of the disease. Patch closure remains the gold standard in management, not least since it allows simultaneous repair of associated intracardiac defects.
INTRODUCTION
Doubly committed ventricular septal defects (VSDs), also called outlet, conal, infundibular, supracristal or subpulmonary VSDs, are quite rare in western countries, where the rate of incidence is reported at ∼5%. They are much more frequent in Asian populations, where they account for one-third of isolated defects [1–8]. We report here our own experience, in which doubly committed defects accounted for almost three-quarters of consecutive patients requiring surgical closure of a VSD. Surgical closure using a patch, with or without simultaneous surgery on the aortic valve, has for decades been considered the gold standard for the treatment of this specific lesion [9–16].
The increased paediatric cardiac activities in Asia, together with a remarkable interest in the potential to close the doubly committed defects with a device inserted percutaneously, or with a per-ventricular hybrid technique, have resulted in several recent reports [17–20], followed not only by prompt surgical replies [21, 22], but also by reviews of the specific phenotypic morphology of the various defects opening between the ventricular outlets [23], as well as the diagnostic approaches [24]. The availability of interventional cardiology in a geographical location, with an elevated incidence of patients with doubly committed defects, and the recent beginning of paediatric and congenital cardiac surgery in the same hospital have motivated our retrospective study to evaluate the outcomes using the surgical approach to treat the doubly committed variant. The experience with percutaneous insertion of devices will be reviewed and reported separately.
MATERIALS AND METHODS
Between October 2013, when our new service of paediatric cardiac surgery was opened, and December 2014, 24 patients were referred for surgical closure of VSDs. Among them, 17 patients (71%), with a median age of 6 years ranging from 2 to 9 years, having a median body weight of 19 kg, ranging from 11 to 56 kg, underwent surgical repair of defects shown to be doubly committed and juxta-arterial on the basis of the presence of fibrous continuity between the leaflets of the aortic and pulmonary valves (Table 1). Patients with associated morphology tetralogy of Fallot-like condition were excluded. All details regarding clinical records, operative findings and the outcomes were retrospectively reviewed.
Demographics of patients undergoing surgical closure of doubly committed and juxta-arterial ventricular septal defects
| Patient characteristics | |
| Total number | 17 |
| Male/female | 10/7 |
| Mean age (years) | 8.6 ± 5.5 (2–19) |
| Mean weight (kg) | 25.5 ± 14.3 (11–56) |
| Mean height (cm) | 121.2 ± 29.9 (65–165) |
| Mean body surface area (m2) | 0.9 ± 0.4 (0.49–1.59) |
| Size of VSD | |
| Small | 0 |
| Moderate | 4 (24%) |
| Large | 13 (76%) |
| Associated defects | |
| Aortic valvar prolapse | 17 (100%) |
| Aortic valvular regurgitation | 11 (65%) |
| RVOT obstruction | 8 (59%) |
| Subaortic obstruction | 5 (29%) |
| Patent arterial duct | 3 (18%) |
| Anomalous coronary artery | 1 (6%) |
| Multiple VSDs | 1 (6%) |
| Double outlet right ventricle | 1 (6%) |
| Degree of aortic valvular regurgitation | |
| Absent | 6 (35%) |
| Trivial | 4 (24%) |
| Mild | 5 (29%) |
| Moderate | 2 (12%) |
| Severe | 0 |
| Follow-up periods, months | 14 ± 4 (6–20) |
| Patient characteristics | |
| Total number | 17 |
| Male/female | 10/7 |
| Mean age (years) | 8.6 ± 5.5 (2–19) |
| Mean weight (kg) | 25.5 ± 14.3 (11–56) |
| Mean height (cm) | 121.2 ± 29.9 (65–165) |
| Mean body surface area (m2) | 0.9 ± 0.4 (0.49–1.59) |
| Size of VSD | |
| Small | 0 |
| Moderate | 4 (24%) |
| Large | 13 (76%) |
| Associated defects | |
| Aortic valvar prolapse | 17 (100%) |
| Aortic valvular regurgitation | 11 (65%) |
| RVOT obstruction | 8 (59%) |
| Subaortic obstruction | 5 (29%) |
| Patent arterial duct | 3 (18%) |
| Anomalous coronary artery | 1 (6%) |
| Multiple VSDs | 1 (6%) |
| Double outlet right ventricle | 1 (6%) |
| Degree of aortic valvular regurgitation | |
| Absent | 6 (35%) |
| Trivial | 4 (24%) |
| Mild | 5 (29%) |
| Moderate | 2 (12%) |
| Severe | 0 |
| Follow-up periods, months | 14 ± 4 (6–20) |
Values are mean ± standard deviation (range) or n (%).
RVOT: right ventricular outflow tract; VSD: ventricular septal defect.
Demographics of patients undergoing surgical closure of doubly committed and juxta-arterial ventricular septal defects
| Patient characteristics | |
| Total number | 17 |
| Male/female | 10/7 |
| Mean age (years) | 8.6 ± 5.5 (2–19) |
| Mean weight (kg) | 25.5 ± 14.3 (11–56) |
| Mean height (cm) | 121.2 ± 29.9 (65–165) |
| Mean body surface area (m2) | 0.9 ± 0.4 (0.49–1.59) |
| Size of VSD | |
| Small | 0 |
| Moderate | 4 (24%) |
| Large | 13 (76%) |
| Associated defects | |
| Aortic valvar prolapse | 17 (100%) |
| Aortic valvular regurgitation | 11 (65%) |
| RVOT obstruction | 8 (59%) |
| Subaortic obstruction | 5 (29%) |
| Patent arterial duct | 3 (18%) |
| Anomalous coronary artery | 1 (6%) |
| Multiple VSDs | 1 (6%) |
| Double outlet right ventricle | 1 (6%) |
| Degree of aortic valvular regurgitation | |
| Absent | 6 (35%) |
| Trivial | 4 (24%) |
| Mild | 5 (29%) |
| Moderate | 2 (12%) |
| Severe | 0 |
| Follow-up periods, months | 14 ± 4 (6–20) |
| Patient characteristics | |
| Total number | 17 |
| Male/female | 10/7 |
| Mean age (years) | 8.6 ± 5.5 (2–19) |
| Mean weight (kg) | 25.5 ± 14.3 (11–56) |
| Mean height (cm) | 121.2 ± 29.9 (65–165) |
| Mean body surface area (m2) | 0.9 ± 0.4 (0.49–1.59) |
| Size of VSD | |
| Small | 0 |
| Moderate | 4 (24%) |
| Large | 13 (76%) |
| Associated defects | |
| Aortic valvar prolapse | 17 (100%) |
| Aortic valvular regurgitation | 11 (65%) |
| RVOT obstruction | 8 (59%) |
| Subaortic obstruction | 5 (29%) |
| Patent arterial duct | 3 (18%) |
| Anomalous coronary artery | 1 (6%) |
| Multiple VSDs | 1 (6%) |
| Double outlet right ventricle | 1 (6%) |
| Degree of aortic valvular regurgitation | |
| Absent | 6 (35%) |
| Trivial | 4 (24%) |
| Mild | 5 (29%) |
| Moderate | 2 (12%) |
| Severe | 0 |
| Follow-up periods, months | 14 ± 4 (6–20) |
Values are mean ± standard deviation (range) or n (%).
RVOT: right ventricular outflow tract; VSD: ventricular septal defect.
The position, presence and size of the defects were determined by echocardiography, with careful attention to distinguish the truly doubly committed VSDs from perimembranous defects with outlet extension, using meticulous echocardiographic observation of the short-axis view of the right ventricular outflow tract at the level of the aortic root. The size of the defect was graded as being small, moderate or large in relation to the dimensions of the aortic valvar orifice, using values of less than 25%, between 25 and 50% or greater than 50%, respectively. When measuring the size of the defect, no account was taken of any portion occluded by a prolapsing leaflet of the aortic valve. Aortic valvular regurgitation (AoVR), assessed by echocardiography and colour flow Doppler investigation, was graded as absent, trivial, mild, moderate or severe in accordance with the standard echocardiography criteria. The details of both the defect and AoVR were also graded by angiography, when available. All echocardiographic and angiographic images were separately reviewed and graded by the two senior clinicians (Abdul Rahim Wong and Antonio F. Corno).
The indication for surgery was based on the size of the VSD (large in 76% of the patients) and/or the presence of associated defects (Table 1).
Survival, incidence and type of complications, duration of stay in the intensive care unit and the hospital, and follow-up were all recorded. All patients have been followed in the paediatric cardiac clinic with a schedule of clinic and echocardiographic evaluation 1 month after hospital discharge, then after 3 months, 6 months and then annually. The latest observation for the purpose of this study took place in June 2015.
Data are expressed as mean ± standard deviation (SD).
Surgery was performed with normothermic high-flow cardiopulmonary bypass. After aortic cross-clamping, and delivery of the first dosage of cold blood cardioplegia, repeated every 20 min when needed, a right atriotomy was performed to introduce a vent into the left heart across the oval fossa, and to explore the intracardiac anatomy. At the beginning of our experience, the defect was approached through a right ventriculotomy (Fig. 1), whereas in the last 5 patients the preferred approach was a longitudinal incision on the pulmonary trunk. The defect was closed with a heterologous bovine pericardial patch, attached with a running 5-0 or 6-0 monofilament suture, by placing the sutures directly at the base of the leaflets of the pulmonary valve, creating an artificial plane in the upper part to leave the aortic leaflets on the left side. Any obstructive tissues in the right ventricular outflow tract were resected through the right ventriculotomy. In the presence of AoVR, and/or subaortic obstruction, these lesions were dealt with the repair of the aortic valve with prolapsing leaflet suspension in the presence of moderate or severe degree of regurgitation and/or subaortic resection with myectomy through an oblique aortotomy directed towards the non-coronary aortic valvar sinus. The right ventriculotomy was closed with a heterologous bovine pericardial patch attached with a running 5-0 monofilament suture, the incision on the pulmonary trunk as well as the aortotomy with double running sutures and after air evacuation and aortic declamping, the right atriotomy was closed with double running sutures. Intraoperative echocardiography was used to rule out any residual shunting, and to assess the presence and degree of any residual AoVR. Oxygen saturation was routinely measured in the right atrium and pulmonary arteries to rule out residual intracardiac shunts, and right ventricular, pulmonary arterial and systemic systolic pressures were measured. The routine perioperative management for surgery using normothermic high-flow cardiopulmonary bypass and its results have previously been reported [25].
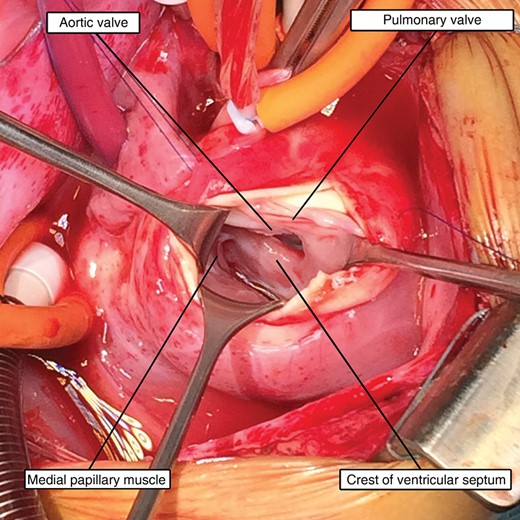
Intraoperative photograph showing the defect approached through a longitudinal incision in the pulmonary trunk, with continuity between the leaflets of the aortic and pulmonary valves.
RESULTS
The mean duration of cardiopulmonary bypass was 88 ± 31.1 min, ranging from 67 to 201 min, and the mean aortic cross-clamp time was 46 ± 17.9 min, ranging from 32 to 100 min. The results of surgical closure are given in Table 2.
Outcome of patients undergoing surgical closure of doubly committed and juxta-arterial ventricular septal defects (n = 17)
| Mean CPB time (min) | 88 ± 31.1 |
| Mean aortic cross-clamp time (min) | 46 ± 17.9 |
| Mean ICU stay (days) | 2.6 ± 1.2 |
| Mean hospital stay (days) | 6.8 ± 0.8 |
| Degree of aortic valvular regurgitation | |
| Absent | 11 (65%) |
| Trivial | 5 (29%) |
| Mild | 1 (6%) |
| Moderate | 0 |
| Severe | 0 |
| Residual shunt | |
| Absent | 15 (88%) |
| Trivial | 2 (12%) |
| Mild | 0 |
| Moderate | 0 |
| Severe | 0 |
| Mean CPB time (min) | 88 ± 31.1 |
| Mean aortic cross-clamp time (min) | 46 ± 17.9 |
| Mean ICU stay (days) | 2.6 ± 1.2 |
| Mean hospital stay (days) | 6.8 ± 0.8 |
| Degree of aortic valvular regurgitation | |
| Absent | 11 (65%) |
| Trivial | 5 (29%) |
| Mild | 1 (6%) |
| Moderate | 0 |
| Severe | 0 |
| Residual shunt | |
| Absent | 15 (88%) |
| Trivial | 2 (12%) |
| Mild | 0 |
| Moderate | 0 |
| Severe | 0 |
Values are mean ± standard deviation or n (%).
CPB: cardiopulmonary bypass; ICU: intensive care unit.
Outcome of patients undergoing surgical closure of doubly committed and juxta-arterial ventricular septal defects (n = 17)
| Mean CPB time (min) | 88 ± 31.1 |
| Mean aortic cross-clamp time (min) | 46 ± 17.9 |
| Mean ICU stay (days) | 2.6 ± 1.2 |
| Mean hospital stay (days) | 6.8 ± 0.8 |
| Degree of aortic valvular regurgitation | |
| Absent | 11 (65%) |
| Trivial | 5 (29%) |
| Mild | 1 (6%) |
| Moderate | 0 |
| Severe | 0 |
| Residual shunt | |
| Absent | 15 (88%) |
| Trivial | 2 (12%) |
| Mild | 0 |
| Moderate | 0 |
| Severe | 0 |
| Mean CPB time (min) | 88 ± 31.1 |
| Mean aortic cross-clamp time (min) | 46 ± 17.9 |
| Mean ICU stay (days) | 2.6 ± 1.2 |
| Mean hospital stay (days) | 6.8 ± 0.8 |
| Degree of aortic valvular regurgitation | |
| Absent | 11 (65%) |
| Trivial | 5 (29%) |
| Mild | 1 (6%) |
| Moderate | 0 |
| Severe | 0 |
| Residual shunt | |
| Absent | 15 (88%) |
| Trivial | 2 (12%) |
| Mild | 0 |
| Moderate | 0 |
| Severe | 0 |
Values are mean ± standard deviation or n (%).
CPB: cardiopulmonary bypass; ICU: intensive care unit.
Of the 17 patients undergoing surgical closure, in 4 (24%) the defect was of moderate size, being large in the remaining 13 (76%). All the patients presented with prolapsed leaflets of the aortic valve. The presence and degree of AoVR before the surgical procedure and its associated intracardiac defects are given in Table 1. Two or more of the malformations were present in 5 patients (29%). In 2 patients, the evidence of endocarditis was observed on the leaflets of the aortic and pulmonary valves; in both patients, the macroscopic evidence of endocarditis was removed and the gap in the semilunar valve leaflets was closed with a small patch of heterologous bovine pericardium. Surgical closure in 1 patient was undertaken after an unsuccessful attempt at interventional closure.
In 2 patients with trivial residual defects, the step-up in oxygen saturation between right atrium and pulmonary artery was 4 and 5%, respectively, in the presence of normal systolic right ventricular pressures, respectively, 22 and 23 mmHg. The presence and degree of AoVR after the surgical procedure are reported in Table 2. No complications were noted during and after the surgical procedure. Mean durations of stay in the intensive care unit and hospital stay were 2.6 ± 1.2 days, ranging from 2 to 7 days, and 6.8 ± 0.8 days, ranging from 6 to 9 days, respectively.
The mean duration of follow-up was 14 ± 4 months, ranging from 6 to 20 months, with no early or late deaths and without any evidence of complications or deterioration in clinical conditions, including no evidence of increase of AoVR and no appearance of pulmonary valve regurgitation in any case.
DISCUSSION
The doubly committed VSD, representing failure of muscularization of the subpulmonary infundibular sleeve, cannot exist in an otherwise normal heart [21]. In the normal heart, the pulmonary root is lifted away from the base of the heart by the free-standing infundibulum. In consequence of the deficient infundibulum, there is a fibrous continuity between the leaflets of the aortic and pulmonary valves, with the defect not only committed to the aorta, but also to the pulmonary trunk, hence its doubly committed phenotype [1, 3, 5–8, 16, 21] (Fig. 1). Owing to the lack of anatomical muscular support provided by the subpulmonary infundibulum, the right coronary leaflet, and occasionally the non-coronary leaflet, of the aortic valve tend to prolapse through the defect [5, 8–10, 14, 15, 21]. During systole, the Venturi effect drags the sagging aortic leaflet, or leaflets, into the low-pressure cavity of the right ventricle. Over the course of time, this results in progressive AoVR [10, 11, 13–15, 20, 21]. Such prolapse of the aortic valve may initially reduce the size of the defect, rendering it ‘functionally’ restrictive, but morphologically the defect is usually large, and generally does not tend to undergo spontaneous closure [13, 17, 21]. Eventually, an aneurysm of the supporting aortic sinus of Valsalva can develop, with rupture of the sinus in adulthood, a progression frequently encountered in the Asian populations [11]. Because of these peculiar characteristics of the morphology and natural history, the management of the doubly committed defect is different from that usually undertaken for the more common perimembranous lesions.
Diagnosis
Potential mistakes in distinguishing a doubly committed VSD from perimembranous defects with a muscularized but hypoplastic subpulmonary infundibulum should be eliminated with meticulous echocardiographic observation of the short-axis view of the right ventricular outflow tract at the level of the aortic root [1, 2] or with more sophisticated cardiac computed tomography [24]. Advances in prenatal diagnosis could also now permit anticipation of the diagnosis and allow for a better follow-up, with more appropriate timing for closure of the defect.
Age and indication for closure
The optimal timing for closure of the doubly committed defects remains controversial. The relatively low risk of the procedure needs to be weighed against the benefits of the prevention of severe AoVR [13]. The closure of the defect, with or without repair of the aortic valve in the case of surgical treatment, should be performed at the first appearance of AoVR, or if there is progression of previously observed trivial aortic regurgitation [5, 7, 10, 11, 13–15]. The seeming presence of a ‘functionally’ restrictive defect on echocardiography, with most of the area of the morphologically large defect closed by the prolapsing aortic valve leaflet, can be misleading and can cause a dangerous delay in referral for treatment. When a doubly committed defect has been reliably diagnosed, closure should ideally be undertaken before the onset of aortic regurgitation, as observed in a quite large percentage of our patients, due to the relatively late referral. Such regurgitation very rarely appears before the age of 3 or 4 years [11–13]. This finding was confirmed in our experience, where the only patient referred for surgery before the age of 3 years also had severe obstruction of the right ventricular outflow tract.
Surgical closure, with or without simultaneous repair of the aortic valve, is still considered the gold standard for the treatment of this congenital heart defect [5, 7, 9–17, 20, 21]. The approach through a right atriotomy, with retraction or detachment of the antero-superior leaflet of the tricuspid valve [22], does not always allow for adequate exposure of the upper part of the defect. This means that either a longitudinal right ventriculotomy, or a longitudinal incision in the pulmonary trunk, or an oblique incision of the ascending aorta, will likely be needed, with the last approach also allowing direct repair of the aortic valve [5, 7, 9–16]. Such patch closure realigns the ventricular septum and provides support for the leaflets of the aortic valve, possibly avoiding further surgery.
In our experience (Table 2), the degree of AoVR was substantially reduced when compared with the preoperative state, remaining stable through the entire period of follow-up, which is, however, relatively short. The surgical approach, furthermore, allows for the simultaneous repair of associated intracardiac defects, not uncommon in our population of patients (Table 1). Since the repair of associated intracardiac defects is not possible with the interventional cardiology approach, unless for closure of a persistently patent arterial duct or atrial septal defect, the presence of the most frequently associated malformations, such as right ventricular outflow tract obstruction, and/or subaortic obstruction, is a contraindication for the interventional catheterization approach.
Study limitations
The main limitation of our study is the relatively short duration of follow-up, reflecting the fact that paediatric and congenital cardiac surgery only became available at the centre after October 2013. A potential limitation is that the age of the patients at the time of surgery was much older than is usually the case for those treated in western centres. This reflects the ongoing evolution of health care in Asian countries.
CONCLUSIONS
The incidence of doubly committed VSDs is unusually high in our local population. It is frequently associated with AoVR and other associated malformations. Early detection is crucial to prevent further progression of the disease. Patch closure remains the gold standard in managing these defects, not least because this approach allows simultaneous repair of associated intracardiac defects.
Conflict of interest: none declared.




