-
PDF
- Split View
-
Views
-
Cite
Cite
Yuki Matsumura, Yuki Owada, Takumi Yamaura, Satoshi Muto, Jun Osugi, Mika Hoshino, Mitsunori Higuchi, Tetsuya Ohira, Hiroyuki Suzuki, Mitsukazu Gotoh, Epidermal growth factor receptor gene mutation as risk factor for recurrence in patients with surgically resected lung adenocarcinoma: a matched-pair analysis, Interactive CardioVascular and Thoracic Surgery, Volume 23, Issue 2, August 2016, Pages 216–222, https://doi.org/10.1093/icvts/ivw116
Close - Share Icon Share
Abstract
Epidermal growth factor receptor (EGFR) mutation is a robust prognostic factor in patients with lung adenocarcinoma (ADC). However, the role of EGFR mutation status as a recurrence-risk factor remains unknown because the presence of such mutations is associated with other background characteristics. We therefore conducted a matched-pair analysis to compare recurrence-free survival (RFS) in matched cohorts of patients with lung ADC.
We enrolled 379 patients who underwent surgical resection for lung ADC between 2005 and 2012. We determined the EGFR mutation status of each tumour. Matching their age, gender, smoking history and pathological stage (pStage), we compared RFS between matched cohorts with and without EGFR mutation (n = 86 each).
The median age was 67 years, there were 39 (45%) men, 39 (45%) ex- or current smokers and pStage I: 71 (83%), II: 5 (6%), III: 8 (9%), IV: 2 (2%) in each group. The 3- and 5-year RFS rates in patients with mutant and wild-type EGFR were 85 and 78%, and 74 and 60%, respectively, with significant differences between the groups (P = 0.040). Multivariate analysis identified vascular invasion and lymphatic permeation, but not EGFR mutation status, as independent risk factors for recurrence.
EGFR-gene mutation might be a favourable recurrence-risk factor in patients with surgically resected lung ADC, but further studies in larger cohorts are needed to verify this hypothesis.
INTRODUCTION
The identification of epidermal growth factor receptor (EGFR) gene mutations and introduction of EGFR tyrosine kinase inhibitors (EGFR–TKI) have dramatically changed the treatment of advanced or recurrent lung adenocarcinoma (ADC) over the last decade [1, 2]. The value of EGFR–TKIs has been demonstrated in Phase III randomized trials [3, 4], and they are currently the first-choice treatment for patients with advanced or recurrent lung ADCs harbouring EGFR mutations [2, 5]. Regarding surgically resected ADCs, some retrospective studies also reported that mutant EGFR was a favourable prognostic factor for postoperative overall survival (OS) [6, 7], but were unable to demonstrate whether EGFR mutation itself makes effects on the postoperative survival of ADCs, partly because of well-known biases in clinical backgrounds between lung ADC patients with mutant and wild-type EGFR. EGFR mutations are more common in women and in non-smokers, and many reports have shown better survival in these patients, irrespective of their EGFR status [8, 9]. Furthermore, EGFR–TKIs appear to prolong post-recurrence survival in surgically resected lung ADC in patients harbouring EGFR mutations [6, 10], making it impossible to determine if the prolonged survival of EGFR-mutated ADC patients was the result of EGFR–TKIs or EGFR mutation itself.
We reduced these biases in patient backgrounds by carrying out a matched-pair analysis to compare recurrence-free survival (RFS) between lung ADC patients with and without EGFR mutations, free from the effects of EGFR–TKIs. The primary aim of the study was thus to clarify the value of EGFR mutation status as a recurrence-risk factor in patients with surgically resected lung ADCs, using matched-pair analysis.
PATIENTS AND METHODS
Patient selection
A total of 601 consecutive patients underwent surgical resection for primary lung cancer in Fukushima Medical University between January 2005 and December 2012, of which 546 (91%) had lobectomies. Among them, 406 (68%) had ADCs that were examined for EGFR mutation status. Patients were excluded if they had undergone incomplete resection, or had anaplastic lymphoma kinase fusion genes. We excluded also the patients who took EGFR–TKIs before recurrence because EGFR–TKIs were reported to make effects on RFS. The patients in whom EGFR mutation status could not be determined because of insufficient specimens were also excluded. A total of 379 patients (93%) were finally enrolled in this study (Supplementary Fig. 1). The study was approved by the institutional review board of Fukushima Medical University in July 2015. Most patients underwent preoperative evaluations including physical examination, chest X-ray, chest and upper abdomen computed tomography (CT), brain magnetic resonance imaging (MRI) and positron emission tomography (PET).
Histopathological examination
Resected specimens were immediately examined macroscopically, and lobar and sublobar lymph nodes were removed in the operating room. Whole specimens were then fixed in 10% formalin and sectioned horizontally at ∼5–10-mm intervals. We routinely created paraffin-embedded sections of several surfaces of the main tumour. Other nodules off the main tumour were examined macroscopically and pathologically. Serial 4-µm sections were stained with haematoxylin and eosin for routine histopathological work-up. Elastica–Masson staining was routinely used to visualize elastic fibres in all sections containing tumour cells for evaluating vascular invasion, lymphatic permeation and pleural invasion. We re-evaluated the invasiveness of the enrolled lung ADCs in terms of adenocarcinoma in situ (AIS), minimally invasive adenocarcinoma (MIA) and invasive adenocarcinoma (IA), and classified their pathological stage (pStage) according to the TNM classification of malignant tumours of the Union for International Cancer Control (UICC, 7th edition) [11] and the newly published World Health Organization (WHO) classification [12].
Examination of epidermal growth factor receptor mutation status
We cut five 10-μm-thick sections from formalin-fixed paraffin-embedded tissues containing a large portion of tumour tissue. We then extracted tumour DNA from the sections and determined the EGFR mutation status using the PCR-Invader method (BML, Tokyo, Japan), according to the manufacturer's protocol [13]. Among the 379 enrolled patients, tumour EGFR mutation status had already been assessed in 271 (72%), and we evaluated the EGFR mutation statuses of the remaining 108 (28%) patients upon the current analysis.
Follow-up and evaluation of recurrence
Postoperative adjuvant therapy was basically administered in patients with pStage IB or higher. pStage IB was treated with tegafur (UFT; Taiho, Tokyo, Japan), and pStage II or more with platinum-doublet chemotherapy, according to the Japanese guideline for the diagnosis and treatment of lung cancer, annually published by the Japan Lung Cancer Society [5] and past evidence-based reports [14–17]. All patients were followed up at our outpatient clinic, including examinations every 3–6 months during the first 2 years after surgery, and every 6–12 months thereafter. Follow-up evaluation included physical examination, chest radiography and blood examination, including tumour markers. Chest and upper abdominal CT scans were taken every 6–12 months. Further evaluations, such as brain MRI and PET–CT, were performed if any symptoms or signs of recurrence were detected. Treatment after recurrence was determined after consultation with other thoracic surgeons, oncologists and radiologists. The date of recurrence was defined as the date of histological proof, or of diagnosis based on clinicoradiological findings. Recurrence was categorized as intrathoracic or extrathoracic recurrence. The former included recurrence in the bronchial stump, chest wall, residual lung field, pleural dissemination and hilar, mediastinal and cervical lymph nodes, whereas the latter, which was similar to distant metastasis, was defined as metastasis to extrathoracic organs, including the brain, bone, liver, adrenal gland, subcutaneous tissue and others. Patients with both intrathoracic and extrathoracic recurrences were classified as having distant metastases.
Statistical analysis
This study was a retrospective observational analysis, and a pilot study in a single institution. So we did not set the sample size, and collected the medical records of all consecutive patients during the designated period. Matched-pair analysis was performed on the enrolled 379 patients in relation to age (±3 years), sex, smoking history (never smoker versus ex- or current smoker) and pStage (I versus II versus III versus IV). Matching was performed by one of the authors (Tetsuya Ohira) using SAS version 9.1 (SAS Institute, Cary, NC, USA). We designed the current study according to the recently published statistical and data reporting guidelines of the EJCTS and ICVTS [18]. Because we considered our study to be one of the case–control studies, the STROBE statement about case–control studies, which is described in the guideline was utilized.
Differences between categorized groups were evaluated using Fisher's exact test or Pearson's χ2 test. RFS was defined as the interval in months from the date of resection to the date of first recurrence or last follow-up. OS was defined as the interval in months between the date of surgical intervention and the date of death due to any cause or last follow-up. Observations were censored at the last follow-up when the patient was alive or was lost to follow-up.
OS and RFS curves were plotted by the Kaplan–Meier method and differences in univariate analyses of survival were calculated using the log-rank test. Cox regression analysis was used for both univariate and multivariate analyses. All P-values were two-sided, and P-values <0.05 were considered statistically significant. Statistical analysis was performed using SPSS statistical software (IBM, SPSS statistics, version 23, SPSS, Inc., Chicago, IL, USA).
RESULTS
Clinicopathological characteristics and survival in all patients
The clinicopathological characteristics of all enrolled patients are given in Table 1 (n = 379). A total of 192 (51%) patients harboured EGFR mutations, which consisted of 102 (53%) exon21 L858Rs, 68 (35%) exon19 deletions, 11 (6%) exon20 T790Ms and 16 (8%) other mutations. Five patients (3%) had both exon21 L858R and exon20 T790M. The median age of the patients with EGFR mutant and wild-type tumours was 67 years (range: 37–87) and 69 years (26–88), respectively (P = 0.103). There were 67 (35%) men in the mutant-EGFR group, and significantly more (118, 63%) in the wild-type-EGFR group (P < 0.001). Smoking history and pleural invasion differed significantly between the groups (P < 0.001 and 0.036, respectively). However, the number of patients with IA (according to the WHO classification) and the number of patients who received postoperative adjuvant therapies were similar in both groups (P = 0.923 and 0.568, respectively).
| Characteristic . | EGFR . | EGFR . | P-value . | |
|---|---|---|---|---|
| Mutant . | Wild-type . | |||
| n = 192 (%) . | n = 187 (%) . | |||
| Median age (years) | Range | 69 (26–88) | 67 (37–87) | 0.103 |
| Sex | Male | 67 (35) | 118 (63) | <0.001 |
| Smoking history | Ex- or current | 55 (29) | 125 (67) | <0.001 |
| Never | 137 (71) | 62 (33) | ||
| Serum CEA | >5 mg/dl | 33 (17) | 45 (24) | 0.101 |
| Median FEV1.0 | l (range) | 2.1 (0.8–3.9) | 2.2 (1.0–5.0) | 0.426 |
| Procedure | Lobectomy | 175 (91) | 170 (91) | 1.000 |
| Segmentectomy | 12 (6) | 8 (4) | Lobar vs sublobar | |
| Wedge resection | 5 (3) | 9 (5) | ||
| Pathological stage | IA | 115 (60) | 91(49) | 0.159 |
| IB | 34 (18) | 42 (22) | I vs II more | |
| II | 16 (8) | 27 (14) | ||
| III | 23 (12) | 20 (11) | ||
| IV | 4 (2) | 7 (4) | ||
| WHO classification | AIS | 8 (4) | 17 (9) | 0.923 |
| MIA | 18 (9) | 9 (5) | IA vs others | |
| IA | 166 (87) | 161 (86) | ||
| Pleural invasion | Present | 34 (18) | 50 (27) | 0.036 |
| Vascular invasion | Present | 44 (23) | 51 (27) | 0.345 |
| Lymphatic permeation | Present | 57 (30) | 63 (34) | 0.442 |
| Adjuvant therapy | Not performed | 141 (73) | 132 (71) | 0.568 |
| Platinum double | 23 (12) | 24 (13) | Not vs others | |
| Oral drugs | 26 (14) | 27 (14) | ||
| Others | 2 (1) | 4 (2) | ||
| EGFR mutation | Exon21 L858R | 102 (53) | NA | |
| Exon19 del | 68 (35) | NA | ||
| Exon20 T790M | 11 (6)a | NA | ||
| Others | 16 (8) | NA | ||
| EGFR–TKI | Administered | 39 (20) | 10 (5) | <0.001 |
| Characteristic . | EGFR . | EGFR . | P-value . | |
|---|---|---|---|---|
| Mutant . | Wild-type . | |||
| n = 192 (%) . | n = 187 (%) . | |||
| Median age (years) | Range | 69 (26–88) | 67 (37–87) | 0.103 |
| Sex | Male | 67 (35) | 118 (63) | <0.001 |
| Smoking history | Ex- or current | 55 (29) | 125 (67) | <0.001 |
| Never | 137 (71) | 62 (33) | ||
| Serum CEA | >5 mg/dl | 33 (17) | 45 (24) | 0.101 |
| Median FEV1.0 | l (range) | 2.1 (0.8–3.9) | 2.2 (1.0–5.0) | 0.426 |
| Procedure | Lobectomy | 175 (91) | 170 (91) | 1.000 |
| Segmentectomy | 12 (6) | 8 (4) | Lobar vs sublobar | |
| Wedge resection | 5 (3) | 9 (5) | ||
| Pathological stage | IA | 115 (60) | 91(49) | 0.159 |
| IB | 34 (18) | 42 (22) | I vs II more | |
| II | 16 (8) | 27 (14) | ||
| III | 23 (12) | 20 (11) | ||
| IV | 4 (2) | 7 (4) | ||
| WHO classification | AIS | 8 (4) | 17 (9) | 0.923 |
| MIA | 18 (9) | 9 (5) | IA vs others | |
| IA | 166 (87) | 161 (86) | ||
| Pleural invasion | Present | 34 (18) | 50 (27) | 0.036 |
| Vascular invasion | Present | 44 (23) | 51 (27) | 0.345 |
| Lymphatic permeation | Present | 57 (30) | 63 (34) | 0.442 |
| Adjuvant therapy | Not performed | 141 (73) | 132 (71) | 0.568 |
| Platinum double | 23 (12) | 24 (13) | Not vs others | |
| Oral drugs | 26 (14) | 27 (14) | ||
| Others | 2 (1) | 4 (2) | ||
| EGFR mutation | Exon21 L858R | 102 (53) | NA | |
| Exon19 del | 68 (35) | NA | ||
| Exon20 T790M | 11 (6)a | NA | ||
| Others | 16 (8) | NA | ||
| EGFR–TKI | Administered | 39 (20) | 10 (5) | <0.001 |
CEA: carcinoembryonic antigen; NA: not available; EGFR: epidermal growth factor receptor; AIS: adenocarcinoma in situ; MIA: minimally invasive adenocarcinoma; IA: invasive adenocarcinoma; TKI: tyrosine kinase inhibitor.
aFive patients had both exon21 L858R and exon20 T790M.
| Characteristic . | EGFR . | EGFR . | P-value . | |
|---|---|---|---|---|
| Mutant . | Wild-type . | |||
| n = 192 (%) . | n = 187 (%) . | |||
| Median age (years) | Range | 69 (26–88) | 67 (37–87) | 0.103 |
| Sex | Male | 67 (35) | 118 (63) | <0.001 |
| Smoking history | Ex- or current | 55 (29) | 125 (67) | <0.001 |
| Never | 137 (71) | 62 (33) | ||
| Serum CEA | >5 mg/dl | 33 (17) | 45 (24) | 0.101 |
| Median FEV1.0 | l (range) | 2.1 (0.8–3.9) | 2.2 (1.0–5.0) | 0.426 |
| Procedure | Lobectomy | 175 (91) | 170 (91) | 1.000 |
| Segmentectomy | 12 (6) | 8 (4) | Lobar vs sublobar | |
| Wedge resection | 5 (3) | 9 (5) | ||
| Pathological stage | IA | 115 (60) | 91(49) | 0.159 |
| IB | 34 (18) | 42 (22) | I vs II more | |
| II | 16 (8) | 27 (14) | ||
| III | 23 (12) | 20 (11) | ||
| IV | 4 (2) | 7 (4) | ||
| WHO classification | AIS | 8 (4) | 17 (9) | 0.923 |
| MIA | 18 (9) | 9 (5) | IA vs others | |
| IA | 166 (87) | 161 (86) | ||
| Pleural invasion | Present | 34 (18) | 50 (27) | 0.036 |
| Vascular invasion | Present | 44 (23) | 51 (27) | 0.345 |
| Lymphatic permeation | Present | 57 (30) | 63 (34) | 0.442 |
| Adjuvant therapy | Not performed | 141 (73) | 132 (71) | 0.568 |
| Platinum double | 23 (12) | 24 (13) | Not vs others | |
| Oral drugs | 26 (14) | 27 (14) | ||
| Others | 2 (1) | 4 (2) | ||
| EGFR mutation | Exon21 L858R | 102 (53) | NA | |
| Exon19 del | 68 (35) | NA | ||
| Exon20 T790M | 11 (6)a | NA | ||
| Others | 16 (8) | NA | ||
| EGFR–TKI | Administered | 39 (20) | 10 (5) | <0.001 |
| Characteristic . | EGFR . | EGFR . | P-value . | |
|---|---|---|---|---|
| Mutant . | Wild-type . | |||
| n = 192 (%) . | n = 187 (%) . | |||
| Median age (years) | Range | 69 (26–88) | 67 (37–87) | 0.103 |
| Sex | Male | 67 (35) | 118 (63) | <0.001 |
| Smoking history | Ex- or current | 55 (29) | 125 (67) | <0.001 |
| Never | 137 (71) | 62 (33) | ||
| Serum CEA | >5 mg/dl | 33 (17) | 45 (24) | 0.101 |
| Median FEV1.0 | l (range) | 2.1 (0.8–3.9) | 2.2 (1.0–5.0) | 0.426 |
| Procedure | Lobectomy | 175 (91) | 170 (91) | 1.000 |
| Segmentectomy | 12 (6) | 8 (4) | Lobar vs sublobar | |
| Wedge resection | 5 (3) | 9 (5) | ||
| Pathological stage | IA | 115 (60) | 91(49) | 0.159 |
| IB | 34 (18) | 42 (22) | I vs II more | |
| II | 16 (8) | 27 (14) | ||
| III | 23 (12) | 20 (11) | ||
| IV | 4 (2) | 7 (4) | ||
| WHO classification | AIS | 8 (4) | 17 (9) | 0.923 |
| MIA | 18 (9) | 9 (5) | IA vs others | |
| IA | 166 (87) | 161 (86) | ||
| Pleural invasion | Present | 34 (18) | 50 (27) | 0.036 |
| Vascular invasion | Present | 44 (23) | 51 (27) | 0.345 |
| Lymphatic permeation | Present | 57 (30) | 63 (34) | 0.442 |
| Adjuvant therapy | Not performed | 141 (73) | 132 (71) | 0.568 |
| Platinum double | 23 (12) | 24 (13) | Not vs others | |
| Oral drugs | 26 (14) | 27 (14) | ||
| Others | 2 (1) | 4 (2) | ||
| EGFR mutation | Exon21 L858R | 102 (53) | NA | |
| Exon19 del | 68 (35) | NA | ||
| Exon20 T790M | 11 (6)a | NA | ||
| Others | 16 (8) | NA | ||
| EGFR–TKI | Administered | 39 (20) | 10 (5) | <0.001 |
CEA: carcinoembryonic antigen; NA: not available; EGFR: epidermal growth factor receptor; AIS: adenocarcinoma in situ; MIA: minimally invasive adenocarcinoma; IA: invasive adenocarcinoma; TKI: tyrosine kinase inhibitor.
aFive patients had both exon21 L858R and exon20 T790M.
The median follow-up period for all patients was 52 months (range: 1–118), during which time 108 (27%) patients had recurrence. Figure 1 shows RFS and OS curves according to EGFR mutation status. The 3- and 5-year RFS rates of patients with mutant and wild-type EGFR were 77 and 70%, and 64 and 55%, respectively. These survival curves differed significantly between the groups (P = 0.003). The 3- and 5-year OS rates of patients with mutant and wild-type EGFR were 92 and 87%, and 80 and 72%, respectively, and their survival curves also differed significantly (P < 0.001). Univariate and multivariate analyses identified pStage, pleural invasion and lymphatic permeation as independent recurrence-risk factors (Supplementary Table 1), and age, pStage, pleural invasion, vascular invasion and lymphatic permeation as independent prognostic factors (Supplementary Table 2). Among all the patients, EGFR mutation was a significant recurrence-risk and prognostic factor according to univariate analysis, but not identified as an independent factor by multivariate analysis. In the present study, no patients with AIS and MIA had recurrence, so we therefore did not include the WHO classification in the statistical analysis.
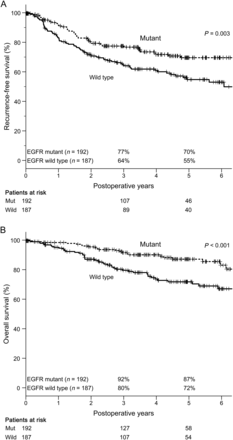
RFS and OS of all patients in relation to EGFR mutation status (n = 379). (A) The 3- and 5-year RFS rates of patients with and without EGFR mutation were 77 and 70% and 64 and 55%, respectively. These survival curves were significantly different (P = 0.003). (B) The 3- and 5-year OS rates of patients with and without EGFR mutation were 92 and 87% and 80 and 72%, respectively. These survival curves were also significantly different (P < 0.001). RFS: recurrence-free survival; OS: overall survival; EGFR: epidermal growth factor receptor.
Clinicopathological characteristics and survival in pair-matched patients
Matched-pair analysis generated two groups with mutant and wild-type EGFR, respectively (n = 86 each). The clinicopathological characteristics of both groups are given in Table 2. The groups were perfectly matched in terms of sex, smoking history and pStage (P = 1.000, each), and closely matched in terms of age (P = 0.939). The median age, number of male patients and number of ex- or current smoker was 67 years, 39 (45%), 39 (45%) in each group, respectively. There were patients with pStage I: 71 (83%), II: 5 (6%), III: 8 (9%), IV: 2 (2%) in each group. There were no significant differences between the groups in terms of any other recorded factors.
| Characteristic . | . | EGFR . | EGFR . | P-value . |
|---|---|---|---|---|
| Mutant . | Wild-type . | |||
| n = 86 (%) . | n = 86 (%) . | |||
| Median age (years) | Range | 67 (43–85) | 67 (43–87) | 0.939 |
| Sex | Male | 39 (45) | 39 (45) | 1.000 |
| Smoking history | Ex- or current | 39 (45) | 39 (45) | 1.000 |
| Never | 47 (55) | 47 (55) | ||
| Serum CEA | >5 mg/dl | 9 (13) | 13 (19) | 0.402 |
| Median FEV1.0 | l (range) | 2.3 (0.8–3.9) | 2.2 (1.0–3.5) | 0.426 |
| Procedure | Lobectomy | 74 (92) | 76 (96) | 0.432 |
| Segmentectomy | 3 (4) | 1 (1) | Lobar vs sublobar | |
| Wedge resection | 3 (4) | 2 (3) | ||
| Pathological stage | IA | 56 (65) | 51 (59) | 1.000 |
| IB | 15 (17) | 20 (23) | I vs II more | |
| II | 5 (6) | 5 (6) | ||
| III | 8 (9) | 8 (9) | ||
| IV | 2 (2) | 2 (2) | ||
| WHO classification | AIS | 4 (5) | 10 (12) | 0.845 |
| MIA | 10 (12) | 5 (6) | IA vs others | |
| IA | 72 (83) | 71 (82) | ||
| Pleural invasion | Present | 7 (12) | 14 (23) | 0.536 |
| Vascular invasion | Present | 11 (16) | 17 (25) | 0.566 |
| Lymphatic permeation | Present | 17 (24) | 24 (35) | 0.305 |
| Adjuvant therapy | Not performed | 64 (74) | 62 (72) | 0.863 |
| Platinum double | 8 (9) | 8 (9) | Not vs others | |
| Oral drugs | 14 (17) | 16 (19) | ||
| EGFR mutation | Exon21 L858R | 48 (56) | NA | |
| Exon19 del | 31 (36) | NA | ||
| Exon20 T790M | 3 (3) | NA | ||
| Others | 4 (5) | NA | ||
| EGFR–TKI | Administered | 12 (14) | 6 (7) | 0.001 |
| Characteristic . | . | EGFR . | EGFR . | P-value . |
|---|---|---|---|---|
| Mutant . | Wild-type . | |||
| n = 86 (%) . | n = 86 (%) . | |||
| Median age (years) | Range | 67 (43–85) | 67 (43–87) | 0.939 |
| Sex | Male | 39 (45) | 39 (45) | 1.000 |
| Smoking history | Ex- or current | 39 (45) | 39 (45) | 1.000 |
| Never | 47 (55) | 47 (55) | ||
| Serum CEA | >5 mg/dl | 9 (13) | 13 (19) | 0.402 |
| Median FEV1.0 | l (range) | 2.3 (0.8–3.9) | 2.2 (1.0–3.5) | 0.426 |
| Procedure | Lobectomy | 74 (92) | 76 (96) | 0.432 |
| Segmentectomy | 3 (4) | 1 (1) | Lobar vs sublobar | |
| Wedge resection | 3 (4) | 2 (3) | ||
| Pathological stage | IA | 56 (65) | 51 (59) | 1.000 |
| IB | 15 (17) | 20 (23) | I vs II more | |
| II | 5 (6) | 5 (6) | ||
| III | 8 (9) | 8 (9) | ||
| IV | 2 (2) | 2 (2) | ||
| WHO classification | AIS | 4 (5) | 10 (12) | 0.845 |
| MIA | 10 (12) | 5 (6) | IA vs others | |
| IA | 72 (83) | 71 (82) | ||
| Pleural invasion | Present | 7 (12) | 14 (23) | 0.536 |
| Vascular invasion | Present | 11 (16) | 17 (25) | 0.566 |
| Lymphatic permeation | Present | 17 (24) | 24 (35) | 0.305 |
| Adjuvant therapy | Not performed | 64 (74) | 62 (72) | 0.863 |
| Platinum double | 8 (9) | 8 (9) | Not vs others | |
| Oral drugs | 14 (17) | 16 (19) | ||
| EGFR mutation | Exon21 L858R | 48 (56) | NA | |
| Exon19 del | 31 (36) | NA | ||
| Exon20 T790M | 3 (3) | NA | ||
| Others | 4 (5) | NA | ||
| EGFR–TKI | Administered | 12 (14) | 6 (7) | 0.001 |
CEA: carcinoembryonic antigen; NA: not available; EGFR: epidermal growth factor receptor; AIS: adenocarcinoma in situ; MIA: minimally invasive adenocarcinoma; IA: invasive adenocarcinoma; TKI: tyrosine kinase inhibitor.
| Characteristic . | . | EGFR . | EGFR . | P-value . |
|---|---|---|---|---|
| Mutant . | Wild-type . | |||
| n = 86 (%) . | n = 86 (%) . | |||
| Median age (years) | Range | 67 (43–85) | 67 (43–87) | 0.939 |
| Sex | Male | 39 (45) | 39 (45) | 1.000 |
| Smoking history | Ex- or current | 39 (45) | 39 (45) | 1.000 |
| Never | 47 (55) | 47 (55) | ||
| Serum CEA | >5 mg/dl | 9 (13) | 13 (19) | 0.402 |
| Median FEV1.0 | l (range) | 2.3 (0.8–3.9) | 2.2 (1.0–3.5) | 0.426 |
| Procedure | Lobectomy | 74 (92) | 76 (96) | 0.432 |
| Segmentectomy | 3 (4) | 1 (1) | Lobar vs sublobar | |
| Wedge resection | 3 (4) | 2 (3) | ||
| Pathological stage | IA | 56 (65) | 51 (59) | 1.000 |
| IB | 15 (17) | 20 (23) | I vs II more | |
| II | 5 (6) | 5 (6) | ||
| III | 8 (9) | 8 (9) | ||
| IV | 2 (2) | 2 (2) | ||
| WHO classification | AIS | 4 (5) | 10 (12) | 0.845 |
| MIA | 10 (12) | 5 (6) | IA vs others | |
| IA | 72 (83) | 71 (82) | ||
| Pleural invasion | Present | 7 (12) | 14 (23) | 0.536 |
| Vascular invasion | Present | 11 (16) | 17 (25) | 0.566 |
| Lymphatic permeation | Present | 17 (24) | 24 (35) | 0.305 |
| Adjuvant therapy | Not performed | 64 (74) | 62 (72) | 0.863 |
| Platinum double | 8 (9) | 8 (9) | Not vs others | |
| Oral drugs | 14 (17) | 16 (19) | ||
| EGFR mutation | Exon21 L858R | 48 (56) | NA | |
| Exon19 del | 31 (36) | NA | ||
| Exon20 T790M | 3 (3) | NA | ||
| Others | 4 (5) | NA | ||
| EGFR–TKI | Administered | 12 (14) | 6 (7) | 0.001 |
| Characteristic . | . | EGFR . | EGFR . | P-value . |
|---|---|---|---|---|
| Mutant . | Wild-type . | |||
| n = 86 (%) . | n = 86 (%) . | |||
| Median age (years) | Range | 67 (43–85) | 67 (43–87) | 0.939 |
| Sex | Male | 39 (45) | 39 (45) | 1.000 |
| Smoking history | Ex- or current | 39 (45) | 39 (45) | 1.000 |
| Never | 47 (55) | 47 (55) | ||
| Serum CEA | >5 mg/dl | 9 (13) | 13 (19) | 0.402 |
| Median FEV1.0 | l (range) | 2.3 (0.8–3.9) | 2.2 (1.0–3.5) | 0.426 |
| Procedure | Lobectomy | 74 (92) | 76 (96) | 0.432 |
| Segmentectomy | 3 (4) | 1 (1) | Lobar vs sublobar | |
| Wedge resection | 3 (4) | 2 (3) | ||
| Pathological stage | IA | 56 (65) | 51 (59) | 1.000 |
| IB | 15 (17) | 20 (23) | I vs II more | |
| II | 5 (6) | 5 (6) | ||
| III | 8 (9) | 8 (9) | ||
| IV | 2 (2) | 2 (2) | ||
| WHO classification | AIS | 4 (5) | 10 (12) | 0.845 |
| MIA | 10 (12) | 5 (6) | IA vs others | |
| IA | 72 (83) | 71 (82) | ||
| Pleural invasion | Present | 7 (12) | 14 (23) | 0.536 |
| Vascular invasion | Present | 11 (16) | 17 (25) | 0.566 |
| Lymphatic permeation | Present | 17 (24) | 24 (35) | 0.305 |
| Adjuvant therapy | Not performed | 64 (74) | 62 (72) | 0.863 |
| Platinum double | 8 (9) | 8 (9) | Not vs others | |
| Oral drugs | 14 (17) | 16 (19) | ||
| EGFR mutation | Exon21 L858R | 48 (56) | NA | |
| Exon19 del | 31 (36) | NA | ||
| Exon20 T790M | 3 (3) | NA | ||
| Others | 4 (5) | NA | ||
| EGFR–TKI | Administered | 12 (14) | 6 (7) | 0.001 |
CEA: carcinoembryonic antigen; NA: not available; EGFR: epidermal growth factor receptor; AIS: adenocarcinoma in situ; MIA: minimally invasive adenocarcinoma; IA: invasive adenocarcinoma; TKI: tyrosine kinase inhibitor.
In these matched groups, 38 (22%) patients had recurrent disease during the follow-up period. The RFS and OS curves of the pair-matched patients according to EGFR mutation status are shown in Fig. 2. The 3- and 5-year RFS rates in the mutant and wild-type EGFR groups were 85 and 78% and 74 and 60%, respectively, both of which differed significantly between the two groups (P = 0.040). The equivalent 3- and 5-year OS rates were 95 and 89% and 82 and 77%, respectively, which also differed significantly (P = 0.016). Given that patients were matched for age, sex, smoking history and pStage, we performed univariate and multivariate analyses of RFS and OS in relation to other factors (Tables 3 and 4). Vascular invasion and lymphatic permeation were identified as independent recurrence-risk factors in RFS, and vascular invasion and EGFR mutation status as independent prognostic factors in OS.
Univariate and multivariate analyses of clinicopathological factors associated with recurrence-free survival in pair-matched patients
| Variable . | Favourable . | Unfavourable . | Univariate analysis . | Multivariate analysis . | ||||
|---|---|---|---|---|---|---|---|---|
| HR . | 95% CI . | P-value . | HR . | 95% CI . | P-value . | |||
| Pleural invasion | Absent | Present | 3.92 | 2.08–7.37 | <0.001 | 1.58 | 0.75–3.30 | 0.221 |
| Vascular invasion | Absent | Present | 4.05 | 2.24–7.32 | <0.001 | 2.10 | 1.03–4.29 | 0.036 |
| Lymphatic permeation | Absent | Present | 4.59 | 2.56–8.26 | <0.001 | 2.86 | 1.47–5.57 | 0.001 |
| EGFR mutation | Mutant | Wild-type | 1.86 | 1.02–3.38 | 0.040 | 1.63 | 0.89–3.01 | 0.108 |
| Variable . | Favourable . | Unfavourable . | Univariate analysis . | Multivariate analysis . | ||||
|---|---|---|---|---|---|---|---|---|
| HR . | 95% CI . | P-value . | HR . | 95% CI . | P-value . | |||
| Pleural invasion | Absent | Present | 3.92 | 2.08–7.37 | <0.001 | 1.58 | 0.75–3.30 | 0.221 |
| Vascular invasion | Absent | Present | 4.05 | 2.24–7.32 | <0.001 | 2.10 | 1.03–4.29 | 0.036 |
| Lymphatic permeation | Absent | Present | 4.59 | 2.56–8.26 | <0.001 | 2.86 | 1.47–5.57 | 0.001 |
| EGFR mutation | Mutant | Wild-type | 1.86 | 1.02–3.38 | 0.040 | 1.63 | 0.89–3.01 | 0.108 |
HR: hazard ratio; CI: confidence interval; EGFR: epidermal growth factor receptor.
Univariate and multivariate analyses of clinicopathological factors associated with recurrence-free survival in pair-matched patients
| Variable . | Favourable . | Unfavourable . | Univariate analysis . | Multivariate analysis . | ||||
|---|---|---|---|---|---|---|---|---|
| HR . | 95% CI . | P-value . | HR . | 95% CI . | P-value . | |||
| Pleural invasion | Absent | Present | 3.92 | 2.08–7.37 | <0.001 | 1.58 | 0.75–3.30 | 0.221 |
| Vascular invasion | Absent | Present | 4.05 | 2.24–7.32 | <0.001 | 2.10 | 1.03–4.29 | 0.036 |
| Lymphatic permeation | Absent | Present | 4.59 | 2.56–8.26 | <0.001 | 2.86 | 1.47–5.57 | 0.001 |
| EGFR mutation | Mutant | Wild-type | 1.86 | 1.02–3.38 | 0.040 | 1.63 | 0.89–3.01 | 0.108 |
| Variable . | Favourable . | Unfavourable . | Univariate analysis . | Multivariate analysis . | ||||
|---|---|---|---|---|---|---|---|---|
| HR . | 95% CI . | P-value . | HR . | 95% CI . | P-value . | |||
| Pleural invasion | Absent | Present | 3.92 | 2.08–7.37 | <0.001 | 1.58 | 0.75–3.30 | 0.221 |
| Vascular invasion | Absent | Present | 4.05 | 2.24–7.32 | <0.001 | 2.10 | 1.03–4.29 | 0.036 |
| Lymphatic permeation | Absent | Present | 4.59 | 2.56–8.26 | <0.001 | 2.86 | 1.47–5.57 | 0.001 |
| EGFR mutation | Mutant | Wild-type | 1.86 | 1.02–3.38 | 0.040 | 1.63 | 0.89–3.01 | 0.108 |
HR: hazard ratio; CI: confidence interval; EGFR: epidermal growth factor receptor.
Univariate and multivariate analyses of clinicopathological factors associated with overall survival in pair-matched patients
| Variable . | Favourable . | Unfavourable . | Univariate analysis . | Multivariate analysis . | ||||
|---|---|---|---|---|---|---|---|---|
| HR . | 95% CI . | P-value . | HR . | 95% CI . | P-value . | |||
| Pleural invasion | Absent | Present | 4.27 | 1.92–9.51 | <0.001 | 1.54 | 0.61–3.92 | 0.361 |
| Vascular invasion | Absent | Present | 5.51 | 2.58–11.77 | <0.001 | 3.57 | 1.42–8.98 | 0.006 |
| Lymphatic permeation | Absent | Present | 3.79 | 1.77–8.13 | <0.001 | 1.79 | 0.74–4.32 | 0.173 |
| EGFR mutation | Mutant | Wild-type | 2.67 | 1.17–6.11 | 0.016 | 2.48 | 1.07–5.76 | 0.034 |
| Variable . | Favourable . | Unfavourable . | Univariate analysis . | Multivariate analysis . | ||||
|---|---|---|---|---|---|---|---|---|
| HR . | 95% CI . | P-value . | HR . | 95% CI . | P-value . | |||
| Pleural invasion | Absent | Present | 4.27 | 1.92–9.51 | <0.001 | 1.54 | 0.61–3.92 | 0.361 |
| Vascular invasion | Absent | Present | 5.51 | 2.58–11.77 | <0.001 | 3.57 | 1.42–8.98 | 0.006 |
| Lymphatic permeation | Absent | Present | 3.79 | 1.77–8.13 | <0.001 | 1.79 | 0.74–4.32 | 0.173 |
| EGFR mutation | Mutant | Wild-type | 2.67 | 1.17–6.11 | 0.016 | 2.48 | 1.07–5.76 | 0.034 |
HR: hazard ratio; CI: confidence interval; EGFR: epidermal growth factor receptor.
Univariate and multivariate analyses of clinicopathological factors associated with overall survival in pair-matched patients
| Variable . | Favourable . | Unfavourable . | Univariate analysis . | Multivariate analysis . | ||||
|---|---|---|---|---|---|---|---|---|
| HR . | 95% CI . | P-value . | HR . | 95% CI . | P-value . | |||
| Pleural invasion | Absent | Present | 4.27 | 1.92–9.51 | <0.001 | 1.54 | 0.61–3.92 | 0.361 |
| Vascular invasion | Absent | Present | 5.51 | 2.58–11.77 | <0.001 | 3.57 | 1.42–8.98 | 0.006 |
| Lymphatic permeation | Absent | Present | 3.79 | 1.77–8.13 | <0.001 | 1.79 | 0.74–4.32 | 0.173 |
| EGFR mutation | Mutant | Wild-type | 2.67 | 1.17–6.11 | 0.016 | 2.48 | 1.07–5.76 | 0.034 |
| Variable . | Favourable . | Unfavourable . | Univariate analysis . | Multivariate analysis . | ||||
|---|---|---|---|---|---|---|---|---|
| HR . | 95% CI . | P-value . | HR . | 95% CI . | P-value . | |||
| Pleural invasion | Absent | Present | 4.27 | 1.92–9.51 | <0.001 | 1.54 | 0.61–3.92 | 0.361 |
| Vascular invasion | Absent | Present | 5.51 | 2.58–11.77 | <0.001 | 3.57 | 1.42–8.98 | 0.006 |
| Lymphatic permeation | Absent | Present | 3.79 | 1.77–8.13 | <0.001 | 1.79 | 0.74–4.32 | 0.173 |
| EGFR mutation | Mutant | Wild-type | 2.67 | 1.17–6.11 | 0.016 | 2.48 | 1.07–5.76 | 0.034 |
HR: hazard ratio; CI: confidence interval; EGFR: epidermal growth factor receptor.
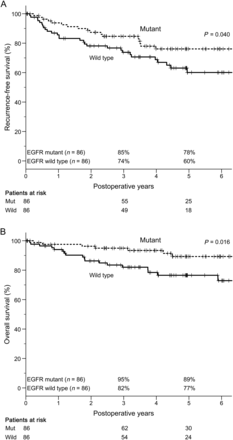
RFS and OS of pair-matched patients in relation to EGFR mutation status (n = 172). (A) The 3- and 5-year RFS rates of patients with and without EGFR mutation were 85 and 78% and 74 and 60%, respectively. These survival curves were significantly different (P = 0.040). (B) The 3- and 5-year OS rates of patients with and without EGFR mutation were 95 and 89% and 82 and 77%, respectively. These survival curves were also significantly different (P = 0.016). RFS: recurrence-free survival; OS: overall survival; EGFR: epidermal growth factor receptor.
DISCUSSION
We sometimes observe that patients with lung ADCs harbouring EGFR mutations have rapid recurrence after surgical resection. However, the association between EGFR mutation status and recurrence remains unclear. Some previous studies have addressed the association between EGFR mutation status and survival in patients with surgically resected non-small-cell lung cancer (NSCLC) [6, 7, 19–21]. Kim et al. [6] demonstrated that age, pStage and smoking status, but not EGFR mutation status, were significant prognostic factors for OS and RFS according to a Cox's proportional hazard model in 636 patients with ADC. Kosaka et al. [7] also analysed OS in 397 ADC patients who underwent surgical resection between 2000 and 2005, and reported that EGFR mutation status was a significant favourable prognostic factor according to univariate, but not multivariate analysis. From the review about EGFR mutation as a prognostic factor in NSCLC, some papers reported that EGFR mutation was a prognostic factor, but others did not, regarding both RFS and OS; thus the issue remained controversial [19]. Our current study has the advantage of being a matched-pair analysis, thus reducing bias due to other background characteristics, and enabling us to analyse RFS strictly in relation to EGFR mutation status. The primary results demonstrate better RFS in matched patients with mutant EGFR tumours compared with wild-type. These suggest that the presence of EGFR mutation might be a favourable recurrence-risk factor in patients with surgically resected lung ADC.
To the best of our knowledge, this is the first study to compare the survival of patients with lung ADC in relation to EGFR using matched-pair analysis. However, some aspects of this study need to be discussed. The first point is about which factors should be matched. There is limited evidence that the clinical factors, such as age, gender and smoking history are postoperative recurrence-risk factors [6, 7]. However, considering the primary aim of this study was analysing the RFS strictly due to EGFR mutation status, matching these factors is considered to serve the purpose because they have strong relation with harbouring EGFR mutation status. Microscopic findings, such as pleural invasion, vascular invasion and lymphatic permeation were well-reported independent prognostic factors, so we might have also considered to match these findings, but Table 2 shows us there were no significant differences between the two matched groups. As for the second point, it is still unclear if the current results allow us to conclude that mutated EGFR is a favourable recurrence-risk factor. In accordance with previous studies [6, 7], EGFR mutation was not an independent recurrence factor in both the whole patients and the pair-matched ones in the current multivariate analysis. If all the clinicopathological factors except for EGFR mutation status were matched, the RFS curves between the patients with or without EGFR mutant might not reach significant difference. The third point is whether it is appropriate to combine various types of EGFR mutation together as a single risk factor. Some reports proposed that OS and response to EGFR–TKI were different due to the types of EGFR mutation [19, 21, 22]. However, as mentioned, the primary purpose of the current analysis is to clarify how patients whose lung ADCs acquired EGFR mutation differed from the others. So we think it is reasonable to put all types of EGFR mutation together on analysis. We also analysed survivals according to the types of EGFR mutation but we did not report them because they were not related with the current arguments.
The difference in OS curves between patients with mutant and wild-type EGFR was greater than that for RFS curves, even in the matched patients. Indeed, multivariate analysis identified EGFR mutation as an independent prognostic factor for OS, but not for RFS, in the matched-pair cohorts. These results were largely attributable to the fact that patients whose tumours harboured EGFR mutations were likely to be more sensitive to EGFR–TKIs than those with wild-type EGFR. On the other hand, some reports have suggested that proliferative signalling via receptor tyrosine kinases differs between EGFR mutant and wild-type lung ADCs [23, 24], implying that such differences might lead the proliferative and invasive potentials of cancer nests to affect their clinical behaviours.
We chose the matched-pair analysis in the current research because we wanted to make the clinical and pathological variables more even before comparing the RFS due to EGFR mutation status. In the Cox proportional hazard model, we can estimate the effect of one clinicopatholgical factor on survival while adjusting those of other factors, while matched-pair analysis enables us to compare survivals after making one or some clinicopatholgical factors even between the two groups. However, matched-pair analysis also has some limitations in terms of reduced patient numbers and representativeness. Because this method selects patients from the whole cohort, it is necessary to decide which factors to match. Matching factors unrelated to EGFR mutation status or recurrence would not only reduce the number of analysed patients unnecessarily, but also derive the unrelated results to EGFR mutation. Given that some factors, such as sex and smoking history, are known to be associated with EGFR mutation status, we considered matched-pair analysis a suitable approach for the current research.
All of the tumours in the enrolled patients were re-evaluated pathologically according to the newly published WHO classification of lung ADC, and we found no difference in the rates of invasive ADC between patients with mutant and wild-type EGFR. Yanagawa et al. [25] reported frequencies of EGFR mutation of 62% in AIS, 60% in MIA and 51% in IA among 486 patients with lung ADC, results which were similar to those in the current study.
The major limitation of this study was its retrospective nature, which meant that the follow-up procedure and methods for examining recurrence were not standardized. Additionally, as mentioned in the Methods, this was a pilot study, so we did not plan the sample size, and collected the medical records of as many patients as possible in our institution. Calculating the required sample size based on the current study to have 80% statistical power at a two-sided significance level of P = 0.05, we needed 179 patients for each group. As a result, our present study was found to have about half the number of the required patients (86/179, 48%). For resolving this limitation, we currently have a multi-institutional observational analysis in progress.
In conclusion, EGFR-gene-mutation status might be a favourable recurrence-risk factor in patients with surgically resected lung ADC. However, further studies in larger cohorts are needed to verify this result.
SUPPLEMENTARY MATERIAL
Supplementary material is available at ICVTS online.
ACKNOWLEDGEMENTS
The authors thank Yukiko Kikuta for preparing the paraffin sections from formalin-fixed paraffin-embedded tissue for DNA extraction.
Conflict of interest: none declared.
REFERENCES




