-
PDF
- Split View
-
Views
-
Cite
Cite
Riken Kawachi, Rie Matsuwaki, Keisei Tachibana, Shin Karita, Yoko Nakazato, Ryota Tanaka, Yasushi Nagashima, Hidefumi Takei, Haruhiko Kondo, Thoracoscopic modified pleural tent for spontaneous pneumothorax, Interactive CardioVascular and Thoracic Surgery, Volume 23, Issue 2, August 2016, Pages 190–194, https://doi.org/10.1093/icvts/ivw107
Close - Share Icon Share
Abstract
We developed a modified pleural tent (m-tent) procedure and used it in our hospital in almost 30 consecutive patients with spontaneous pneumothorax. The objective of this study was to clarify the feasibility and effectiveness of a thoracoscopic m-tent for the treatment of spontaneous pneumothorax.
From July 2013 to November 2014, 107 patients with spontaneous pneumothorax were treated in our institution. Eighty-nine of these patients were analysed retrospectively. The inclusion criteria for thoracoscopic m-tent for spontaneous pneumothorax were multiple and widespread bullae, postoperative relapse and secondary spontaneous pneumothorax. The surgical procedures were usually performed through three ports. After bullectomy, an m-tent is made to strip the parietal pleura off the chest wall from about the level of the fourth or fifth rib to the apex, and two or three ligations are then applied to fix the pleural tent and lung parenchyma. Patients in whom an m-tent was not indicated underwent bullectomy plus coverage using absorbable materials.
Twenty-seven patients underwent bullectomy plus m-tent (m-tent group) and 62 underwent bullectomy plus coverage over a staple line using an absorbable material such as a polyglycolic acid sheet or nitrocellulose sheet (coverage group). No severe postoperative complications were observed in either group. The m-tent and coverage groups showed significant differences in operation time (129 vs 86 min, mean), haemorrhage (12.8 vs 7.2 ml), postoperative hospital stay (3.7 vs 2.9 days) and postoperative painkiller intake (8.6 vs 6.8 days). Recurrence was observed in 1 (3.7%) and 2 patients (3.2%), respectively.
The thoracoscopic m-tent procedure requires a longer operation, a longer hospital stay and greater painkiller intake. However, these differences are acceptable, and an m-tent should be considered as an option for pleural reinforcement in spontaneous pneumothorax, especially in patients who are complicated with severe pulmonary emphysema, widespread bullae or recurrent pneumothorax.
INTRODUCTION
Postoperative prolonged air leakage and the recurrence of bullae that can lead to recurrent pneumothorax are troublesome problems. The surgical treatment for spontaneous pneumothorax is sometimes accompanied by immediate postoperative air leakage and recurrence. Bullectomy alone, performed thoracoscopically, has been associated with high postoperative recurrence rates, and various procedures have been used to reduce these rates; e.g. pleural abrasion, pleurectomy, mesh coverage and chemical pleurodesis. [1–6]. One of the causes of recurrence has been reported to be recurrent bullae in the staple line, and this demonstrates that the application of tension on stapling can make the pleura more vulnerable [3, 7]. A variety of procedures that reinforce the staple line and apex area have been reported [2–6]. A pleural tent has been used in the treatment of tuberculosis, post-upper lobectomy and lung volume reduction surgery (LVRS) for severe chronic obstructive pulmonary disease (COPD) [8, 9]. To solve these problems, we have developed a thoracoscopic modified pleural tent (m-tent) procedure for patients with spontaneous pneumothorax. The purpose of the present study was to analyse the efficacy and feasibility of the m-tent procedure by comparing an m-tent group and a group that was treated with absorbable mesh materials and coverage of the staple line.
PATIENTS AND METHODS
Patients
From July 2013 to November 2014, 107 patients with spontaneous pneumothorax were treated in our institution. Haemothorax, open thoracotomy and pulmonary resection that required greater than bullectomy were excluded. Eighteen patients were excluded from this study because of a procedure under thoracotomy in 11, pneumohaemothorax in 5, lobectomy in 1 and catamenial pneumothorax in 1. Consequently, 89 patients were included in this study and analysed retrospectively. Two different treatment procedures were applied to the patients: a modified tent (m-tent) group and a coverage group. All patients routinely received daily treatment with Loxoprofen sodium postoperatively. Epidural anaesthesia was not routinely used. Postoperative pain was defined as pain that required painkiller intake for more than 3 weeks or additional painkiller. The median follow-up time was 519 days [range: 258–786 days, standard deviation (SD): 149].
Surgical procedures
Thoracoscopic modified pleural tent procedure
Under separate lung ventilation, the surgical procedure starts in the lateral decubitus position. Three ports placed in the axilla are used to perform the procedures, including bulla resection, and to produce a modified pleural tent. After the resection or ligation of bulla using staples or ligation devices, a modified pleural tent is made to drape over the staple line and pleura at the apex, since this prevents air leaks and reinforces the visceral pleura. Firstly, the parietal pleura is incised with a cautery from the internal thoracic vein anteriorly to the sympathetic nerve trunk posteriorly (Fig. 1). The cut edge of the parietal pleura is then grasped gently with forceps and dissected bluntly using an instrument, such as a peanut dissector. The edge of the parietal pleura is further retracted and stripped from the chest wall. This must be performed carefully to avoid tearing of the pleura, which might reduce the quality of the tent. The dissection is carried anteriorly, posteriorly and superiorly to the apex of the thoracic cavity (Fig. 1). We usually do not strip the surface of the innominate vein or subclavian artery. The pleural tent stretches down to cover the upper lobe on the right side and the upper segment on the left side (Fig. 2). In the case of bullae in the superior segment of the lower lobe, a greater amount of parietal pleura would need to be mobilized to cover the superior segment. The edge of the pleura is then tied to the edge of the right upper lobe at two or three sites. Haemostasis is secured over the raw surface of the chest wall using a cautery, and a 28-Fr chest tube is placed posteriorly through the posterior trocar site. The lung should be inflated and complete expansion of all the lung parenchyma should be confirmed. The inclusion criteria for thoracoscopic m-tent for spontaneous pneumothorax were multiple and widespread bullae, postoperative relapse and secondary spontaneous pneumothorax.
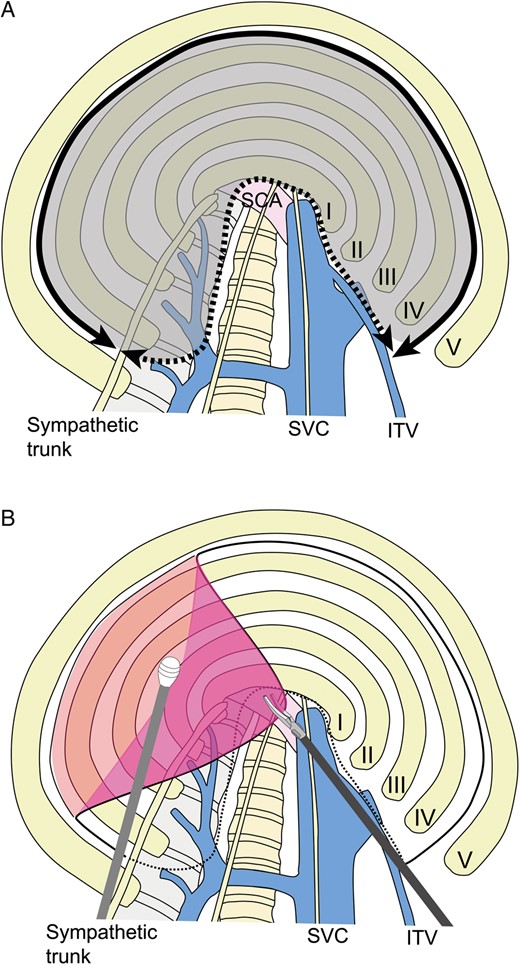
(A) Incision of parietal pleura and range of mobilization. Parietal pleura are incised with an electric cautery from the internal thoracic vein anteriorly to the sympathetic nerve trunk posteriorly (solid line). Parietal pleura are mobilized to the superior vena cava anteriorly and the vertebral body posteriorly (dotted line). Parietal pleura (dark area) are stripped. SCA: subclavian artery; SVC: superior vena cava; ITV: internal thoracic vein. (B) Mobilization of parietal pleura. The cut edge of the parietal pleura is grasped with forceps and dissected bluntly using an instrument, such as a peanut dissector.
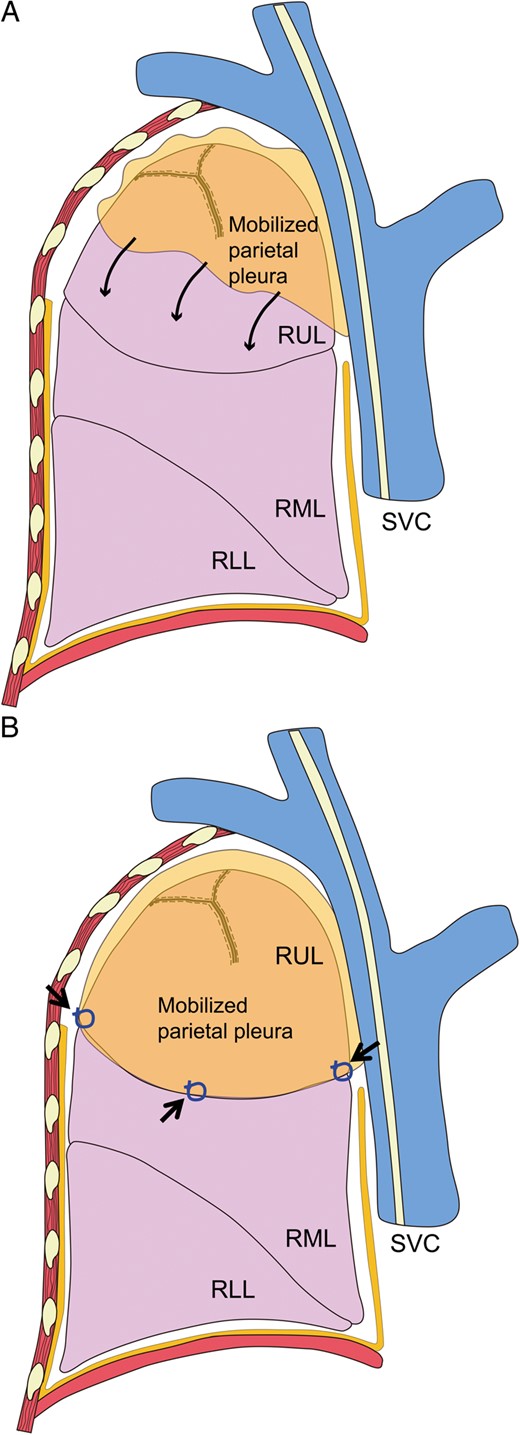
(A) Stripped pleura covering the right upper lobe or upper segment. The stripped parietal pleura stretches down to cover the upper lobe on the right side and the upper segment on the left side (thin arrows). (B) The edge of the pleura is tied to the edge of the right upper lobe at two or three sites (thick arrows). RUL: right upper lobe; RML: right middle lobe; RLL: right lower lobe; SVC: superior vena cava.
Coverage procedure
In the other patients (coverage group), in whom the m-tent procedure was not performed, a polyglycolic acid sheet (NEOVEIL®, GUNZE Limited, 10 × 5 cm) or a nitrocellulose sheet (SURGICEL®, Johnson & Johnson Products, Inc., 20.3 × 10.2 cm) was used as the coverage material. The staple line was fully covered with these materials after bullectomy. In several of these patients, the mesh was spritzed with 10–20 ml of autologous blood.
The decision regarding which surgical procedure should be performed was made based on intrathoracic findings and the surgeons' preference.
Statistical analysis
All the data were analysed using IBM SPSS Statistics software, Version 19 (SPSS, Inc., Chicago, IL, USA). Descriptive statistics were expressed as mean ± SD. Continuous variables were analysed by the Mann–Whitney U-test. Categorical variables were expressed in terms of frequency and analysed by Fisher's exact test.
RESULTS
Patients
The characteristics of both groups are given in Table 1. The m-tent group included older patients and more secondary pneumothorax patients than the coverage group. None of the patients underwent a simultaneous bilateral m-tent procedure. The m-tent procedure was performed more frequently in secondary pneumothorax than in primary pneumothorax. Persistent air leak and ipsilateral recurrence were frequent surgical indications in both the groups.
Clinical characteristics of patients treated by a modified pleural tent (m-tent group) or coverage with absorbable materials (coverage group)
| . | Modified pleural tent (n = 27) . | Coverage (n = 62) . | P-value . |
|---|---|---|---|
| Age (years) | 0.006 | ||
| Mean (range, SD) | 35.0 (16–85, 18.4) | 27.4 (13–79, 19.1) | |
| Gender (%) | 0.052 | ||
| Male | 27 (100) | 53 (86) | |
| Female | 0 (0) | 9 (15) | |
| Side (%) | 0.346 | ||
| Right | 11 (41) | 30 (48) | |
| Left | 16 (59) | 29 (47) | |
| Bilateral (simultaneous) | 0 (0) | 3 (5) | |
| Smoking status (pack-year) | 0.055 | ||
| Mean (range, SD) | 7.8 (0–60, 13.1) | 7.6 (0–129, 20.3) | |
| Type of pneumothoraxa (%) | 0.075 | ||
| Primary | 19 (70) | 54 (87) | |
| Secondary | 8 (30) | 8 (13) | |
| Treatment history (%) | 0.647 | ||
| None | 14 (52) | 28 (45) | |
| Underwent | 13 (48) | 34 (55) | |
| Ipsilateral | 7 (26) | 19 (31) | |
| Observation | 3 (11) | 6 (10) | |
| Drainage | 3 (11) | 13 (21) | |
| Operation | 1 (4) | 0 (0) | |
| Contralateral | 1 (4) | 8 (13) | |
| Observation | 0 (0) | 1 (2) | |
| Drainage | 0 (0) | 2 (3) | |
| Operation | 1 (4) | 5 (8) | |
| Bilateral | 5 (19) | 7 (11) | |
| Observation | 1 (4) | 0 (0) | |
| Drainage | 0 (0) | 3 (5) | |
| Operation | 4 (4) | 4 (7) | |
| Surgical indications (%) | 0.269 | ||
| Persistent air leak | 7 (26) | 27 (44) | |
| Ipsilateral recurrence | 12 (44) | 23 (37) | |
| Postoperative recurrence | 5 (19) | 4 (7) | |
| Contralateral recurrence | 1 (4) | 4 (7) | |
| Others | 7 (26) | 8 (13) | |
| Status of bullae (%) | <0.001 | ||
| Single | 0 (0) | 39 (63) | |
| Multiple or widespread | 27 (100) | 23 (37) |
| . | Modified pleural tent (n = 27) . | Coverage (n = 62) . | P-value . |
|---|---|---|---|
| Age (years) | 0.006 | ||
| Mean (range, SD) | 35.0 (16–85, 18.4) | 27.4 (13–79, 19.1) | |
| Gender (%) | 0.052 | ||
| Male | 27 (100) | 53 (86) | |
| Female | 0 (0) | 9 (15) | |
| Side (%) | 0.346 | ||
| Right | 11 (41) | 30 (48) | |
| Left | 16 (59) | 29 (47) | |
| Bilateral (simultaneous) | 0 (0) | 3 (5) | |
| Smoking status (pack-year) | 0.055 | ||
| Mean (range, SD) | 7.8 (0–60, 13.1) | 7.6 (0–129, 20.3) | |
| Type of pneumothoraxa (%) | 0.075 | ||
| Primary | 19 (70) | 54 (87) | |
| Secondary | 8 (30) | 8 (13) | |
| Treatment history (%) | 0.647 | ||
| None | 14 (52) | 28 (45) | |
| Underwent | 13 (48) | 34 (55) | |
| Ipsilateral | 7 (26) | 19 (31) | |
| Observation | 3 (11) | 6 (10) | |
| Drainage | 3 (11) | 13 (21) | |
| Operation | 1 (4) | 0 (0) | |
| Contralateral | 1 (4) | 8 (13) | |
| Observation | 0 (0) | 1 (2) | |
| Drainage | 0 (0) | 2 (3) | |
| Operation | 1 (4) | 5 (8) | |
| Bilateral | 5 (19) | 7 (11) | |
| Observation | 1 (4) | 0 (0) | |
| Drainage | 0 (0) | 3 (5) | |
| Operation | 4 (4) | 4 (7) | |
| Surgical indications (%) | 0.269 | ||
| Persistent air leak | 7 (26) | 27 (44) | |
| Ipsilateral recurrence | 12 (44) | 23 (37) | |
| Postoperative recurrence | 5 (19) | 4 (7) | |
| Contralateral recurrence | 1 (4) | 4 (7) | |
| Others | 7 (26) | 8 (13) | |
| Status of bullae (%) | <0.001 | ||
| Single | 0 (0) | 39 (63) | |
| Multiple or widespread | 27 (100) | 23 (37) |
SD: standard deviation.
aA primary spontaneous pneumothorax occurs without a precipitating event in the absence of clinical lung disease. In contrast, secondary spontaneous pneumothorax is defined as a pneumothorax that occurs as a complication of underlying lung disease.
Clinical characteristics of patients treated by a modified pleural tent (m-tent group) or coverage with absorbable materials (coverage group)
| . | Modified pleural tent (n = 27) . | Coverage (n = 62) . | P-value . |
|---|---|---|---|
| Age (years) | 0.006 | ||
| Mean (range, SD) | 35.0 (16–85, 18.4) | 27.4 (13–79, 19.1) | |
| Gender (%) | 0.052 | ||
| Male | 27 (100) | 53 (86) | |
| Female | 0 (0) | 9 (15) | |
| Side (%) | 0.346 | ||
| Right | 11 (41) | 30 (48) | |
| Left | 16 (59) | 29 (47) | |
| Bilateral (simultaneous) | 0 (0) | 3 (5) | |
| Smoking status (pack-year) | 0.055 | ||
| Mean (range, SD) | 7.8 (0–60, 13.1) | 7.6 (0–129, 20.3) | |
| Type of pneumothoraxa (%) | 0.075 | ||
| Primary | 19 (70) | 54 (87) | |
| Secondary | 8 (30) | 8 (13) | |
| Treatment history (%) | 0.647 | ||
| None | 14 (52) | 28 (45) | |
| Underwent | 13 (48) | 34 (55) | |
| Ipsilateral | 7 (26) | 19 (31) | |
| Observation | 3 (11) | 6 (10) | |
| Drainage | 3 (11) | 13 (21) | |
| Operation | 1 (4) | 0 (0) | |
| Contralateral | 1 (4) | 8 (13) | |
| Observation | 0 (0) | 1 (2) | |
| Drainage | 0 (0) | 2 (3) | |
| Operation | 1 (4) | 5 (8) | |
| Bilateral | 5 (19) | 7 (11) | |
| Observation | 1 (4) | 0 (0) | |
| Drainage | 0 (0) | 3 (5) | |
| Operation | 4 (4) | 4 (7) | |
| Surgical indications (%) | 0.269 | ||
| Persistent air leak | 7 (26) | 27 (44) | |
| Ipsilateral recurrence | 12 (44) | 23 (37) | |
| Postoperative recurrence | 5 (19) | 4 (7) | |
| Contralateral recurrence | 1 (4) | 4 (7) | |
| Others | 7 (26) | 8 (13) | |
| Status of bullae (%) | <0.001 | ||
| Single | 0 (0) | 39 (63) | |
| Multiple or widespread | 27 (100) | 23 (37) |
| . | Modified pleural tent (n = 27) . | Coverage (n = 62) . | P-value . |
|---|---|---|---|
| Age (years) | 0.006 | ||
| Mean (range, SD) | 35.0 (16–85, 18.4) | 27.4 (13–79, 19.1) | |
| Gender (%) | 0.052 | ||
| Male | 27 (100) | 53 (86) | |
| Female | 0 (0) | 9 (15) | |
| Side (%) | 0.346 | ||
| Right | 11 (41) | 30 (48) | |
| Left | 16 (59) | 29 (47) | |
| Bilateral (simultaneous) | 0 (0) | 3 (5) | |
| Smoking status (pack-year) | 0.055 | ||
| Mean (range, SD) | 7.8 (0–60, 13.1) | 7.6 (0–129, 20.3) | |
| Type of pneumothoraxa (%) | 0.075 | ||
| Primary | 19 (70) | 54 (87) | |
| Secondary | 8 (30) | 8 (13) | |
| Treatment history (%) | 0.647 | ||
| None | 14 (52) | 28 (45) | |
| Underwent | 13 (48) | 34 (55) | |
| Ipsilateral | 7 (26) | 19 (31) | |
| Observation | 3 (11) | 6 (10) | |
| Drainage | 3 (11) | 13 (21) | |
| Operation | 1 (4) | 0 (0) | |
| Contralateral | 1 (4) | 8 (13) | |
| Observation | 0 (0) | 1 (2) | |
| Drainage | 0 (0) | 2 (3) | |
| Operation | 1 (4) | 5 (8) | |
| Bilateral | 5 (19) | 7 (11) | |
| Observation | 1 (4) | 0 (0) | |
| Drainage | 0 (0) | 3 (5) | |
| Operation | 4 (4) | 4 (7) | |
| Surgical indications (%) | 0.269 | ||
| Persistent air leak | 7 (26) | 27 (44) | |
| Ipsilateral recurrence | 12 (44) | 23 (37) | |
| Postoperative recurrence | 5 (19) | 4 (7) | |
| Contralateral recurrence | 1 (4) | 4 (7) | |
| Others | 7 (26) | 8 (13) | |
| Status of bullae (%) | <0.001 | ||
| Single | 0 (0) | 39 (63) | |
| Multiple or widespread | 27 (100) | 23 (37) |
SD: standard deviation.
aA primary spontaneous pneumothorax occurs without a precipitating event in the absence of clinical lung disease. In contrast, secondary spontaneous pneumothorax is defined as a pneumothorax that occurs as a complication of underlying lung disease.
Operations
Twenty-seven patients underwent bullectomy plus a modified pleural tent (m-tent group) and 62 underwent bullectomy plus pleural coverage using a polyglycolic acid sheet in 16 patients (+ autologous blood in 12) and a nitrocellulose sheet in 38 (+ autologous blood in 34), or both in 9 (all 9 used autologous blood) (coverage group). No severe postoperative complications were observed in either group. The m-tent group showed significant differences in blood loss, operation time, postoperative drainage duration and hospital stay (Table 2).
Results in patients treated by a modified pleural tent (m-tent group) or coverage with absorbable materials (coverage group)
| . | Modified pleural tent (n = 27) . | Coverage (n = 62) . | P-value . |
|---|---|---|---|
| Blood loss (ml) | 0.012 | ||
| Mean (range, SD) | 12.8 (1–104, 21.1) | 7.2 (1–210, 26.4) | |
| Operation time (min) | <0.001 | ||
| Mean (range, SD) | 129.0 (72–237, 33.6) | 86.1 (37–185, 29.0) | |
| Postoperative hospital stay (days) | <0.001 | ||
| Mean (range, SD) | 3.7 (2–10, 1.5) | 2.9 (2–9, 1.4) | |
| Postoperative drainage (days) | 0.032 | ||
| Mean (range, SD) | 1.9 (1–4, 1.0) | 1.6 (1–9, 1.4) | |
| Drainage duration ≥3 days (%) | 7 (26) | 5 (8) | 0.039 |
| Postoperative pain (%)a | 11 (41) | 14 (23) | 0.122 |
| Painkiller intake ≥3 weeks (%) | 6 (22) | 8 (13) | 0.343 |
| Additional painkiller (%) | 10 (37) | 9 (15) | 0.025 |
| Recurrence (%) | 1 (4) | 2 (3) | 1.0 |
| Require drainage (%) | 1 (4) | 0 (0) | 0.303 |
| . | Modified pleural tent (n = 27) . | Coverage (n = 62) . | P-value . |
|---|---|---|---|
| Blood loss (ml) | 0.012 | ||
| Mean (range, SD) | 12.8 (1–104, 21.1) | 7.2 (1–210, 26.4) | |
| Operation time (min) | <0.001 | ||
| Mean (range, SD) | 129.0 (72–237, 33.6) | 86.1 (37–185, 29.0) | |
| Postoperative hospital stay (days) | <0.001 | ||
| Mean (range, SD) | 3.7 (2–10, 1.5) | 2.9 (2–9, 1.4) | |
| Postoperative drainage (days) | 0.032 | ||
| Mean (range, SD) | 1.9 (1–4, 1.0) | 1.6 (1–9, 1.4) | |
| Drainage duration ≥3 days (%) | 7 (26) | 5 (8) | 0.039 |
| Postoperative pain (%)a | 11 (41) | 14 (23) | 0.122 |
| Painkiller intake ≥3 weeks (%) | 6 (22) | 8 (13) | 0.343 |
| Additional painkiller (%) | 10 (37) | 9 (15) | 0.025 |
| Recurrence (%) | 1 (4) | 2 (3) | 1.0 |
| Require drainage (%) | 1 (4) | 0 (0) | 0.303 |
SD: standard deviation.
aPostoperative pain was defined as pain that required painkiller intake longer than 3 weeks or additional painkiller.
Results in patients treated by a modified pleural tent (m-tent group) or coverage with absorbable materials (coverage group)
| . | Modified pleural tent (n = 27) . | Coverage (n = 62) . | P-value . |
|---|---|---|---|
| Blood loss (ml) | 0.012 | ||
| Mean (range, SD) | 12.8 (1–104, 21.1) | 7.2 (1–210, 26.4) | |
| Operation time (min) | <0.001 | ||
| Mean (range, SD) | 129.0 (72–237, 33.6) | 86.1 (37–185, 29.0) | |
| Postoperative hospital stay (days) | <0.001 | ||
| Mean (range, SD) | 3.7 (2–10, 1.5) | 2.9 (2–9, 1.4) | |
| Postoperative drainage (days) | 0.032 | ||
| Mean (range, SD) | 1.9 (1–4, 1.0) | 1.6 (1–9, 1.4) | |
| Drainage duration ≥3 days (%) | 7 (26) | 5 (8) | 0.039 |
| Postoperative pain (%)a | 11 (41) | 14 (23) | 0.122 |
| Painkiller intake ≥3 weeks (%) | 6 (22) | 8 (13) | 0.343 |
| Additional painkiller (%) | 10 (37) | 9 (15) | 0.025 |
| Recurrence (%) | 1 (4) | 2 (3) | 1.0 |
| Require drainage (%) | 1 (4) | 0 (0) | 0.303 |
| . | Modified pleural tent (n = 27) . | Coverage (n = 62) . | P-value . |
|---|---|---|---|
| Blood loss (ml) | 0.012 | ||
| Mean (range, SD) | 12.8 (1–104, 21.1) | 7.2 (1–210, 26.4) | |
| Operation time (min) | <0.001 | ||
| Mean (range, SD) | 129.0 (72–237, 33.6) | 86.1 (37–185, 29.0) | |
| Postoperative hospital stay (days) | <0.001 | ||
| Mean (range, SD) | 3.7 (2–10, 1.5) | 2.9 (2–9, 1.4) | |
| Postoperative drainage (days) | 0.032 | ||
| Mean (range, SD) | 1.9 (1–4, 1.0) | 1.6 (1–9, 1.4) | |
| Drainage duration ≥3 days (%) | 7 (26) | 5 (8) | 0.039 |
| Postoperative pain (%)a | 11 (41) | 14 (23) | 0.122 |
| Painkiller intake ≥3 weeks (%) | 6 (22) | 8 (13) | 0.343 |
| Additional painkiller (%) | 10 (37) | 9 (15) | 0.025 |
| Recurrence (%) | 1 (4) | 2 (3) | 1.0 |
| Require drainage (%) | 1 (4) | 0 (0) | 0.303 |
SD: standard deviation.
aPostoperative pain was defined as pain that required painkiller intake longer than 3 weeks or additional painkiller.
Postoperative parameters
The m-tent group showed a longer postoperative hospital stay, a longer postoperative drain duration and more frequent postoperative pain than the coverage group (Table 2). The mean painkiller intake was 8.6 days (m-tent group) versus 6.8 days (coverage group). One patient required painkiller intake for 3 months. Except for this patient, painkiller intake was complete within 1 month. In addition to postoperative pain, depression that required psychiatric drug therapy developed in 2 patients in the m-tent group and 1 patient in the coverage group. Recurrence was observed in 1 and 2 patients, respectively. One patient who underwent the m-tent procedure had ipsilateral recurrent pneumothorax and needed drainage. Bullae were localized in both the upper and lower lobes in this patient. Nitrocellulose sheet coverage was performed in the lower lobe, but the bullae in the lower lobe caused recurrence.
Comment
We developed a modified pleural tent (m-tent) procedure that was based on a pleural tent procedure and parietal pleurectomy. The main purpose of this method is coverage and reinforcement of the staple line and the apex, in which bullae could recur. The pleural tent procedure was originally developed for tuberculosis and has recently been used for LVRS, post-upper lobectomy etc. [8, 9]. The idea of controlling the residual intrathoracic space and stopping possible air leakage was first proposed for tuberculosis by Miscall et al. in 1956 [9]. This procedure has been demonstrated to be effective for reducing air leakage and the hospital stay after upper lobectomy in a randomized setting [8]. Venuta et al. reported a thoracoscopic pleural tent in LVRS, and this technique could also be performed less invasively [10]. On the other hand, parietal pleurectomy has frequently been performed for spontaneous pneumothorax. Pleurectomy that elicits adhesion between the chest wall and lung parenchyma would prevent lung collapse. However, adhesion may not occur unless the lung fully expands. Consequently, the recurrence rate has been reported to be approximately 5–10%, even in bullectomy and pleurectomy [11]. To reduce recurrence, we developed this m-tent procedure, which combines a pleural tent and pleurectomy. The mobilized parietal pleura completely cover the upper lobe, which reinforces the staple line and visceral pleura. The original method for a pleural tent reduces the residual pleural space by mobilizing the parietal pleura. An advantage of the m-tent procedure is that it encourages the prompt reinforcement of visceral pleura, and this reduces subsequent recurrence. Adhesion between the pleural tent and chest wall helps to prevent postoperative recurrence in the late period.
This study compared the coverage procedure with absorbable materials, which has been used as our routine technique, and the m-tent procedure. With the m-tent procedure, there was more blood loss, more postoperative pain, longer drainage duration and a longer hospital stay. In addition to postoperative pain, depression developed in 2 patients and they required psychiatric drug therapy. These findings demonstrated that an m-tent is more invasive than coverage, and therefore its application should be considered carefully.
The recurrence rate was similar between the two groups even though the m-tent group included more complicated patients, e.g. COPD, than the coverage group. This indicates that the m-tent procedure can potentially prevent pneumothorax from recurring. The mobilized parietal pleura should reinforce the strength of the visceral pleura and lead to adhesion to the chest wall. The recurrence rate in the m-tent group was 3.7%, and this seems to be acceptable although the m-tent group included more secondary pneumothorax patients. However, a longer follow-up time will be needed to evaluate recurrence correctly.
Our modified pleural tent procedure has a few problems, such as the size of parietal pleura mobilization. In this procedure, we create a modified pleural tent that covers the upper lobe and the upper division or superior segment of the lower lobe where a bulla is likely to occur. As the extent of pleural dissection is increased, lung function could become more restricted, and a longer observation period should be mandatory. In addition, in this procedure, mediastinal pleura, especially parietal pleura on the superior vena cava, are not stripped off, and a longer observation will be needed to evaluate how this could influence the results. Especially in younger patients, indications for this procedure should be carefully determined. Secondly, this technique requires an additional procedure to resect bullae, and this could result in a longer operation time and postoperative pain that includes not only somatic and visceral pain but also intercostal neuropathic pain. Consequently, a gentle and careful manoeuvre is mandatory. While these kinds of pain were observed, fortunately they did not last beyond a month. Thirdly, the m-tent procedure seems to be difficult to apply in cases with multiple bullae in the lower lobe, although the upper lobe or upper segment can be covered with the m-tent. Coverage or some other procedure should be performed in such patients. A patient who underwent the m-tent procedure and had recurrence postoperatively was observed, and bullae were localized in the lower lobe.
In conclusion, a video-assisted thoracic surgery modified pleural tent is a valid and useful procedure for spontaneous pneumothorax with emphysematous lung parenchyma and widespread bullae, since this procedure can be performed with comparative safety. However, it can be associated with more severe postoperative pain.
Conflict of interest: none declared.
REFERENCES
APPENDIX. CONFERENCE DISCUSSION
Dr C. Zirafa(Pisa, Italy): I have two questions for you. First, what features must the patients have who could take advantage of your M-tent technique? The second question is, do you usually perform pleurodesis or pleurectomy to treat recurrent pneumothorax?
Dr Kawachi: Our study was unable to demonstrate that the M-tent technique offered a definite advantage because the patients had completely different backgrounds and thus it is difficult to compare them to each other.
Before we started our modified pleural tent, we performed parietal pleurectomy. Due to the greater resection, patients would often require a few months for full expansion. In several patients, recurrence and prolonged air leakage developed postoperatively. Therefore, we developed this procedure. As I noted earlier, this procedure was indicated for patients with multiple and widespread bullae, relapse and severe spontaneous pneumothorax. Since this was a retrospective study, I don't know whether patients strictly must have these features. However, except for the long operation time and postoperative relapse, the two groups were quite similar, so I believe the procedure could be acceptable for such patients.
To answer your other question, in our hospital chemical pleurodesis is not performed for spontaneous pneumothorax, especially primary spontaneous pneumothorax. As I said, parietal pleurectomy before we started our modified pleural tent procedure. After that, we stopped performing parietal pleurectomy.
Dr H. Fallouh(Cardiff, United Kingdom): Presumably, this study included a bit of a learning curve with this procedure, just for people who want to go and try it out at home. Any tricks you do to avoid trapping lungs down with your sheet of pleura? Have you had a problem with lung trapping in such a procedure.
Dr Kawachi: As I stated in the presentation, we incise the parietal pleura on the fourth or fifth rib. We have to evaluate the size of the upper lobe. If the mobilized parietal pleura is too small, the lung could become trapped. So, yes, we try to make the modified pleural tent relatively large.
Dr H. Elkhayat(Assiut, Egypt): I have two questions. First, why don't you use staplers? You just ligate it. The second question is, when you use the coverage, you said that you put 10 ml to 20 ml of blood for the coverage. I know from the Okada experience in segmentectomy that for the Japanese coverage, they put fibrin glue with thrombin on it. Why do you choose blood for these cases?
Dr Kawachi: Our patients usually have multiple bullae in the apex, so we have to cut the bullae using an automatic stapler. If the bullae are separated from each other, we sometimes ligate.
Author notes
Presented at the 29th Annual Meeting of the European Association for Cardio-Thoracic Surgery, Amsterdam, Netherlands, 3–7 October 2015.





Comments
© The Author 2016. Published by Oxford University Press on behalf of the European Association for Cardio-Thoracic Surgery. All rights reserved
We read the article entitled "Thoracoscopic modified pleural tent for spontaneous pneumothorax" by Kawachi et al. [1]. When we looked to the clinical characteristics of the patients, we saw that approximately 82% of the patients (73 of 89 patients) was treated for primary spontaneous pneumothorax. As primary spontaneous pneumothorax is generally seen in young patients, we want to point out the patient consent about the surgical treatment strategy of primary spontaneous pneumothorax.
The surgery for primary spontaneous pneumothorax can be divided into two stages. In the first stage, the diseased part of the lung is stapled via VATS. This stage is generally done in the same way among the surgeons. In the second part, one of the recurrence prevention procedures is generally performed. Pleurectomy and pleural abrasion are the most used techniques. These techniques and all the other procédures, including the authors' procedure, have advantages and disadvantages associated with them. As there is no consensus about which procedure is the best, we think that for this second stage, the final decision should be left to the patient. Patient should be informed about the recurrence rates and the degree of the recurrence preventive adhesions in each procedure. Relative comparison of the procedures should be expressed to the patient and her/his family. After obtaining the consent, the procedure could be carried out without hesitation.
We thank the authors for their article about their innovative model which could be used in the treatment of secondary pneumothorax.
Reference
[1] Kawachi R, Matsuwaki R, Tachibana K, Karita S, Nakazato Y, Tanaka R et al. . Thoracoscopic modified pleural tent for spontaneous pneumothorax. Interact CardioVasc Thorac Surg 18 April 2016; doi: 10.1093/icvts/ivw107.
Conflict of interest: none declared.