-
PDF
- Split View
-
Views
-
Cite
Cite
Laura Donahoe, John Granton, Karen McRae, John Thenganatt, Jacov Moric, Shaf Keshavjee, Marc de Perrot, Role of extracorporeal life support after pulmonary endarterectomy: a single-centre experience, Interactive CardioVascular and Thoracic Surgery, Volume 23, Issue 1, July 2016, Pages 74–78, https://doi.org/10.1093/icvts/ivw075
Close - Share Icon Share
Abstract
Extracorporeal life support (ECLS) for rescue after pulmonary endarterectomy (PEA) has become a viable option. This study aims to present a single-centre experience looking at the indications and outcome of ECLS after PEA.
Retrospective analysis of all patients undergoing PEA from January 2008 to January 2015 in our institution.
Among 144 consecutive patients undergoing PEA for chronic thromboembolic pulmonary hypertension, 6 (4%) received ECLS postoperatively for right ventricular (RV) failure (n = 3), severe hypoxaemia (n = 2) and haemorrhagic pulmonary oedema (n = 1). ECLS configuration was central veno-arterial (cVA) in 3 patients, peripheral VA (pVA) in 1 and veno-venous (VV) in 2. One patient with cVA was switched to VV after 5 days. Overall ECLS duration ranged between 3 and 39 (median 5) days. ECLS patients had higher preoperative total pulmonary vascular resistance (TPR) compared with non-ECLS patients (1477 ± 671 vs 954 ± 462 Dynes.s.cm−5, P = 0.009) and more frequently required hospital admission for RV failure before surgery (50 vs 9%, P = 0.02). The overall in-hospital mortality rate for all patients was 2% (3/144), including one ECLS patient on pVA. The remaining 5 ECLS patients (83%) were discharged from the hospital and are alive after a median follow-up of 11 (range 6–27) months. Two ECLS patients (40%) are on therapy for residual PH compared with 13 (10%) in the non-ECLS patients (P = 0.09).
ECLS is a safe and important rescue option after PEA. The use of ECLS may expand eligibility for PEA by allowing sicker patients to undergo surgery.
INTRODUCTION
Pulmonary endarterectomy (PEA) is the treatment of choice for patients with chronic thromboembolic pulmonary hypertension (CTEPH). As the disease diagnosis becomes more widespread and the surgical treatment becomes more available, the number of patients referred for surgery is increasing. With a larger number of patients comes a wider variety of severity of disease, from minimally symptomatic with minor haemodynamic dysfunction to severely symptomatic with florid heart failure. The indications for PEA are thus evolving, and include patients at higher risk, demonstrated by increased pulmonary vascular resistance (PVR) levels, more distal disease and worsening right ventricular (RV) failure. The acuity of patients proceeding to PEA increases, so does the potential for increased mortality. Extracorporeal life support (ECLS), with central and peripheral veno-arterial (pVA) and peripheral veno-venous (VV) cannulation, may provide a means to support these high-risk patients postoperatively, thereby potentially decreasing mortality. We reviewed all patients who underwent PEA at a single centre in order to determine the characteristics of those requiring ECLS as well as the overall survival for patients who require ECLS.
MATERIALS AND METHODS
After approval by the institutional review ethics board, all patients who were referred for consideration of PEA at Toronto General Hospital from January 2008 to January 2015 were reviewed. Typical work-up for patients being considered for PEA includes history and physical examination, screening blood work (complete blood count, electrolytes and extended electrolytes, liver function tests, coagulation profile, brain natriuretic peptide, blood type and screen and sickle cell screen for patients deemed at risk), ventilation/perfusion (V/Q) scan, CT-pulmonary angiogram (CTPA), invasive pulmonary angiogram, transthoracic echocardiogram, left- (in patients 40 years and older) and right heart catheterization, pulmonary function tests, 6-min walk test, peripheral venous and femoral and carotid arterial Doppler ultrasounds. Patients with an established diagnosis of CTEPH based on CTPA and/or invasive pulmonary angiogram were selected for PEA regardless of the severity of the PVR. Patients with evidence of sub-segmental disease only on CTPA and invasive pulmonary angiogram were occasionally not considered surgical candidates due to distal disease. However, discrepancy between extent of disease and PVR (i.e. out-of-proportion PH) was not used as an exclusion criterion. After determining the reasons, patients who did not proceed to surgery were excluded from analysis. Patients underwent PEA according to the standard technique using deep hypothermic circulatory arrest as previously described [1]. The charts from all patients who underwent PEA were examined, and divided into two groups: those who did not need ECLS postoperatively and those who required ECLS postoperatively. Data were collected prospectively and retrospectively reviewed.
The type of ECLS was determined by the primary cause of failure either cardiac or pulmonary. VA ECMO was used for patients with haemodynamic compromise, whereas VV ECMO was used for patients with severe hypoxaemia in the absence of haemodynamic problems. Peripheral VA ECMO through the femoral vessels was considered for patients presenting with haemodynamic instability during the postoperative course, while central VA ECMO was selected for patients presenting with haemodynamic instability after weaning cardiopulmonary bypass (CPB). In general, these patients could not be weaned from CPB despite nitric oxide and optimal inotropic supports, and therefore the cannulation sites on the ascending aorta and the right atrium were preserved and connected to the ECLS circuit. Patients on central VA ECMO had their sternum left opened with the skin closed and were maintained on antibiotics until the sternum was closed. Careful haemostasis was performed before closing the skin and transferring the patient to the intensive care unit.
Demographic, treatment data and adverse events were reported as mean ± standard deviation or as median and range. Categorical variables were compared by χ2 analysis or Fisher's exact test and continuous variables by Student's t-test. Statview V (Abacus Concept, Berkeley, CA, USA) was used for all analyses. P-value <0.05 was considered significant.
RESULTS
Among a total of 174 patients with V/Q scan demonstrating mismatched perfusion defects referred to Toronto General Hospital for consideration of PEA between January 2008 and January 2015, 144 (83%) proceeded to surgery (Fig. 1). Thirty patients did not proceed to surgery due to distal disease (n = 9, 5%), comorbidities (n = 8, 5%), absence of disease on CTPA and invasive pulmonary angiogram (n = 5, 3%) and other reasons (n = 8, 5%).
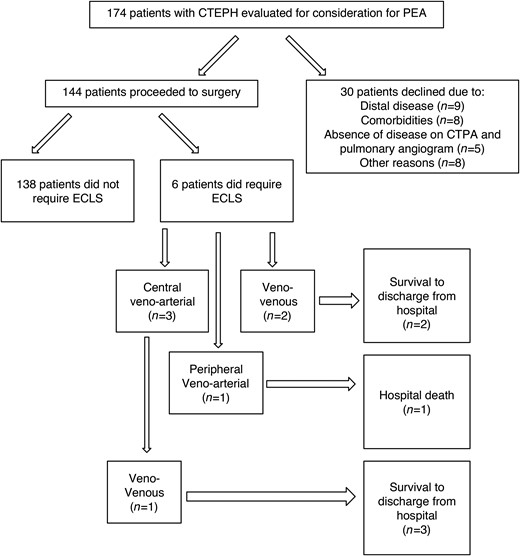
CONSORT diagram showing the flow of patients. CTEPH: chronic thromboembolic pulmonary hypertension; PEA: pulmonary endarterectomy; ECLS: extracorporeal life support.
Patients undergoing PEA had a median age of 59 years and were evenly distributed between males and females (Table 1). A total of 119 patients (83%) received no medical therapy for their PH prior to PEA. Twenty-five patients were on monotherapy (n = 21), dual therapy (n = 2) or tri-therapy (n = 2). Hospital admission for decompensated right heart failure was observed in 16 patients.
| Age (years) | 59 (18–85) |
| Gender (M/F) | 72/72 |
| History of hospital admission for decompensated right heart failure | 16 |
| 6-min walk distance (m) | 374 ± 134 |
| BNP (pg/ml) | 282 ± 378 |
| Preoperative PH therapy (no/yes) | 119/25 |
| Preoperative mean PA pressure (mmHg) | 44 ± 13 |
| Preoperative cardiac index (l/min/m2) | 2.1 ± 0.6 |
| Preoperative TPR (Dynes.s.cm−5) | 977 ± 481 |
| Preoperative TPR >1200 Dynes.s.cm−5 (n) | 39 |
| Age (years) | 59 (18–85) |
| Gender (M/F) | 72/72 |
| History of hospital admission for decompensated right heart failure | 16 |
| 6-min walk distance (m) | 374 ± 134 |
| BNP (pg/ml) | 282 ± 378 |
| Preoperative PH therapy (no/yes) | 119/25 |
| Preoperative mean PA pressure (mmHg) | 44 ± 13 |
| Preoperative cardiac index (l/min/m2) | 2.1 ± 0.6 |
| Preoperative TPR (Dynes.s.cm−5) | 977 ± 481 |
| Preoperative TPR >1200 Dynes.s.cm−5 (n) | 39 |
ECLS: extracorporeal life support; BNP: brain natriuretic peptide; PA: pulmonary arterial; TPR: total pulmonary vascular resistance.
| Age (years) | 59 (18–85) |
| Gender (M/F) | 72/72 |
| History of hospital admission for decompensated right heart failure | 16 |
| 6-min walk distance (m) | 374 ± 134 |
| BNP (pg/ml) | 282 ± 378 |
| Preoperative PH therapy (no/yes) | 119/25 |
| Preoperative mean PA pressure (mmHg) | 44 ± 13 |
| Preoperative cardiac index (l/min/m2) | 2.1 ± 0.6 |
| Preoperative TPR (Dynes.s.cm−5) | 977 ± 481 |
| Preoperative TPR >1200 Dynes.s.cm−5 (n) | 39 |
| Age (years) | 59 (18–85) |
| Gender (M/F) | 72/72 |
| History of hospital admission for decompensated right heart failure | 16 |
| 6-min walk distance (m) | 374 ± 134 |
| BNP (pg/ml) | 282 ± 378 |
| Preoperative PH therapy (no/yes) | 119/25 |
| Preoperative mean PA pressure (mmHg) | 44 ± 13 |
| Preoperative cardiac index (l/min/m2) | 2.1 ± 0.6 |
| Preoperative TPR (Dynes.s.cm−5) | 977 ± 481 |
| Preoperative TPR >1200 Dynes.s.cm−5 (n) | 39 |
ECLS: extracorporeal life support; BNP: brain natriuretic peptide; PA: pulmonary arterial; TPR: total pulmonary vascular resistance.
Of the 144 consecutive patients who underwent PEA in the study period, 6 patients (4%) required ECLS postoperatively (Table 2). Three patients were placed on central veno-arterial (cVA) ECLS, 1 patient was placed on pVA ECLS, and 2 patients were placed on VV ECLS (Fig. 1). Indications for ECLS included RV failure (n = 3), severe hypoxaemia (n = 2) and haemorrhagic pulmonary oedema (n = 1). Patients requiring cVA ECMO had severe residual pulmonary hypertension with mean PA pressure >40 mmHg immediately after coming off CPB despite nitric oxide. Patients requiring VV ECMO or pVA ECMO had mild residual pulmonary hypertension with the mean PA pressure of 35 mmHg or less. One patient who was placed initially on cVA ECLS was switched to VV after 5 days. Overall ECLS duration ranged between 3 and 39 (median 5) days. One patient on VV ECLS was extubated while on ECLS (Fig. 2). All patients who required ECLS post PEA experienced at least one complication during the duration of their hospital stay (Table 2).
| Patients . | Age (years) . | Preop TPR (Dynes.s.cm−5) . | ECLS mode . | Indication for ECLS . | Duration of ECLS (days) . | Extubation on ECLS . | Complications . | Outcome . | PH therapy . |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 64 | 1285 | Peripheral VA | RV failure, Pulmonary oedema | 39 | No | Renal failure, sepsis | death | - |
| 2 | 52 | 929 | VV (dual lumen) | Severe hypoxaemia | 5 | Yes | SVT | Alive 27 months | No |
| 3 | 57 | 1508 | Central VA, then VV (dual lumen) | RV failure, hypoxaemia | 5 (VA), 11 (VV) | No | Chest bleeding, subdural haematoma, VFib | Alive 12 months | Yes |
| 4 | 53 | 1675 | Central VA | Haemorrhagic pulmonary oedema | 3 | No | Delirium, pneumonia | Alive 11 months | No |
| 5 | 53 | 800 | VV (dual lumen) | Pneumonia | 6 | No | SVT | Alive 9 months | No |
| 6 | 45 | 2667 | Central VA | RV failure | 3 | No | Pneumonia | Alive 6 months | Yes |
| Patients . | Age (years) . | Preop TPR (Dynes.s.cm−5) . | ECLS mode . | Indication for ECLS . | Duration of ECLS (days) . | Extubation on ECLS . | Complications . | Outcome . | PH therapy . |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 64 | 1285 | Peripheral VA | RV failure, Pulmonary oedema | 39 | No | Renal failure, sepsis | death | - |
| 2 | 52 | 929 | VV (dual lumen) | Severe hypoxaemia | 5 | Yes | SVT | Alive 27 months | No |
| 3 | 57 | 1508 | Central VA, then VV (dual lumen) | RV failure, hypoxaemia | 5 (VA), 11 (VV) | No | Chest bleeding, subdural haematoma, VFib | Alive 12 months | Yes |
| 4 | 53 | 1675 | Central VA | Haemorrhagic pulmonary oedema | 3 | No | Delirium, pneumonia | Alive 11 months | No |
| 5 | 53 | 800 | VV (dual lumen) | Pneumonia | 6 | No | SVT | Alive 9 months | No |
| 6 | 45 | 2667 | Central VA | RV failure | 3 | No | Pneumonia | Alive 6 months | Yes |
ECLS: extracorporeal life support; VA: veno-arterial; VV: veno-venous; MV: mechanical ventilation; VFib: ventricular fibrillation; SVT: supraventricular tachycardia; RV failure: right ventricular failure.
| Patients . | Age (years) . | Preop TPR (Dynes.s.cm−5) . | ECLS mode . | Indication for ECLS . | Duration of ECLS (days) . | Extubation on ECLS . | Complications . | Outcome . | PH therapy . |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 64 | 1285 | Peripheral VA | RV failure, Pulmonary oedema | 39 | No | Renal failure, sepsis | death | - |
| 2 | 52 | 929 | VV (dual lumen) | Severe hypoxaemia | 5 | Yes | SVT | Alive 27 months | No |
| 3 | 57 | 1508 | Central VA, then VV (dual lumen) | RV failure, hypoxaemia | 5 (VA), 11 (VV) | No | Chest bleeding, subdural haematoma, VFib | Alive 12 months | Yes |
| 4 | 53 | 1675 | Central VA | Haemorrhagic pulmonary oedema | 3 | No | Delirium, pneumonia | Alive 11 months | No |
| 5 | 53 | 800 | VV (dual lumen) | Pneumonia | 6 | No | SVT | Alive 9 months | No |
| 6 | 45 | 2667 | Central VA | RV failure | 3 | No | Pneumonia | Alive 6 months | Yes |
| Patients . | Age (years) . | Preop TPR (Dynes.s.cm−5) . | ECLS mode . | Indication for ECLS . | Duration of ECLS (days) . | Extubation on ECLS . | Complications . | Outcome . | PH therapy . |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 64 | 1285 | Peripheral VA | RV failure, Pulmonary oedema | 39 | No | Renal failure, sepsis | death | - |
| 2 | 52 | 929 | VV (dual lumen) | Severe hypoxaemia | 5 | Yes | SVT | Alive 27 months | No |
| 3 | 57 | 1508 | Central VA, then VV (dual lumen) | RV failure, hypoxaemia | 5 (VA), 11 (VV) | No | Chest bleeding, subdural haematoma, VFib | Alive 12 months | Yes |
| 4 | 53 | 1675 | Central VA | Haemorrhagic pulmonary oedema | 3 | No | Delirium, pneumonia | Alive 11 months | No |
| 5 | 53 | 800 | VV (dual lumen) | Pneumonia | 6 | No | SVT | Alive 9 months | No |
| 6 | 45 | 2667 | Central VA | RV failure | 3 | No | Pneumonia | Alive 6 months | Yes |
ECLS: extracorporeal life support; VA: veno-arterial; VV: veno-venous; MV: mechanical ventilation; VFib: ventricular fibrillation; SVT: supraventricular tachycardia; RV failure: right ventricular failure.
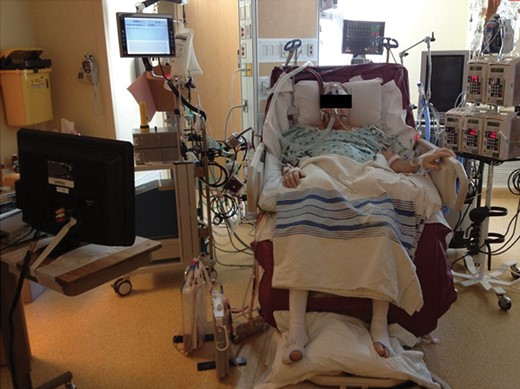
Patient requiring VV ECLS due to severe hypoxia after PEA. The use of a dual lumen VV ECLS allowed extubation and mobilization of the patient during recovery on ECLS. ECLS: extracorporeal life support; PEA: pulmonary endarterectomy; VV: veno-venous.
The age, 6-min walk distance and BNP level were not significantly different between patients who required and did not require ECLS (Table 3). In terms of preoperative physiological variables, patients requiring ECLS had higher preoperative total pulmonary vascular resistance (TPR) compared with non-ECLS patients and more frequently required hospital admission for decompensated right heart failure before surgery. All patients requiring VA ECLS had a preoperative TPR >1200 Dynes.s.cm−5.
| . | ECLS (N = 6) . | Non-ECLS (N = 138) . | P-value . |
|---|---|---|---|
| Age (years) | 54 ± 6 | 57 ± 14 | 0.6 |
| History of hospital admission for decompensated right heart failure (n) | 3 | 13 | 0.02 |
| 6-min walk distance (m) | 341 ± 193 | 375 ± 132 | 0.5 |
| BNP (pg/ml) | 391 ± 611 | 277 ± 367 | 0.5 |
| Mean PA pressure, preop (mmHg) | 52 ± 8 | 44 ± 13 | 0.14 |
| Cardiac Index, preop (l/min/m2) | 1.6 ± 0.4 | 2.1 ± 0.6 | 0.046 |
| TPR preop (Dynes.s.cm−5) | 1477 ± 671 | 954 ± 462 | 0.009 |
| TPR >1200 Dynes.s.cm−5 (n) | 4 | 35 | 0.046 |
| Length of ICU stay (days) | 25 ± 19 | 5 ± 7 | <0.0001 |
| Total hospital stay (days) | 48 ± 34 | 16 ± 13 | <0.0001 |
| . | ECLS (N = 6) . | Non-ECLS (N = 138) . | P-value . |
|---|---|---|---|
| Age (years) | 54 ± 6 | 57 ± 14 | 0.6 |
| History of hospital admission for decompensated right heart failure (n) | 3 | 13 | 0.02 |
| 6-min walk distance (m) | 341 ± 193 | 375 ± 132 | 0.5 |
| BNP (pg/ml) | 391 ± 611 | 277 ± 367 | 0.5 |
| Mean PA pressure, preop (mmHg) | 52 ± 8 | 44 ± 13 | 0.14 |
| Cardiac Index, preop (l/min/m2) | 1.6 ± 0.4 | 2.1 ± 0.6 | 0.046 |
| TPR preop (Dynes.s.cm−5) | 1477 ± 671 | 954 ± 462 | 0.009 |
| TPR >1200 Dynes.s.cm−5 (n) | 4 | 35 | 0.046 |
| Length of ICU stay (days) | 25 ± 19 | 5 ± 7 | <0.0001 |
| Total hospital stay (days) | 48 ± 34 | 16 ± 13 | <0.0001 |
ECLS: extracorporeal life support; BNP: brain natriuretic peptide; PA: pulmonary arterial; TPR: total pulmonary vascular resistance; ICU: intensive care unit.
| . | ECLS (N = 6) . | Non-ECLS (N = 138) . | P-value . |
|---|---|---|---|
| Age (years) | 54 ± 6 | 57 ± 14 | 0.6 |
| History of hospital admission for decompensated right heart failure (n) | 3 | 13 | 0.02 |
| 6-min walk distance (m) | 341 ± 193 | 375 ± 132 | 0.5 |
| BNP (pg/ml) | 391 ± 611 | 277 ± 367 | 0.5 |
| Mean PA pressure, preop (mmHg) | 52 ± 8 | 44 ± 13 | 0.14 |
| Cardiac Index, preop (l/min/m2) | 1.6 ± 0.4 | 2.1 ± 0.6 | 0.046 |
| TPR preop (Dynes.s.cm−5) | 1477 ± 671 | 954 ± 462 | 0.009 |
| TPR >1200 Dynes.s.cm−5 (n) | 4 | 35 | 0.046 |
| Length of ICU stay (days) | 25 ± 19 | 5 ± 7 | <0.0001 |
| Total hospital stay (days) | 48 ± 34 | 16 ± 13 | <0.0001 |
| . | ECLS (N = 6) . | Non-ECLS (N = 138) . | P-value . |
|---|---|---|---|
| Age (years) | 54 ± 6 | 57 ± 14 | 0.6 |
| History of hospital admission for decompensated right heart failure (n) | 3 | 13 | 0.02 |
| 6-min walk distance (m) | 341 ± 193 | 375 ± 132 | 0.5 |
| BNP (pg/ml) | 391 ± 611 | 277 ± 367 | 0.5 |
| Mean PA pressure, preop (mmHg) | 52 ± 8 | 44 ± 13 | 0.14 |
| Cardiac Index, preop (l/min/m2) | 1.6 ± 0.4 | 2.1 ± 0.6 | 0.046 |
| TPR preop (Dynes.s.cm−5) | 1477 ± 671 | 954 ± 462 | 0.009 |
| TPR >1200 Dynes.s.cm−5 (n) | 4 | 35 | 0.046 |
| Length of ICU stay (days) | 25 ± 19 | 5 ± 7 | <0.0001 |
| Total hospital stay (days) | 48 ± 34 | 16 ± 13 | <0.0001 |
ECLS: extracorporeal life support; BNP: brain natriuretic peptide; PA: pulmonary arterial; TPR: total pulmonary vascular resistance; ICU: intensive care unit.
The overall in-hospital mortality rate for all patients who underwent PEA was 2% (3/144). One patient on pVA ECLS died from sepsis after 39 days on ECLS and 2 non-ECLS patients died from pneumonia (n = 2) 29 and 39 days after PEA. The remaining 5 ECLS patients (83%) were discharged from hospital and are alive after a median follow-up of 11 (range 6–27) months. Two ECLS patients (40%) are on therapy for residual PH compared with 13 (10%) in the non-ECLS patients (P = 0.09).
DISCUSSION
Currently, PEA is the treatment of choice for patients with CTEPH [2]. The implications for the surgical CTEPH team are that patients present for surgery at varying stages of disease progression, including some patients who are severely symptomatic with serious haemodynamic derangements already present [1]. Well-known postoperative complications of PEA include persistent right heart failure, residual pulmonary hypertension and pulmonary reperfusion injury resulting in pulmonary oedema and pulmonary haemorrhage [3–5]. Also, massive pulmonary hypertension that develops after weaning of CPB can be secondary to a technical complication [6]. These complications can develop intraoperatively immediately after the endarterectomy is performed, manifested by inability to wean from CPB or severe hypoxia, or days later. ECLS can be a good option for patients experiencing these complications.
Over the last decade, a number of improvements have been made to many components of the ECLS circuits. Firstly, silicon membrane oxygenators have been replaced by hollow-fibre polymethyl pentene (PMP) oxygenators. The advantages of PMP oxygenators include smaller surface area, smaller priming volumes and less activation of blood components as a result of the heparin-coated nature of these membranes [7]. Clinically, this results in patients receiving fewer transfusions of blood products. PMP oxygenators provide superior gas exchange, and are built specifically for long-term use [8]. Secondly, important improvements have been made with the use of a centrifugal pump for the ECLS circuit instead of the older roller pump. Advantages of the centrifugal pump include smaller priming volume, ease of use and the ability to operate for weeks without technical failure [7]. Also, tubing coated with a biocompatible lining has been developed, which decreases systemic inflammatory response and risk of thrombosis [7]. Lastly, the development of a dual lumen catheter (Avalon Elite, Avalon Laboratories, Rancho Dominguez, CA, USA) for VV ECLS provides the advantage of requiring only one site of vascular access, thereby decreasing the potential vascular complications and potentially allowing extubation and more patient mobility [7].
For patients who develop severe refractory hypoxaemia, VV ECLS can be used to support oxygenation in patients with preserved right heart function [9]. The 3 patients included in this study who were placed on VV ECLS had refractory hypoxaemia secondary to pulmonary oedema, pneumonia or the pulmonary artery ‘steal’ phenomenon with associated ventilation/perfusion mismatch. The dual lumen Avalon cannula was used for all patients and allowed us to extubated 1 patient while waiting for resolution of the severe hypoxaemia. One theoretical drawback of VV ECLS compared with VA ECLS is the absence of unloading the pulmonary vascular bed. However, VV ECLS provides advantages on ease of use with less risk of complications compared with VA ECLS. In our PEA and lung transplant experience, VV ECMO did not appear to lead to delay resolution of the pulmonary oedema compared with VA ECMO despite the persistent volume load on the pulmonary vascular bed.
RV dysfunction after PEA can present immediately during attempt to wean from CPB or within 2–3 days postoperatively. The resultant cardiac dysfunction coupled with hypoxaemia is managed with either central or peripheral VA ECLS [9]. In the setting of pulmonary hypertension, RV hypertrophy initially develops to deal with the pressure overload. As the pulmonary hypertension progresses, the RV dilates leads to RV failure and eventually may impair left ventricular function [10]. Although peripheral VA ECLS may seem appealing after PEA as it allows chest closure, central VA ECLS presents advantages when implanted in the operating room after surgery due to the ease of insertion and the immediate decrease in afterload, allowing recovery of RV function [10]. Central VA ECLS was used in 3 patients who had difficulty weaning CPB in the operating room. ECLS was connected using the aortic and one of the venous sites from CPB. ECLS was then slowly weaned over a few days postoperatively before decannulating and closing the sternum. The technique of slow weaning with VA ECLS is increasingly being used in patients with pulmonary arterial hypertension undergoing bilateral lung transplantation and has shown that the RV dysfunction will often improve dramatically during the first few days after surgery [10].
This study demonstrates that ECLS is a good option for patients who develop complications both immediately after PEA and in the early postoperative period. As experience with PEA is increasing, a higher proportion of patients are proceeding to surgery, and surgery is increasingly being offered to patients with more severe haemodynamic derangements [1]. In our study, the patient who was on cVA initially and then VV ECLS had been declined for surgery 4 years before being reconsidered. Even though she had worse pulmonary hypertension and right heart failure, increased experience allowed her to undergo surgical treatment. As can be expected, operating on sicker patients predisposes to both higher rates and increased severity of complications. Having the ability to deal with more severe complications is imperative, and our study has shown that ECLS is a good option for support of these patients in the postoperative period with 83% of the patients being discharged from hospital and doing well after several months of follow-up.
Currently in the literature, there are little data on the use of ECLS after PEA. The largest study describes 20 patients who were placed on VV ECLS postoperatively, with a successful weaning rate of 40% and overall survival rate of 30%. The patients who survived after weaning of ECLS were more likely to have shorter lengths of pre-ECLS ventilator support, cannulation within 120 h of surgery and less severe preoperative pulmonary hypertension [5]. The second largest study reports on the use of cVA ECLS in 7 patients, with significantly higher pre- and postoperative PA pressures, postoperative PVR and preoperative central venous pressure. The mortality rate for patients on cVA ECLS was 43% [4]. There are also a number of case reports of successful ECMO for pulmonary haemorrhage, using both VV and peripheral VA cannulation [11–15].
Each of the studies mentioned above used one mode of ECLS for the management of complications postoperatively. Our series has shown that ECLS post-PEA should be individualized based on patient needs. We believe that VV ECLS is the treatment of choice for pulmonary reperfusion injury, manifested as pulmonary oedema with preserved right heart function, particularly if it occurs in the intensive care unit after PEA. For persistent pulmonary hypertension immediately after surgery and ongoing right heart failure, central VA ECLS was an excellent option in our experience as it provided both oxygenation and unloading of the heart and the pulmonary vasculature to allow recovery of the RV. The RV improved after 2 or 3 days allowing us to wean cVA ECLS completely in 2 patients and switch to VV ECLS in one. Peripheral VA ECMO was used in one patient in our series who developed RV dysfunction postoperatively but pVA could not be weaned and the patient eventually died from sepsis.
This study is limited by the small number of patients who were placed on ECLS in the postoperative period. Also, as a single-centre experience, generalizability of the results is limited.
In conclusion, ECLS is a successful management option for patients who experience complications post-PEA. Although the patients who require ECLS have more severe cardiac and respiratory disease preoperatively, the overall survival rate of 83% for these patients suggests that they can be successfully managed with surgery. The option of placing patients on ECLS after PEA may allow sicker patients to be offered this potentially curative treatment.
Conflict of interest: none declared.
REFERENCES
Author notes
Presented at the 23rd European Conference on General Thoracic Surgery, Lisbon, Portugal, 31 May-3 June 2015.
- extracorporeal membrane oxygenation
- thromboembolic pulmonary hypertension
- hypoxemia
- cerebrovascular accident
- pulmonary edema
- follow-up
- objective (goal)
- heart ventricle
- hospital mortality
- patient discharge
- polyvinyl alcohol
- preoperative care
- surgical procedures, operative
- surgery specialty
- pulmonary vascular resistance
- hospital admission
- pulmonary artery endarterectomy
- patient-ventilator asynchrony




