-
PDF
- Split View
-
Views
-
Cite
Cite
Yoshiyuki Tokuda, Hideki Oshima, Yuji Narita, Tomonobu Abe, Masato Mutsuga, Kazuro Fujimoto, Sachie Terazawa, Hideki Ito, Makoto Hibino, Wataru Uchida, Kimihiro Komori, Akihiko Usui, Extended total arch replacement via the L-incision approach: single-stage repair for extensive aneurysms of the aortic arch, Interactive CardioVascular and Thoracic Surgery, Volume 22, Issue 6, June 2016, Pages 750–755, https://doi.org/10.1093/icvts/ivw034
Close - Share Icon Share
Abstract
Total arch replacement via the L-incision approach (a combination of left anterior thoracotomy and upper median sternotomy) can be used to achieve more extensive replacement.
In the period between 2002 and 2014, 279 total arch replacement procedures were performed. After excluding cases of acute aortic dissection and cases involving concomitant, hybrid or frozen elephant trunk procedures, patients who underwent isolated total arch replacement via an L-incision (n = 29) and via median sternotomy (n = 143) were identified and the data pertaining to their cases were analysed.
Operative mortality was higher in the L-incision group than in the median sternotomy group (6.9 vs 2.1%); however, the difference was not statistically significant. The L-incision group displayed a higher rate of respiratory complications, including pneumonia (28 vs 7.0%, P = 0.0034), the need for tracheostomy (17 vs 2.1%, P = 0.0038) and pulmonary haemorrhage (6.9 vs 0%, P = 0.028). The rate of paraplegia was similar between the groups (0 vs 1.4%, P = 1.00), despite the wider range replaced via the L-incision approach (7.3 ± 1.5 vs 4.7 ± 0.8 anatomical zones, P < 0.001). The rates of other complications and functional recovery were similar. The long-term survival (73 vs 84% at 5 years) and aortic event-free rates (94 vs 96% at 5 years) were similar in both groups.
A combination of left anterior thoracotomy and upper median sternotomy can be applied to the single-stage repair of extended aneurysms with acceptable results in appropriately selected patients.
INTRODUCTION
The management of patients with aortic arch aneurysms is a technical challenge and it remains an area of ongoing development. Owing to the refinements in operative techniques with the use of brain protection such as antegrade cerebral perfusion (ACP), total arch replacement can now be achieved with acceptable surgical outcomes [1–3]. However, in patients who have extensive aortic arch pathology involving the distal arch and the more distal descending aorta, distal anastomosis in deep positions via median sternotomy can be troublesome to perform. Thus, the use of the treatment strategy remains controversial in cases of extended arch aneurysms. A two-stage operation is one treatment option for extensive aortic diseases. Such patients can be treated by total aortic arch replacement using the elephant trunk technique via a median sternotomy followed by the graft replacement of the descending aorta via a left thoracotomy or thoracic endovascular aortic repair (TEVAR) [4]. However, it has been reported that some patients never reach the second-stage operation because they die either shortly after the first-stage operation or from an aneurysm rupture while waiting for the second-stage repair [5].
Alternatively, the L-incision approach (a combination of left anterior thoracotomy and upper median sternotomy) can be used as a single-stage approach in extended total arch replacement operations to achieve better exposure for the distal anastomosis [6–8]. We have applied this approach to the single-stage repair of aortic arch aneurysms extending to the descending aorta. Naturally, the L-incision approach is associated with some possible disadvantages in comparison with median sternotomy, due to its extended incision. The purpose of the present study is to assess the clinical outcomes associated with extended total arch replacement via the L-incision approach. In particular, we compare the outcome of arch repair via the L-incision approach to the outcome of ordinary total arch replacement via median sternotomy to clarify its negative impact.
PATIENTS AND METHODS
During the period from January 2002 to December 2014, 792 open operative repairs of the thoracic aorta (isolated TEVAR procedures excluded) were performed in Nagoya University Hospital. Out of 792 operations, 450 operations involved the aortic arch, of which 279 were total arch replacement procedures. To satisfy the definition of total arch replacement, the aortic arch area between the brachiocephalic artery (BCA) and the left subclavian artery (LSCA) should be replaced and the arch vessels should be reconstructed. So-called proximal hemiarch replacement (e.g. replacement of the lesser curvature of the aortic arch and the ascending aorta) and sub-total distal arch replacement were not regarded as total arch replacement. Cases requiring an urgent repair of an acute aortic dissection (n = 27), cases with concomitant cardiac procedures other than coronary artery bypass grafting (CABG) (n = 50) and cases that involved the concomitant use of the frozen elephant trunk technique (open stent procedures) (n = 26) were excluded from the study. Cases in which total arch replacement was performed as a part of Type III hybrid arch repair (n = 15) were also excluded, as were cases in which total arch replacement was performed via approaches other than median sternotomy or an L-incision (n = 2).
After applying the exclusion criteria, 172 patients were included in the present study: 29 patients who underwent isolated total arch replacement via the L-incision approach (L-incision group) and 143 patients who underwent isolated total arch replacement via median sternotomy (median sternotomy group). Total arch replacement via an L-incision was indicated if the site of the distal anastomosis was expected to be too deep to access via median sternotomy. In such cases, the site of the distal anastomosis is far beyond the reflection point of the aortic arch. The site of the distal anastomosis could be at the level of the pulmonary hilum or even below the level of the hilum. Total arch replacement with concomitant CABG was classified as an isolated total arch replacement, as described above. The records of these patients were retrospectively reviewed and both patient groups were compared. The present retrospective review study was approved by the Institutional Review Board of Nagoya University Graduate School of Medicine (IRB 655-2); the need for individual consent was waived. The indication of aortic repair was based on the standard guidelines and was at the discretion of the multidisciplinary team.
The baseline characteristics of the patients of each group are given in Table 1. Overall, both groups were similar with respect to the individual preoperative risk factors; however, the median sternotomy group displayed higher EuroSCORE II values.
| . | L-incision (n = 29) . | Median sternotomy (n = 143) . | P-value . |
|---|---|---|---|
| Age (years) | 67 ± 9 | 68 ± 10 | 0.55 |
| Male gender | 19 (66%) | 104 (73%) | 0.50 |
| Chronic lung disease (moderate to severe) | 0 (0%) | 4 (2.8%) | 1.00 |
| Renal dysfunction | 3 (10%) | 13 (9%) | 0.73 |
| History of CVA | 4 (14%) | 10 (7.0%) | 0.26 |
| PAD | 8 (28%) | 49 (34%) | 0.53 |
| History or presence of AAA | 5 (17%) | 33 (23%) | 0.63 |
| Diabetes mellitus treatment | 4 (14%) | 23 (16%) | 1.00 |
| LV EF (%) | 65 ± 11 | 66 ± 9 | 0.66 |
| Previous cardiac surgery | 3 (10%) | 35 (25%) | 0.14 |
| Aneurysm type; chronic dissection | 5 (17%) | 26 (18%) | 1.00 |
| Logistic EuroSCORE | 19 ± 11 | 22 ± 17 | 0.20 |
| EuroSCORE II | 3.8 ± 2.2 | 5.8 ± 6.5 | 0.0031 |
| . | L-incision (n = 29) . | Median sternotomy (n = 143) . | P-value . |
|---|---|---|---|
| Age (years) | 67 ± 9 | 68 ± 10 | 0.55 |
| Male gender | 19 (66%) | 104 (73%) | 0.50 |
| Chronic lung disease (moderate to severe) | 0 (0%) | 4 (2.8%) | 1.00 |
| Renal dysfunction | 3 (10%) | 13 (9%) | 0.73 |
| History of CVA | 4 (14%) | 10 (7.0%) | 0.26 |
| PAD | 8 (28%) | 49 (34%) | 0.53 |
| History or presence of AAA | 5 (17%) | 33 (23%) | 0.63 |
| Diabetes mellitus treatment | 4 (14%) | 23 (16%) | 1.00 |
| LV EF (%) | 65 ± 11 | 66 ± 9 | 0.66 |
| Previous cardiac surgery | 3 (10%) | 35 (25%) | 0.14 |
| Aneurysm type; chronic dissection | 5 (17%) | 26 (18%) | 1.00 |
| Logistic EuroSCORE | 19 ± 11 | 22 ± 17 | 0.20 |
| EuroSCORE II | 3.8 ± 2.2 | 5.8 ± 6.5 | 0.0031 |
AAA: abdominal aortic aneurysm; CVA: cerebrovascular accident; EF: ejection fraction; LV: left ventricle; PAD: peripheral arterial disease.
| . | L-incision (n = 29) . | Median sternotomy (n = 143) . | P-value . |
|---|---|---|---|
| Age (years) | 67 ± 9 | 68 ± 10 | 0.55 |
| Male gender | 19 (66%) | 104 (73%) | 0.50 |
| Chronic lung disease (moderate to severe) | 0 (0%) | 4 (2.8%) | 1.00 |
| Renal dysfunction | 3 (10%) | 13 (9%) | 0.73 |
| History of CVA | 4 (14%) | 10 (7.0%) | 0.26 |
| PAD | 8 (28%) | 49 (34%) | 0.53 |
| History or presence of AAA | 5 (17%) | 33 (23%) | 0.63 |
| Diabetes mellitus treatment | 4 (14%) | 23 (16%) | 1.00 |
| LV EF (%) | 65 ± 11 | 66 ± 9 | 0.66 |
| Previous cardiac surgery | 3 (10%) | 35 (25%) | 0.14 |
| Aneurysm type; chronic dissection | 5 (17%) | 26 (18%) | 1.00 |
| Logistic EuroSCORE | 19 ± 11 | 22 ± 17 | 0.20 |
| EuroSCORE II | 3.8 ± 2.2 | 5.8 ± 6.5 | 0.0031 |
| . | L-incision (n = 29) . | Median sternotomy (n = 143) . | P-value . |
|---|---|---|---|
| Age (years) | 67 ± 9 | 68 ± 10 | 0.55 |
| Male gender | 19 (66%) | 104 (73%) | 0.50 |
| Chronic lung disease (moderate to severe) | 0 (0%) | 4 (2.8%) | 1.00 |
| Renal dysfunction | 3 (10%) | 13 (9%) | 0.73 |
| History of CVA | 4 (14%) | 10 (7.0%) | 0.26 |
| PAD | 8 (28%) | 49 (34%) | 0.53 |
| History or presence of AAA | 5 (17%) | 33 (23%) | 0.63 |
| Diabetes mellitus treatment | 4 (14%) | 23 (16%) | 1.00 |
| LV EF (%) | 65 ± 11 | 66 ± 9 | 0.66 |
| Previous cardiac surgery | 3 (10%) | 35 (25%) | 0.14 |
| Aneurysm type; chronic dissection | 5 (17%) | 26 (18%) | 1.00 |
| Logistic EuroSCORE | 19 ± 11 | 22 ± 17 | 0.20 |
| EuroSCORE II | 3.8 ± 2.2 | 5.8 ± 6.5 | 0.0031 |
AAA: abdominal aortic aneurysm; CVA: cerebrovascular accident; EF: ejection fraction; LV: left ventricle; PAD: peripheral arterial disease.
Operative techniques
Our L-incision approach technique is similar to that described in previous reports (Fig. 1) [6–8]. The patient is maintained in the supine position with the left chest elevated slightly (by 10–30°). A double-lumen endotracheal tube is used to permit the deflation of the left lung. First, anterior thoracotomy is performed between the fourth and sixth intercostal spaces (predominantly the fifth intercostal space). The lateral border of the thoracotomy is usually extended to the anterior axillary line. The medial border of the thoracotomy is extended to the sternum. After the division of the left internal thoracic artery, an upper median hemisternotomy is performed by making an L-shaped incision. The right blade of a Dubost sternal retractor is applied along the edge of the right sternum, and the left blade was applied inside the left thoracic cavity. In addition to the placement of the sternal retractor in the left thoracic cavity, the clasp of a Kent retractor is hooked onto the edge of the divided left side of the sternum, and the left anterior chest wall is lifted upwards using a stainless steel wire and an elevating handle (door open technique).
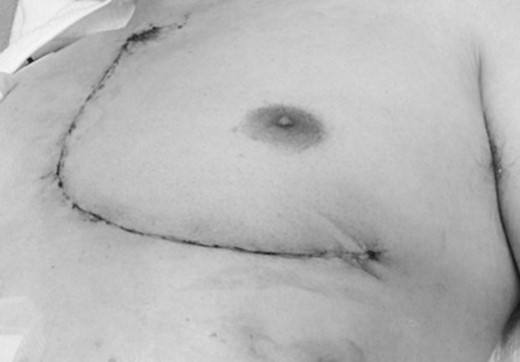
An L-incision made by a combination of left anterior thoracotomy and upper median sternotomy.
In most cases, there is adhesion between the aneurysm and the left lung, thus dissection between the two is required. After the gentle retraction of the left lung, the aortic arch and descending aorta, as well as the ascending aorta and aortic root are exposed. The operative steps of arch replacement are similar in both approaches and have been well documented [3, 9–11]. Currently, our operative technique for performing total arch replacement is as follows: After the pharyngeal temperature decreases to 23°C, with a rectal temperature of less than 28°C, the aortic arch is opened. Balloon-tipped ACP cannulas are inserted into the BCA, the left common carotid artery (LCCA) and the LSCA. The ACP flow was maintained at 15 ml/kg/min using an independent roller pump, and the pressure was maintained between 30 and 40 mmHg. Open distal anastomosis is performed during hypothermic cardiac arrest of the lower body. The stepwise distal anastomosis technique is frequently used to obtain an easy and secure anastomosis [12], especially in operations performed via median sternotomy. In such cases with stepwise distal anastomosis, an inverted tube graft is inserted into the descending aorta; the proximal end is anastomosed to the descending aorta and the distal end of the inserted graft is extracted proximally. The branched aortic arch graft is then anastomosed to the extracted interposed graft. To minimize the risk of spinal cord ischaemia, lower body perfusion was resumed after the completion of distal anastomoses. The proximal aorta and arch vessels are then individually reconstructed using a branched graft. Alternatively, in the earlier period of the study, rather than using ACP, we used the arch-first technique (reconstruction of the arch vessels first, followed by distal anastomosis), which was performed under hypothermic circulatory arrest with retrograde cerebral perfusion. The details of the arch-first technique are described in our previous report [3]. None of the patients received cerebrospinal fluid drainage.
Statistical analysis and definitions
Our definitions of postoperative outcomes, including various postoperative complications were based on the Japan Adult Cardiovascular Surgery Database (JACVSD) protocols [12], which are almost identical to those used in the Society of Thoracic Surgeons (STS) National Database (available online at http://sts.org). To describe the replacement ranges, JACVSD and the Japanese Committee for Stent graft Management use an aortic zone map, which is a modification of the landing zone definitions for TEVAR procedures [13]. Based on this, we divided the aortic arch into four anatomical zones (Z0–Z3) [Z0 (ascending aorta), Z1 (BCA to LCCA), Z2 (LCCA to LSCA) and Z3 (the aortic arch distal to LSCA)] and the following descending aorta into nine anatomical zones (T4–T12, expressed by the level of the corresponding thoracic vertebrae). To describe the width of the replacement ranges, the number of the replacement zones was counted (e.g. Z0–T4 replacement was counted as five zones of replacement). In cases where the zone was even partially replaced, the zone was regarded as having been replaced. In order to evaluate the functional recovery of activities of daily life, the modified Rankin scale [14] was applied on admission to the hospital before the patient underwent surgery and 1 month after surgery. According to the JACVSD criteria, major morbidities include any of the following postoperative complications: stroke, re-exploration, required prolonged postoperative mechanical ventilation (>24 h), renal failure or deep sternal infection. However, because prolonged mechanical ventilation (>24 h) is a very common requirement after aortic operations, we modified this complication with required tracheostomy (modified JACVSD criteria).
Statistical analyses were performed based on our previous report [15] using the JMP version 10 software program (SAS Institute, Inc., Cary, NC, USA). Continuous variables are expressed as the mean ± standard deviation and were compared using Student's t-test or Welch's t-test. Category variables were compared using Fisher's exact test. The survival and aortic event-free rates were estimated using the Kaplan–Meier method, and differences between the groups were determined using a log-rank analysis. Aortic events were defined as aortic dissection, rupture or unplanned reintervention for any aortic pathology. Planned operations for distal aortic lesions were not regarded as aortic events. P-values of <0.05 were considered to be statistically significant.
RESULTS
The intraoperative variables are listed in Table 2. The operation time was longer in the L-incision group (551 ± 29 vs 481 ± 13 min, P = 0.002) and the number of zones of replacement was higher (7.3 ± 1.5 zones vs 4.7 ± 0.8 zones, P < 0.0001), confirming that more lesions were replaced by the L-incision approach. In the L-incision group, distal anastomosis was performed at the level of seventh thoracic vertebra or lower in 31% of the patients (usually the aortic valve is at approximately the same level as the eighth or seventh thoracic vertebra) in comparison with 7.0% of the patients in the median sternotomy group. In these exceptional cases, the lower descending aorta was approached in median sternotomy by entering the left thoracic cavity with the opening of the left pleura.
| . | L-incision (n = 29) . | Median sternotomy (n = 143) . | P-value . |
|---|---|---|---|
| Concomitant CABG | 2 (6.9%) | 22 (15%) | 0.37 |
| Operation time (min) | 551 ± 141 | 481 ± 159 | 0.002 |
| Perfusion time (min) | 241 ± 67 | 248 ± 71 | 0.63 |
| Cardiac arrest time (min) | 119 ± 46 | 139 ± 39 | 0.035 |
| Use of antegrade cerebral perfusion | 18 (62%) | 91 (63%) | 1.00 |
| Number of zones of replacement | 7.3 ± 1.5 | 4.7 ± 0.8 | <0.0001 |
| Distal anastomosis at the level of seventh thoracic vertebra or lower | 9 (31%) | 10 (7.0%) | 0.001 |
| . | L-incision (n = 29) . | Median sternotomy (n = 143) . | P-value . |
|---|---|---|---|
| Concomitant CABG | 2 (6.9%) | 22 (15%) | 0.37 |
| Operation time (min) | 551 ± 141 | 481 ± 159 | 0.002 |
| Perfusion time (min) | 241 ± 67 | 248 ± 71 | 0.63 |
| Cardiac arrest time (min) | 119 ± 46 | 139 ± 39 | 0.035 |
| Use of antegrade cerebral perfusion | 18 (62%) | 91 (63%) | 1.00 |
| Number of zones of replacement | 7.3 ± 1.5 | 4.7 ± 0.8 | <0.0001 |
| Distal anastomosis at the level of seventh thoracic vertebra or lower | 9 (31%) | 10 (7.0%) | 0.001 |
For the zones of replacement, see the main text.
CABG: coronary artery bypass grafting.
| . | L-incision (n = 29) . | Median sternotomy (n = 143) . | P-value . |
|---|---|---|---|
| Concomitant CABG | 2 (6.9%) | 22 (15%) | 0.37 |
| Operation time (min) | 551 ± 141 | 481 ± 159 | 0.002 |
| Perfusion time (min) | 241 ± 67 | 248 ± 71 | 0.63 |
| Cardiac arrest time (min) | 119 ± 46 | 139 ± 39 | 0.035 |
| Use of antegrade cerebral perfusion | 18 (62%) | 91 (63%) | 1.00 |
| Number of zones of replacement | 7.3 ± 1.5 | 4.7 ± 0.8 | <0.0001 |
| Distal anastomosis at the level of seventh thoracic vertebra or lower | 9 (31%) | 10 (7.0%) | 0.001 |
| . | L-incision (n = 29) . | Median sternotomy (n = 143) . | P-value . |
|---|---|---|---|
| Concomitant CABG | 2 (6.9%) | 22 (15%) | 0.37 |
| Operation time (min) | 551 ± 141 | 481 ± 159 | 0.002 |
| Perfusion time (min) | 241 ± 67 | 248 ± 71 | 0.63 |
| Cardiac arrest time (min) | 119 ± 46 | 139 ± 39 | 0.035 |
| Use of antegrade cerebral perfusion | 18 (62%) | 91 (63%) | 1.00 |
| Number of zones of replacement | 7.3 ± 1.5 | 4.7 ± 0.8 | <0.0001 |
| Distal anastomosis at the level of seventh thoracic vertebra or lower | 9 (31%) | 10 (7.0%) | 0.001 |
For the zones of replacement, see the main text.
CABG: coronary artery bypass grafting.
The postoperative morbidities are listed in Table 3. Operative mortality was higher in the L-incision group (6.9 vs 2.1%), but not to a statistically significant extent. The L-incision group displayed a higher rate of respiratory complications, including prolonged ventilation (45 vs 21%, P = 0.010), pneumonia (28 vs 7.0%, P = 0.0034), tracheostomy requirement (17 vs 2.1%, P = 0.0038) and pulmonary haemorrhage (6.9 vs 0%, P = 0.028). The paraplegia rate was similar in both groups (0 vs 1.4%, P = 1.00), despite the wider ranges that were replaced via the L-incision approach. The stroke rate seemed to be higher in the L-incision group, although the difference was not statistically significant (24 vs 13%, P = 0.14). Moreover, the L-incision approach was associated with a longer intensive care unit stay. However, based on the modified JACVSD criteria, if prolonged ventilation (>24 h) was not regarded as a major morbidity, the composite major morbidity rate was similar in both groups. Overall, despite the increased rate of respiratory complications in the L-incision group, the postoperative functional recovery rates (according to the modified Rankin score measurements) of the patients in the two groups were similar.
| . | L-incision (n = 29) . | Median sternotomy (n = 143) . | . |
|---|---|---|---|
| Operative mortality | 2 (6.9%) | 3 (2.1%) | 0.20 |
| Mortality or major morbidity, modified JACVSD criteria | 10 (34%) | 36 (25%) | 0.35 |
| Re-exploration | 3 (10%) | 17 (12%) | 1.00 |
| Renal failure | 4 (14%) | 11 (7.7%) | 0.29 |
| Severe LOS; IABP or delayed sternal/thoracotomy closure | 3 (10%) | 5 (3.5%) | 0.13 |
| Deep sternal wound infection | 1 (3.4%) | 1 (0.7%) | 0.31 |
| Pulmonary haemorrhage | 2 (6.9%) | 0 (0%) | 0.028 |
| Prolonged ventilation (>24 h) | 13 (45%) | 30 (21%) | 0.010 |
| Postoperative ventilation time (h) | 184 ± 204 | 117 ± 344 | 0.16 |
| Tracheostomy | 5 (17%) | 3 (2.1%) | 0.0038 |
| Pneumonia | 8 (28%) | 10 (7.0%) | 0.0034 |
| Stroke | 7 (24%) | 18 (13%) | 0.14 |
| Paraplegia | 0 (0%) | 2 (1.4%) | 1.00 |
| SCI including transient paraparesis | 0 (0%) | 4 (2.8%) | 1.00 |
| Transfusion | 28 (97%) | 129 (90%) | 0.47 |
| Length of ICU stay (days) | 9.3 ± 7.3 | 4.4 ± 4.2 | 0.0012 |
| Compromised ADLs | 8 (28%) | 21 (15%) | 0.11 |
| Modified Rankin scale increase in ≥2 | 12 (41%) | 47 (33%) | 0.40 |
| Postoperative hospital stay (days) | 47 ± 32 | 39 ± 45 | 0.27 |
| . | L-incision (n = 29) . | Median sternotomy (n = 143) . | . |
|---|---|---|---|
| Operative mortality | 2 (6.9%) | 3 (2.1%) | 0.20 |
| Mortality or major morbidity, modified JACVSD criteria | 10 (34%) | 36 (25%) | 0.35 |
| Re-exploration | 3 (10%) | 17 (12%) | 1.00 |
| Renal failure | 4 (14%) | 11 (7.7%) | 0.29 |
| Severe LOS; IABP or delayed sternal/thoracotomy closure | 3 (10%) | 5 (3.5%) | 0.13 |
| Deep sternal wound infection | 1 (3.4%) | 1 (0.7%) | 0.31 |
| Pulmonary haemorrhage | 2 (6.9%) | 0 (0%) | 0.028 |
| Prolonged ventilation (>24 h) | 13 (45%) | 30 (21%) | 0.010 |
| Postoperative ventilation time (h) | 184 ± 204 | 117 ± 344 | 0.16 |
| Tracheostomy | 5 (17%) | 3 (2.1%) | 0.0038 |
| Pneumonia | 8 (28%) | 10 (7.0%) | 0.0034 |
| Stroke | 7 (24%) | 18 (13%) | 0.14 |
| Paraplegia | 0 (0%) | 2 (1.4%) | 1.00 |
| SCI including transient paraparesis | 0 (0%) | 4 (2.8%) | 1.00 |
| Transfusion | 28 (97%) | 129 (90%) | 0.47 |
| Length of ICU stay (days) | 9.3 ± 7.3 | 4.4 ± 4.2 | 0.0012 |
| Compromised ADLs | 8 (28%) | 21 (15%) | 0.11 |
| Modified Rankin scale increase in ≥2 | 12 (41%) | 47 (33%) | 0.40 |
| Postoperative hospital stay (days) | 47 ± 32 | 39 ± 45 | 0.27 |
Compromised ADLs indicates a modified Rankin scale score of ≥4 (unable to walk without assistance) at the time of discharge. A ≥2-point increase in the modified Rankin scale score generally indicates the occurrence of a significant disabling event. Major morbidities according to the modified JACVSD criteria include stroke, re-exploration, the need for tracheostomy, renal failure or deep sternal infection.
ADLs: activities of daily living; IABP: intra-aortic balloon pumping; ICU: intensive care unit; LOS: low output syndrome; SCI: spinal cord ischaemia; JACVSD: Japan Adult Cardiovascular Surgery Database.
| . | L-incision (n = 29) . | Median sternotomy (n = 143) . | . |
|---|---|---|---|
| Operative mortality | 2 (6.9%) | 3 (2.1%) | 0.20 |
| Mortality or major morbidity, modified JACVSD criteria | 10 (34%) | 36 (25%) | 0.35 |
| Re-exploration | 3 (10%) | 17 (12%) | 1.00 |
| Renal failure | 4 (14%) | 11 (7.7%) | 0.29 |
| Severe LOS; IABP or delayed sternal/thoracotomy closure | 3 (10%) | 5 (3.5%) | 0.13 |
| Deep sternal wound infection | 1 (3.4%) | 1 (0.7%) | 0.31 |
| Pulmonary haemorrhage | 2 (6.9%) | 0 (0%) | 0.028 |
| Prolonged ventilation (>24 h) | 13 (45%) | 30 (21%) | 0.010 |
| Postoperative ventilation time (h) | 184 ± 204 | 117 ± 344 | 0.16 |
| Tracheostomy | 5 (17%) | 3 (2.1%) | 0.0038 |
| Pneumonia | 8 (28%) | 10 (7.0%) | 0.0034 |
| Stroke | 7 (24%) | 18 (13%) | 0.14 |
| Paraplegia | 0 (0%) | 2 (1.4%) | 1.00 |
| SCI including transient paraparesis | 0 (0%) | 4 (2.8%) | 1.00 |
| Transfusion | 28 (97%) | 129 (90%) | 0.47 |
| Length of ICU stay (days) | 9.3 ± 7.3 | 4.4 ± 4.2 | 0.0012 |
| Compromised ADLs | 8 (28%) | 21 (15%) | 0.11 |
| Modified Rankin scale increase in ≥2 | 12 (41%) | 47 (33%) | 0.40 |
| Postoperative hospital stay (days) | 47 ± 32 | 39 ± 45 | 0.27 |
| . | L-incision (n = 29) . | Median sternotomy (n = 143) . | . |
|---|---|---|---|
| Operative mortality | 2 (6.9%) | 3 (2.1%) | 0.20 |
| Mortality or major morbidity, modified JACVSD criteria | 10 (34%) | 36 (25%) | 0.35 |
| Re-exploration | 3 (10%) | 17 (12%) | 1.00 |
| Renal failure | 4 (14%) | 11 (7.7%) | 0.29 |
| Severe LOS; IABP or delayed sternal/thoracotomy closure | 3 (10%) | 5 (3.5%) | 0.13 |
| Deep sternal wound infection | 1 (3.4%) | 1 (0.7%) | 0.31 |
| Pulmonary haemorrhage | 2 (6.9%) | 0 (0%) | 0.028 |
| Prolonged ventilation (>24 h) | 13 (45%) | 30 (21%) | 0.010 |
| Postoperative ventilation time (h) | 184 ± 204 | 117 ± 344 | 0.16 |
| Tracheostomy | 5 (17%) | 3 (2.1%) | 0.0038 |
| Pneumonia | 8 (28%) | 10 (7.0%) | 0.0034 |
| Stroke | 7 (24%) | 18 (13%) | 0.14 |
| Paraplegia | 0 (0%) | 2 (1.4%) | 1.00 |
| SCI including transient paraparesis | 0 (0%) | 4 (2.8%) | 1.00 |
| Transfusion | 28 (97%) | 129 (90%) | 0.47 |
| Length of ICU stay (days) | 9.3 ± 7.3 | 4.4 ± 4.2 | 0.0012 |
| Compromised ADLs | 8 (28%) | 21 (15%) | 0.11 |
| Modified Rankin scale increase in ≥2 | 12 (41%) | 47 (33%) | 0.40 |
| Postoperative hospital stay (days) | 47 ± 32 | 39 ± 45 | 0.27 |
Compromised ADLs indicates a modified Rankin scale score of ≥4 (unable to walk without assistance) at the time of discharge. A ≥2-point increase in the modified Rankin scale score generally indicates the occurrence of a significant disabling event. Major morbidities according to the modified JACVSD criteria include stroke, re-exploration, the need for tracheostomy, renal failure or deep sternal infection.
ADLs: activities of daily living; IABP: intra-aortic balloon pumping; ICU: intensive care unit; LOS: low output syndrome; SCI: spinal cord ischaemia; JACVSD: Japan Adult Cardiovascular Surgery Database.
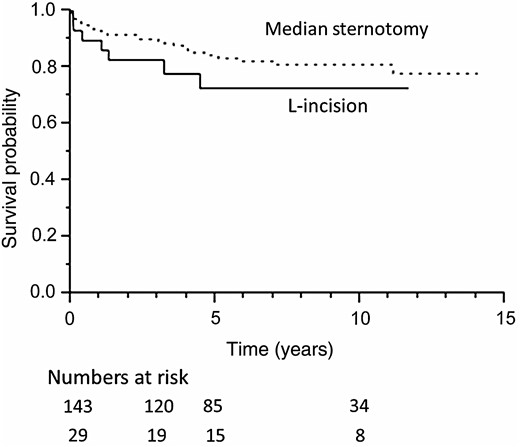
Figure 2 shows the Kaplan–Meier curves for survival in both patient groups. The survival rates in the L-incision and median sternotomy groups were 83 and 90% at 3 years, 73 and 84% at 5 years and 73 and 81% at 10 years (P = 0.25), respectively; the differences were not statistically significant. There were 32 deaths during follow-up. In addition to five postoperative deaths, there were two aorta-related deaths. Both cases involved rupture of an abdominal aortic aneurysm in the median sternotomy group. Other causes of death were malignancy (n = 8), pneumonia and/or respiratory failure (n = 7), cerebrovascular accident (n = 4) and so on.
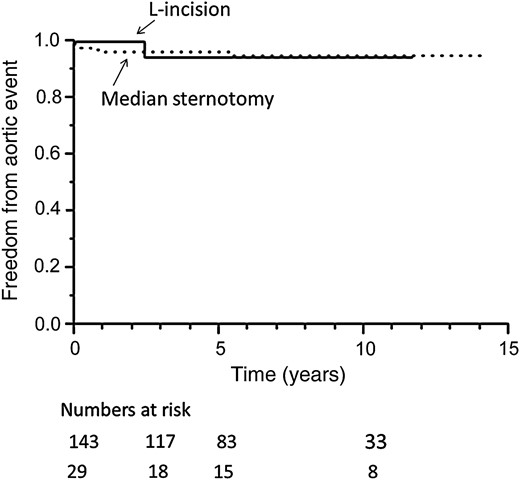
Freedom from aortic events is shown in Fig. 3 and the details of aortic events are described in Table 4. The rates of freedom from aortic events in the L-incision and median sternotomy groups were also similar: 94 and 96% at both 3 and 5 years and 95 and 94% at 10 years, respectively (P = 0.95). Therefore, the long-term results of total arch replacement via the L-incision were equivalent to those of standard total arch replacement via median sternotomy.
| . | L-incision . | Median sternotomy . |
|---|---|---|
| Unplanned aortic intervention (rupture or impending rupture) |
| 3 Repair of the descending aorta via thoracotomy Continuous bleeding from the distal anastomosis site following total arch replacement 1 Rupture of the residual false lumen (early postoperative period) 1 Impending rupture of the descending aorta 1 |
| 1 Repair of the thoracoabdominal aorta Impending rupture of the residual false lumen | ||
| Aorta-related mortality | 2 Rupture of abdominal aortic aneurysm |
| . | L-incision . | Median sternotomy . |
|---|---|---|
| Unplanned aortic intervention (rupture or impending rupture) |
| 3 Repair of the descending aorta via thoracotomy Continuous bleeding from the distal anastomosis site following total arch replacement 1 Rupture of the residual false lumen (early postoperative period) 1 Impending rupture of the descending aorta 1 |
| 1 Repair of the thoracoabdominal aorta Impending rupture of the residual false lumen | ||
| Aorta-related mortality | 2 Rupture of abdominal aortic aneurysm |
TEVAR: thoracic endovascular aortic repair.
| . | L-incision . | Median sternotomy . |
|---|---|---|
| Unplanned aortic intervention (rupture or impending rupture) |
| 3 Repair of the descending aorta via thoracotomy Continuous bleeding from the distal anastomosis site following total arch replacement 1 Rupture of the residual false lumen (early postoperative period) 1 Impending rupture of the descending aorta 1 |
| 1 Repair of the thoracoabdominal aorta Impending rupture of the residual false lumen | ||
| Aorta-related mortality | 2 Rupture of abdominal aortic aneurysm |
| . | L-incision . | Median sternotomy . |
|---|---|---|
| Unplanned aortic intervention (rupture or impending rupture) |
| 3 Repair of the descending aorta via thoracotomy Continuous bleeding from the distal anastomosis site following total arch replacement 1 Rupture of the residual false lumen (early postoperative period) 1 Impending rupture of the descending aorta 1 |
| 1 Repair of the thoracoabdominal aorta Impending rupture of the residual false lumen | ||
| Aorta-related mortality | 2 Rupture of abdominal aortic aneurysm |
TEVAR: thoracic endovascular aortic repair.
DISCUSSION
The performance of a distal anastomosis in a deep position via median sternotomy is a technical challenge in total arch replacement operations, even when using the stepwise anastomosis technique. Approaching the aorta beyond the reflection point of the aortic arch via median sternotomy is generally difficult. In contrast, the descending aorta, even below the level of the pulmonary hilum, can be well exposed in the L-incision approach by retracting the lung. The approach was found to be satisfactory, not only with regard to the access that it provided to the distal side, but also with regard to the exposure of the proximal aorta. The L-incision provides an operative view of the heart, the ascending aorta and the proximal aortic arch. The cannulation of the aorta and right atrium can be performed as in typical cardiac operations. Apart from the inferior wall, the L-incision can provide visualization of the coronary arteries similar to that of median sternotomy. Total arch replacement via an L-incision consists of a number of steps that are similar to the established total arch replacement via median sternotomy [6–9, 15]. Such similarities represent a major advantage of the L-incision approach over the single thoracotomy approach.
However, extended total arch replacement via the L-incision approach is associated with a higher rate of postoperative respiratory complications. Seventeen percent of the patients required tracheostomy and 45% required prolonged ventilation. The pneumonia rate was also higher and intrapulmonary haemorrhages were found to be a characteristic complication of the L-incision group. Preventing intrapulmonary haemorrhage is especially important to maintain the postoperative respiratory function. The left lung should be manipulated particularly gently; such manipulation should preferably be limited to the upper lobe only. When possible (when the patient's oxygenation condition allows), the reinflation of the left lung should be performed after the administration of protamine. Moreover, although the difference was not significant, the operative mortality was slightly higher in the L-incision group than in the median sternotomy group. This difference was clinically important, and we should not overlook the potential invasiveness of extended total arch replacement via an L-incision.
Regarding neurological complications, which may be due to more excessive atherosclerotic changes, the stroke rate tended to be higher in the L-incision group, although the result was not statistically significant. The rates of paraplegia were similar in both groups despite the wider range replaced via the L-incision approach. Fortunately, we did not experience any cases of paraplegia in the present case series of total arch replacement via L-incision. We speculate that this is because of the shorter distal anastomosis time in the L-incision group owing to the better exposure obtained with extended incision. In our procedural steps, distal perfusion was usually resumed as soon after the completion of distal anastomosis as possible using the combination of a balloon-tipped cannula and the side branch of a branched graft or a femoral artery cannula. Unfortunately, our database did not record the anastomosis time or lower body ischaemic time. The cardiac arrest time, which was usually related to the sum of the time required for distal, proximal and left subclavian anastomosis in our surgical steps, was significantly elongated in the median sternotomy group. The longer cardiac arrest time probably reflects the longer distal anastomosis time of the median sternotomy approach. Better exposure and the relatively low incidence of spinal cord ischaemia are advantages of the L-incision approach. However, even via the L-incision approach, it is difficult to perform intercostal artery reconstruction. In the present study, the lowest level of distal anastomosis was at the level of the 10th vertebra. Therefore, in patients treated with the L-incision approach, the site of distal anastomosis should not be too low; it should probably be kept at the level of the 10th vertebra or higher to avoid the need for intercostal artery reconstruction.
Another way to achieve single-stage repair of extensive aortic disease is the hybrid frozen elephant trunk procedure via a median sternotomy. It has been reported, however, that there is a concern of a relatively high rate of postoperative paraplegia following this procedure [16, 17]. According to these reports, ischaemic spinal cord injury may occur in up to one-fourth of patients. Previously, we applied the frozen elephant trunk technique for the repair of extended aneurysms. However, due to the higher incidence rate of spinal cord ischaemia, we discontinued this technique. Currently, the frozen elephant trunk technique is only used to close the entry located at the distal arch during the repair of acute aortic dissection. From this point of view, the relatively low incidence of spinal cord ischaemia in patients undergoing extended total arch replacement via the L-incision approach is noteworthy. Alternatively, to reduce the risk of spinal cord ischaemia, the first-stage elephant trunk procedure followed by endovascular completion may be a two-stage hybrid approach option for repairing extensive arch aneurysms. The short-term results seem to be promising, with a low risk of paraplegia [18–20]. As a drawback, possible inferior mid-term results of the hybrid approach have been reported [18–22]. Owing to its unknown long-term durability, we only apply this hybrid technique to patients with relatively short life expectancies.
The single-stage L-incision approach achieves more extensive graft replacement from the ascending aorta, the aortic arch and the descending aorta in a single session with acceptable long-term results. A thorough assessment of the respiratory status and use of an optimal replacement range are essential for determining the optimal surgical strategy.
In conclusion, the present study showed that the L-incision approach achieves more extensive total arch replacement in return for an increased risk of respiratory complications. The rates of other complications, including spinal cord ischaemia, are acceptable. This technique using a combination of left anterior thoracotomy and upper median sternotomy can be applied to the single-stage repair of extended aortic arch aneurysms in appropriately selected patients.
Conflict of interest: none declared.




