-
PDF
- Split View
-
Views
-
Cite
Cite
Phillip S. Naimo, Yves d'Udekem, Christian P. Brizard, Igor E. Konstantinov, Outcomes of repair of left partial anomalous pulmonary venous connection in children, Interactive CardioVascular and Thoracic Surgery, Volume 21, Issue 2, August 2015, Pages 254–256, https://doi.org/10.1093/icvts/ivv133
Close - Share Icon Share
Abstract
Herein, we report a case series of patients who underwent repair of left partial anomalous pulmonary venous connection (L-PAPVC) via anastomosing the anomalous pulmonary vein (PV) to the left atrial appendage. Fifteen children underwent repair of L-PAPVC between 1980 and 2014. The median age at surgery was 3.6 years (range: 5 days to 17.2 years). Concomitant anomalies were present in 87% (13/15). There were no early deaths. There was 1 late death occurring 63 days following surgical repair due to pneumococcal septicaemia in a patient with prior atrial septal defect closure and Ehlers–Danlos syndrome. The overall survival rate was 93.7% at 15 years. A single patient (1/15, 7%) required reoperation 1 year after L-PAPVC repair for PV stenosis due to several thrombi located throughout the PV. The rate of freedom from PV reoperation was 90% at 10 years. The follow-up was 100% complete with a median time of 11 years (range: 52 days to 20 years). To our knowledge, this is the youngest cohort of patients who have undergone surgical repair of L-PAPVC. Repair of L-PAPVC in children can be achieved via anastomosis of the anomalous vessel to the left atrial (LA) with excellent outcomes. The rate of anastomotic stenosis at the site of implantation on the LA is low.
Left partially anomalous pulmonary venous connection (L-PAPVC) is a rare condition, in which part or all of the left pulmonary veins (PVs) drain indirectly into the right atrium (Fig. 1A). Patients with isolated L-PAPVC are often asymptomatic, although some may have a left-to-right shunt and develop pulmonary hypertension or right ventricular (RV) failure [1, 2].
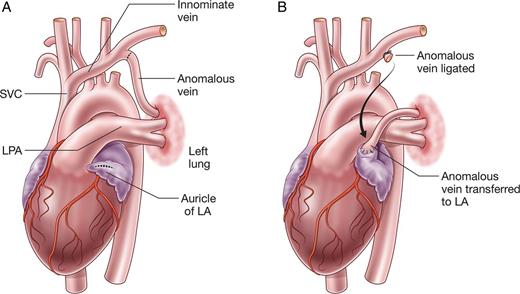
Although surgical repair of L-PAPVC has been performed with excellent results [3, 4], there are limited data on the outcomes of repair in children, particularly the rate of anastomotic stenosis following implantation of the anomalous vein onto the left atrial (LA) appendage. We, therefore, sought to review the outcomes of these rare patients at the Royal Children's Hospital.
CASE SERIES
Between 1980 and 2014, 15 patients underwent repair of L-PAPVC via anastomosis of the anomalous vein to the LA (Fig. 1B). Surgery was performed via median sternotomy (n = 13) with cardiopulmonary bypass (CPB) or posterolateral thoracotomy (n = 2) without CPB. The LA was opened by an oblique incision from the LA appendage to the posterior LA in 11 patients, and the LA appendage was amputated in 4 patients. Anastomosis was achieved with continuous Prolene suture (6/0, n = 6; 7/0, n = 7; 8/0, n = 2) in all patients.
Patient characteristics and surgical procedures are summarized in Table 1. The median age at surgery was 1.4 years (interquartile range: 110 days to 4.4 years) and the median weight at surgery was 12 kg (interquartile range: 4.9–15.1 kg). Concurrent repair of concomitant cardiovascular anomalies was undertaken in 80% (12/15) of patients. Postoperatively, 1 patient with concomitant tetralogy of Fallot, left pulmonary artery sling and tracheal stenosis required extracorporeal membrane oxygenation for 6 days due to respiratory failure and poor cardiac function. This patient is doing well at 18 years after surgery.
| Patient no. . | Years . | Sex . | Age at repair . | Weight (kg) . | Concomitant anomalies . | Prior surgery . | Concomitant repair with L-PAPVC repair . | Reoperations . | Status . |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 1980 | M | 299 days | 7.6 | None | None | None | None | Alive |
| 2 | 1992 | F | 60 days | 3.6 | CoA; PDA; VSD | None | CoA repair; PDA ligation; VSD closure | None | Alive |
| 3 | 1994 | M | 99 days | 5.2 | Hypoplastic RV; PDA; tricuspid atresia; VSD; hypoplastic left lung | None | PA banding | 1. PV stenosis relief 2. MBTS 3. BCPC and RPA patch augmentation 4. Take-down of BCPC and rerouting of LPA to SVC | Alive |
| 4 | 1996 | F | 1.2 years | 8.8 | PA sling; TOF | MBTS 9 months earlier | PA sling repair; VSD closure | RVOT resection and 19 mm aortic homograft in RVOT to function with the native valve | Alive |
| 5 | 1998 | M | 4.8 years | 15.5 | R-PAPVC | None | R-PAPVC repair | None | Alive |
| 6 | 1999 | M | 4.4 years | 16.0 | None | None | None | None | Alive |
| 7 | 2000 | M | 17.1 years | 40.3 | ASD; Ehlers–Danlos syndrome | ASD repair 6 years earlier | None | None | Late death |
| 8 | 2000 | M | 3.6 years | 13.3 | ASD | None | ASD closure | None | Alive |
| 9 | 2001 | M | 12.2 years | 38.0 | ASD | None | ASD closure | None | Alive |
| 10 | 2001 | F | 4.1 years | 14.8 | CoA; PDA | None | CoA repair; PDA ligation | None | Alive |
| 11 | 2001 | M | 25 days | 3.5 | ASD; VSD | None | ASD closure | None | Alive |
| 12 | 2002 | F | 1.4 years | 10.5 | ASD; cor triatriatum; pulmonary valve stenosis; R-PAPVC | None | ASD closure; cor triatriatum repair; R-PAPVC repair | None | Alive |
| 13 | 2007 | M | 5 days | 2.6 | ASD; CoA; PDA; VSD | None | ASD closure; CoA repair; PDA ligation; VSD closure; PA banding | PA band tightening and plication of left hemi-diaphragm | Alive |
| 14 | 2011 | F | 4.3 years | 16.8 | Bicuspid aortic valve; CoA; PDA; ASD | CoA repair; PDA ligation 4 years earlier | ASD closure | None | Alive |
| 15 | 2011 | F | 112 days | 5.1 | TOF; R-PAPVC | None | TOF repair; R-PAPVC repair | 1. Warden procedure 2. RVOT resection | Alive |
| Patient no. . | Years . | Sex . | Age at repair . | Weight (kg) . | Concomitant anomalies . | Prior surgery . | Concomitant repair with L-PAPVC repair . | Reoperations . | Status . |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 1980 | M | 299 days | 7.6 | None | None | None | None | Alive |
| 2 | 1992 | F | 60 days | 3.6 | CoA; PDA; VSD | None | CoA repair; PDA ligation; VSD closure | None | Alive |
| 3 | 1994 | M | 99 days | 5.2 | Hypoplastic RV; PDA; tricuspid atresia; VSD; hypoplastic left lung | None | PA banding | 1. PV stenosis relief 2. MBTS 3. BCPC and RPA patch augmentation 4. Take-down of BCPC and rerouting of LPA to SVC | Alive |
| 4 | 1996 | F | 1.2 years | 8.8 | PA sling; TOF | MBTS 9 months earlier | PA sling repair; VSD closure | RVOT resection and 19 mm aortic homograft in RVOT to function with the native valve | Alive |
| 5 | 1998 | M | 4.8 years | 15.5 | R-PAPVC | None | R-PAPVC repair | None | Alive |
| 6 | 1999 | M | 4.4 years | 16.0 | None | None | None | None | Alive |
| 7 | 2000 | M | 17.1 years | 40.3 | ASD; Ehlers–Danlos syndrome | ASD repair 6 years earlier | None | None | Late death |
| 8 | 2000 | M | 3.6 years | 13.3 | ASD | None | ASD closure | None | Alive |
| 9 | 2001 | M | 12.2 years | 38.0 | ASD | None | ASD closure | None | Alive |
| 10 | 2001 | F | 4.1 years | 14.8 | CoA; PDA | None | CoA repair; PDA ligation | None | Alive |
| 11 | 2001 | M | 25 days | 3.5 | ASD; VSD | None | ASD closure | None | Alive |
| 12 | 2002 | F | 1.4 years | 10.5 | ASD; cor triatriatum; pulmonary valve stenosis; R-PAPVC | None | ASD closure; cor triatriatum repair; R-PAPVC repair | None | Alive |
| 13 | 2007 | M | 5 days | 2.6 | ASD; CoA; PDA; VSD | None | ASD closure; CoA repair; PDA ligation; VSD closure; PA banding | PA band tightening and plication of left hemi-diaphragm | Alive |
| 14 | 2011 | F | 4.3 years | 16.8 | Bicuspid aortic valve; CoA; PDA; ASD | CoA repair; PDA ligation 4 years earlier | ASD closure | None | Alive |
| 15 | 2011 | F | 112 days | 5.1 | TOF; R-PAPVC | None | TOF repair; R-PAPVC repair | 1. Warden procedure 2. RVOT resection | Alive |
ASD: atrial septal defect; BCPC: bidirectional cavopulmonary connection; CoA: coarctation of aorta; LA: left atrium; LPA: left pulmonary artery; MBTS: modified Blalock–Taussig shunt; PA: pulmonary artery; PAPVC: partially anomalous pulmonary venous connection; PDA: patent ductus arteriosus; PV: pulmonary vein; RA: right atrium; RPA: right pulmonary artery; RV: right ventricle; RVOT: right ventricular outflow tract; SVC: superior vena cava; TOF: tetralogy of Fallot; VSD: ventricular septal defect.
| Patient no. . | Years . | Sex . | Age at repair . | Weight (kg) . | Concomitant anomalies . | Prior surgery . | Concomitant repair with L-PAPVC repair . | Reoperations . | Status . |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 1980 | M | 299 days | 7.6 | None | None | None | None | Alive |
| 2 | 1992 | F | 60 days | 3.6 | CoA; PDA; VSD | None | CoA repair; PDA ligation; VSD closure | None | Alive |
| 3 | 1994 | M | 99 days | 5.2 | Hypoplastic RV; PDA; tricuspid atresia; VSD; hypoplastic left lung | None | PA banding | 1. PV stenosis relief 2. MBTS 3. BCPC and RPA patch augmentation 4. Take-down of BCPC and rerouting of LPA to SVC | Alive |
| 4 | 1996 | F | 1.2 years | 8.8 | PA sling; TOF | MBTS 9 months earlier | PA sling repair; VSD closure | RVOT resection and 19 mm aortic homograft in RVOT to function with the native valve | Alive |
| 5 | 1998 | M | 4.8 years | 15.5 | R-PAPVC | None | R-PAPVC repair | None | Alive |
| 6 | 1999 | M | 4.4 years | 16.0 | None | None | None | None | Alive |
| 7 | 2000 | M | 17.1 years | 40.3 | ASD; Ehlers–Danlos syndrome | ASD repair 6 years earlier | None | None | Late death |
| 8 | 2000 | M | 3.6 years | 13.3 | ASD | None | ASD closure | None | Alive |
| 9 | 2001 | M | 12.2 years | 38.0 | ASD | None | ASD closure | None | Alive |
| 10 | 2001 | F | 4.1 years | 14.8 | CoA; PDA | None | CoA repair; PDA ligation | None | Alive |
| 11 | 2001 | M | 25 days | 3.5 | ASD; VSD | None | ASD closure | None | Alive |
| 12 | 2002 | F | 1.4 years | 10.5 | ASD; cor triatriatum; pulmonary valve stenosis; R-PAPVC | None | ASD closure; cor triatriatum repair; R-PAPVC repair | None | Alive |
| 13 | 2007 | M | 5 days | 2.6 | ASD; CoA; PDA; VSD | None | ASD closure; CoA repair; PDA ligation; VSD closure; PA banding | PA band tightening and plication of left hemi-diaphragm | Alive |
| 14 | 2011 | F | 4.3 years | 16.8 | Bicuspid aortic valve; CoA; PDA; ASD | CoA repair; PDA ligation 4 years earlier | ASD closure | None | Alive |
| 15 | 2011 | F | 112 days | 5.1 | TOF; R-PAPVC | None | TOF repair; R-PAPVC repair | 1. Warden procedure 2. RVOT resection | Alive |
| Patient no. . | Years . | Sex . | Age at repair . | Weight (kg) . | Concomitant anomalies . | Prior surgery . | Concomitant repair with L-PAPVC repair . | Reoperations . | Status . |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 1980 | M | 299 days | 7.6 | None | None | None | None | Alive |
| 2 | 1992 | F | 60 days | 3.6 | CoA; PDA; VSD | None | CoA repair; PDA ligation; VSD closure | None | Alive |
| 3 | 1994 | M | 99 days | 5.2 | Hypoplastic RV; PDA; tricuspid atresia; VSD; hypoplastic left lung | None | PA banding | 1. PV stenosis relief 2. MBTS 3. BCPC and RPA patch augmentation 4. Take-down of BCPC and rerouting of LPA to SVC | Alive |
| 4 | 1996 | F | 1.2 years | 8.8 | PA sling; TOF | MBTS 9 months earlier | PA sling repair; VSD closure | RVOT resection and 19 mm aortic homograft in RVOT to function with the native valve | Alive |
| 5 | 1998 | M | 4.8 years | 15.5 | R-PAPVC | None | R-PAPVC repair | None | Alive |
| 6 | 1999 | M | 4.4 years | 16.0 | None | None | None | None | Alive |
| 7 | 2000 | M | 17.1 years | 40.3 | ASD; Ehlers–Danlos syndrome | ASD repair 6 years earlier | None | None | Late death |
| 8 | 2000 | M | 3.6 years | 13.3 | ASD | None | ASD closure | None | Alive |
| 9 | 2001 | M | 12.2 years | 38.0 | ASD | None | ASD closure | None | Alive |
| 10 | 2001 | F | 4.1 years | 14.8 | CoA; PDA | None | CoA repair; PDA ligation | None | Alive |
| 11 | 2001 | M | 25 days | 3.5 | ASD; VSD | None | ASD closure | None | Alive |
| 12 | 2002 | F | 1.4 years | 10.5 | ASD; cor triatriatum; pulmonary valve stenosis; R-PAPVC | None | ASD closure; cor triatriatum repair; R-PAPVC repair | None | Alive |
| 13 | 2007 | M | 5 days | 2.6 | ASD; CoA; PDA; VSD | None | ASD closure; CoA repair; PDA ligation; VSD closure; PA banding | PA band tightening and plication of left hemi-diaphragm | Alive |
| 14 | 2011 | F | 4.3 years | 16.8 | Bicuspid aortic valve; CoA; PDA; ASD | CoA repair; PDA ligation 4 years earlier | ASD closure | None | Alive |
| 15 | 2011 | F | 112 days | 5.1 | TOF; R-PAPVC | None | TOF repair; R-PAPVC repair | 1. Warden procedure 2. RVOT resection | Alive |
ASD: atrial septal defect; BCPC: bidirectional cavopulmonary connection; CoA: coarctation of aorta; LA: left atrium; LPA: left pulmonary artery; MBTS: modified Blalock–Taussig shunt; PA: pulmonary artery; PAPVC: partially anomalous pulmonary venous connection; PDA: patent ductus arteriosus; PV: pulmonary vein; RA: right atrium; RPA: right pulmonary artery; RV: right ventricle; RVOT: right ventricular outflow tract; SVC: superior vena cava; TOF: tetralogy of Fallot; VSD: ventricular septal defect.
The follow-up was 100% complete with a median time of 11 years (range: 52 days to 20 years). There were no early deaths. There was 1 late death 63 days postoperatively due to pneumococcal septicaemia in a patient with prior ASD repair and Ehlers–Danlos syndrome. It was noted that this patient had not had a pneumococcal vaccination. The overall survival rate was 93 ± 7% (95% confidence interval: 59, 99) at 15 years.
Four patients underwent a total of nine reoperations. All of these patients had concomitant cardiovascular anomalies. Echocardiography identified anastomotic PV stenosis in 1 patient 81 days postoperatively. Cardiac catheterization confirmed anastomotic stenosis. However, during surgery, the anastomotic site was widely patent and the stenosis was located at the entry of the left PVs into the confluence of PVs, and was due to thrombi. The thrombi were removed and the PVs were enlarged with a patch of autologous pericardium. At the last follow-up, echocardiogram demonstrated no anastomotic stenosis in any patient. Additionally, RV dilation had regressed in all but 1 patient, though RV function was normal in all patients. Mild tricuspid regurgitation was present in 2 patients.
DISCUSSION
To our knowledge, we describe the youngest cohort of patients who have undergone repair of L-PAPVC. Mortality and anastomotic stenosis following repair of L-PAPVC is low. A single patient required reoperation for suspected anastomotic stenosis on the LA appendage. However, during surgery, the anastomosis was patent and the stenosis was due to thrombi in the confluence of PVs. Alsoufi et al. [4] described 22 patients who underwent L-PAPVC repair between 1982 and 2006 with a median age of 5.3 years, with no deaths or anastomotic stenosis, after a mean follow-up of 9.1 years. Similarly, ElBardissi et al. [3] described 27 patients who underwent L-PAPVC repair between 1954 and 2006 with a median age of 33 years, with no deaths or anastomotic stenosis, after a mean follow-up of 10.6 years.
Indications for surgery in asymptomatic patients with L-PAPVC has been similar to that of ASD repair and the surgery was recommended in patients with a pulmonary-to-systemic flow ratio (Qp:Qs) >1.5 [5]. ElBardissi et al. [3] suggested that surgical repair take place early in patients with RV dilation or mild to moderate tricuspid regurgitation, or in early stages of pulmonary vascular disease, to prevent pulmonary hypertension. We would agree that the isolated L-PAPVC should be repaired when Qp:Qs >1.5 or with clinical signs of RV dilatation as described by ElBardissi et al. [3]. However, in patients with concomitant cardiovascular anomalies that require surgical repair, simultaneous repair of L-PAPVC can be done safely and with excellent outcomes.
Repair of L-PAPVC in younger children can be achieved via anastomosis of the anomalous vein to the LA appendage with excellent outcomes. The rate of anastomotic stenosis is low. Large, multi-institutional studies are required to determine definitive guidelines for surgical indications.
Conflict of interest: none declared.
REFERENCES
- ehlers-danlos syndrome
- partial anomalous pulmonary venous connection
- left atrium
- left auricular appendage
- congenital anomaly of pulmonary veins
- lung
- anastomotic stenosis
- congenital heart disease
- anastomosis, surgical
- child
- follow-up
- repeat surgery
- septicemia
- surgical procedures, operative
- survival rate
- thrombus
- pulmonary vein stenosis
- closure of defect of interatrial septum




