-
PDF
- Split View
-
Views
-
Cite
Cite
Jacopo Vannucci, Elisa Scarnecchia, Lucio Cagini, Francesco Puma, Pneumoperitoneum as a valuable option in the treatment of post lower lobectomy bronchopleural fistula, Interactive CardioVascular and Thoracic Surgery, Volume 21, Issue 1, July 2015, Pages 121–123, https://doi.org/10.1093/icvts/ivv079
Close - Share Icon Share
Abstract
Although the incidence of post-lobectomy bronchopleural fistula has decreased over years, it remains a threatening complication in lung surgery. Once the fistula is diagnosed, treatment options are several. Conservative versus operative treatment is currently a matter of debate generally regarding timing, patient's condition and fistula's size. Although prompt resurgery is strongly suggested for early onset large fistulas, the clinical scenario may suggest a cautious conduct and conservative treatment could be advocated and repeated. Endoscopic management is now widely employed for limited, small bronchial dehiscence while pneumoperitoneum has surprisingly never been reported for this purpose, despite its potential. We report a case of a complete right lower lobe bronchial stump reopening, successfully treated by pneumoperitoneum.
INTRODUCTION
Post-lobectomy bronchopleural fistula (PL-BPF), although rare, still represents a challenging issue for thoracic surgeons. Early, large PL-BPF is better treated with reoperation rather than by conservative procedures. Impaired general condition and active infection may suggest a non-operative approach. Endoscopic treatment is reported to be successfully feasible only for small fistulas [1]. The unusual case of conservative management for a huge PL-BPF by means of pneumoperitoneum is reported.
CASE REPORT
A 57-year old male heavy smoker was referred to our service for the contralateral relapse of pulmonary adenocarcinoma. Four years earlier, the patient was submitted to targeted therapy (Gefitinib) for an epidermal growth factor receptor (EGFR) mutant cT1N2M0 adenocarcinoma of the left lower lobe. After a 20-month period of complete response, the disease relapsed at primitive site. In 2011, the patient underwent a thoracotomic left lower lobectomy and radical systematic lymphadenectomy with the definitive diagnosis of T2N0M0 primary adenocarcinoma. Gefitinib standard therapy was continued.
In 2014, a cT2N0M0 cancer of the right lower lobe was identified. The needle biopsy showed an EGFR wild-type pulmonary adenocarcinoma. Cardiopulmonary tests were in the normal range. Cervical videomediastinoscopy (frozen section: negative) and three-port video-assisted thoracic surgery (VATS) right lower lobectomy with mediastinal lymph node dissection were performed. The bronchial suture was technically faulty due to stapler malfunction. Air leak test was negative and the stump suture was reinforced by three absorbable stitches (Vicryl 3–0). On Day 1, the patient underwent VATS reoperation for haemothorax (1600 ml) caused by mild bleeding from the subcarinal region, site of scar tissue, resulting from previous surgery. At Day 4, septic fever and severe bilateral pneumonia appeared; the drained fluids changed into purulent features. Massive, continuous air leak, detected throughout the whole respiratory cycle, emerged in postoperative day 7.
Videobronchoscopy revealed a complete dehiscence of the bronchial suture (Video 1). Chest computed tomography (CT) scan confirmed endoscopic findings and bilateral pneumonia (Fig. 1A). The patient's general condition seriously declined with the signs and symptoms of severe sepsis. The extremely critical conditions prevented any invasive procedure and the initial treatment was unavoidably limited to a posterobasal 28Ch chest tube. Microbiology assessment revealed Pseudomonas Aeruginosa on bronchial washing, Acinetobacter Baumannii on pleural fluid and Escherichia Coli on sputum. Intravenous targeted antimicrobial therapy was consequently given and nasogastric catheter feeding was started. Since Day 15, with resolution of the bilateral pneumonia, both septic condition and oxygenation gradually improved, while the massive air leakage remained unchanged, forcing to keep the drain suction pressure at −20 cm H2O. At Day 28, once a non-critical condition was recovered, a local percutaneous infiltration of lidocaine hydrochloride 0.5% solution was performed in the left lower abdominal quadrant. An 18-gauge paracentesis teflon needle was inserted, followed by insufflation of 700 ml of air. Pneumoperitoneum was repeated on Day 33 with extra 800 ml of air. Air leak suddenly decreased, occurring only during coughing. Chest X-ray confirmed the pleural space obliteration with consistent right diaphragmatic elevation (Fig. 2).
Video 1: Videobronchoscopy: complete right lower lobe bronchial stump's dehiscence. Beyond the fistula, diaphragm and purulent cavity are observed.
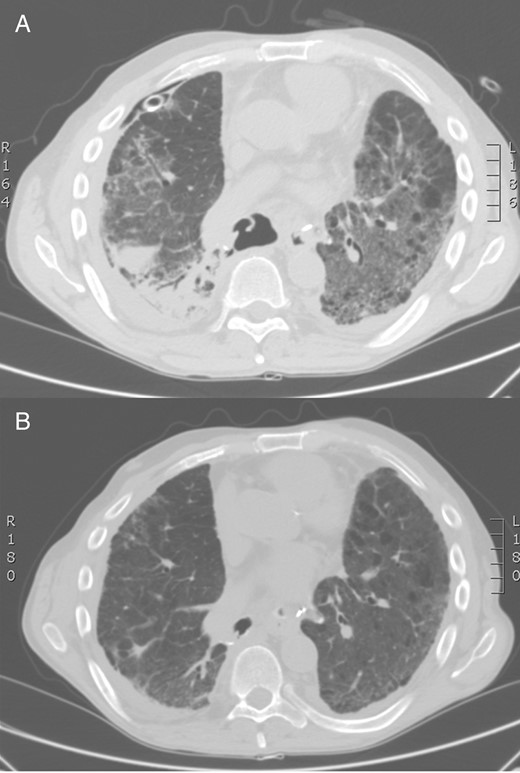
Chest CT scan: large bronchial suture dehiscence after right lower lobectomy and bilateral pneumonia (A). Control chest CT scan: bronchial stump's closure, sealed by diaphragm (B). CT: computed tomography.
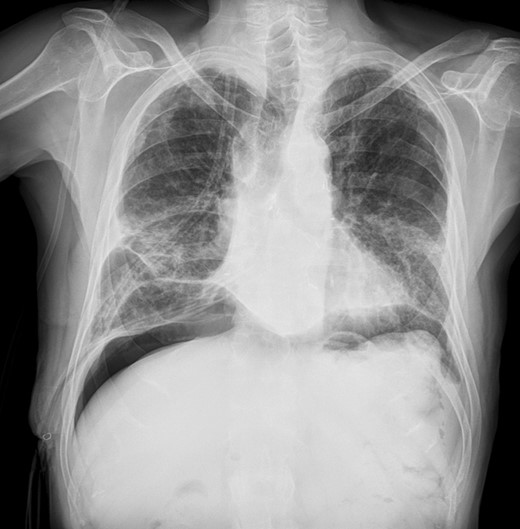
Chest X-ray after repeated pneumoperitoneum (roughly 1500 ml in two separate steps).
On the 60th day after the first operation, the patient was discharged with the posterior chest tube in place with only occasional air leakage. Videobronchoscopy was performed 15 days after hospital discharge: the giant BPF was absolutely sealed by the diaphragm (Video 2). Chest CT scan confirmed the complete pleural space obliteration (Fig. 1B). Chest tube was then removed. The patient is asymptomatic, in good general condition, 11 months after surgery. The tumour was staged as primary adenocarcinoma pT2N1M0.
Video 2: Control videobronchoscopy: bronchial stump closure with patched diaphragm proximity after repeated pneumoperitoneum. No residual fistula can be demonstrated.
DISCUSSION
Early post-resectional bronchopleural fistula is typically related to technical issues. After the evolution of new technologies and materials, a significant decrease of such complication has been reported with considerable reduction of the mortality rate [2].
Large PL-BPF with acute onset generally requires different types of surgical interventions [3]. The conservative approach is preferable for small fistulas, potentially manageable by endoscopic treatment.
A prompt surgical repair of the bronchial dehiscence was impossible for the life-threatening conditions of our patient, which were not only related to the complication: in fact, the severe sepsis, the recent reoperation for bleeding, the concomitant bilateral pneumonia and the bilateral lower lobectomies sustained actually contraindicated any invasive procedure. After improvement of the clinical conditions, we tried to solve the basal pneumothorax by pneumoperitoneum. This simple, bedside, minimally invasive procedure was strikingly effective and the temporary diaphragmatic elevation allowed the pleural cavity obliteration [4] and resulted in the complete and stable closure of the giant fistula in about 1 month. The current availability of plastic-sheathed needles makes pneumoperitoneum a technically simple manoeuver, with low risk. The procedure did not cause paralytic ileus and was well tolerated, producing only a slight transitory abdominal tension. The inoculated air tends to accumulate preferentially below the hemidiaphragm of the operated side, without causing significant ventilatory disorders to the contralateral lung. A theoretical contraindication could be the presence of important peritoneal adhesions and therefore it should be performed with caution in case of previous major abdominal surgery.
Pneumoperitoneum is not a new technique; it was described in the past especially for tuberculosis, and for the treatment of postoperative air leakage [4, 5]. The use of pneumoperitoneum in the treatment of PL-BPF has not been previously reported in the literature. The procedure can be very effective for solving a basal pneumothorax, and with the appropriate chest drainage, may allow the conservative treatment of large BPF after lower lobectomy.
Conflict of interest: none declared.




