-
PDF
- Split View
-
Views
-
Cite
Cite
Christoph Huber, Fabien Praz, Crochan J. O'Sullivan, Bettina Langhammer, Steffen Gloekler, Stefan Stortecky, Regula S. von Allmen, Bernhard Meier, Thierry Carrel, Lars Englberger, Stephan Windecker, Peter Wenaweser, Transcarotid aortic valve-in-valve implantation for degenerated stentless aortic root conduits with severe regurgitation: a case series, Interactive CardioVascular and Thoracic Surgery, Volume 20, Issue 6, June 2015, Pages 694–700, https://doi.org/10.1093/icvts/ivv053
Close - Share Icon Share
Abstract
Transcatheter aortic valve implantation (TAVI) is routinely performed via the transfemoral and the transapical route. Subclavian and direct aortic access are described alternatives for TAVI. Recently, the transcarotid approach has been shown to be feasible among patients with limited vascular access and severe native aortic valve stenosis. We aim to investigate the feasibility of transcatheter aortic valve-in-valve implantation via the transcarotid access in patients with severe aortic regurgitation due to degenerated stentless Shelhigh conduits using the 29 mm Medtronic CoreValve bioprosthesis.
Three patients with complex vascular anatomy undergoing transcatheter valve-in-valve implantation via the transcarotid route were enrolled in the study. The procedure was performed under general anaesthesia using surgical cut-down to facilitate vascular access. Immediate procedural results as well as echocardiographic and clinical outcomes after 30 days and 6 months of the follow-up were recorded and analysed.
All three patients underwent unproblematic TAVI and experienced dramatic improvement of symptoms. Mean transvalvular gradient was 3, 6 and 11 mmHg, respectively. Effective orifice area ranged between 1.7 and 2.2 cm2. Only mild paravalvular regurgitation was detected by echocardiography after 30 days of the follow-up.
The transcarotid approach can be safely performed for valve-in-valve procedures using the Medtronic CoreValve in patients with limited vascular access. It enables accurate positioning and implantation of the prosthesis.
INTRODUCTION
Transcatheter aortic valve implantation (TAVI) has become a routine minimally invasive procedure to treat severe aortic valve stenosis in inoperable and surgical high-risk patients. According to a recently published randomized trial, TAVI was shown to be superior to medical treatment in this specific population of patients [1]. Valve-in-valve interventions have emerged as a promising treatment option for patients with degenerated aortic and mitral bioprostheses. However, patients with limited vascular access due to severe calcification or tortuosity, prior interventions of the iliofemoral vasculature, as well as Type B and operated type A aortic dissections may not be amenable to the transfemoral approach. In this case, the main alternative is the transapical access [2, 3]. Alternatively, the transsubclavian [4] and transaortic [5, 6] routes are increasingly used after examination of the anatomy by the Heart Team. Formally, a patent left internal thoracic artery coronary bypass graft represents a contraindication for the left-sided transsubclavian access due to the risk of compromising the blood flow in the bypass graft following insertion of the 18 French (F) delivery sheath. A pacemaker or an implantable internal defibrillator in the subclavian region are other potential restrictions.
The transapical and the direct aortic accesses requires thoracotomy and ministernotomy, respectively, both performed under general anaesthesia. This may favour the apparition of respiratory complications in patients with pre-existing chronic obstructive pulmonary disease [7, 8]. In all these situations, the transcarotid access, first described in 2010 [9], may offer an attractive alternative.
In this case series, we report the first transcarotid aortic valve-in-valve implantation for severe aortic regurgitation in three patients with degenerated stentless Shelhigh conduits using the 29 mm Medtronic CoreValve bioprosthesis (Medtronic, Inc., Minneapolis, MN, USA).
METHODS
Patient selection
All three patients were referred for TAVI because of symptomatic severe regurgitation of the degenerated bioprosthesis. According to our standard diagnostic work-up, all patients underwent transthoracic echocardiography (TTE), right and left cardiac catheterization, contrast-enhanced electrocardiogram-gated multislice computed tomography (MSCT) and Doppler ultrasound of the carotid and the vertebral arteries prior to the intervention. Analyses of the potential access options and measurements of the aortic annulus were performed using the 3mensio ValvesTM software (3mensio Medical Imaging, Bilthoven, The Netherlands).
After Heart Team discussion, all three patients were considered too high risk for an open-heart redo valve surgery because of chronic renal failure on haemodialysis and severely reduced left ventricular ejection fraction (LVEF) in the first patient, prior ischaemic stroke and frailty (body mass index 19.6 kg/m2) in the second patient and advanced age and reduced mobility in the third patient. Implantation via the transfemoral and the subclavian accesses were deemed either not feasible or associated with a notably increased risk of vascular complications. After selection of the transcarotid route, cerebral magnetic resonance (MR) angiography was performed to demonstrate patency of the circle of Willis. A diameter of the carotid artery of 7 mm or greater was assumed to be adequate for accommodation of the 18F delivery sheath.
Device selection
Device selection was based on the anatomic characteristics of the previously replaced aortic root. The absence of calcifications and the relatively large anatomy of the degenerated bioprosthetic valve (measured annulus size according to angio-CT: 24–25 mm) in all three patients precluded implantation of a balloon-expandable valve. Implantation of the only TAVI system approved for treatment of aortic regurgitation, the JenaValve (Jenavalve Technology, Inc., Munich, Germany), was deemed unfeasible because of the insufficient amount of anchoring tissue offered by the degenerated leaflets of the valved conduits compromising device stabilization. The same limitation would have applied when using the Medtronic Engager device. Furthermore, at the time of the procedures, no data supporting the treatment of AR with the Symetis ACURATE TA bioprosthesis (Symetis, Ecublens, Switzerland) were available. As a consequence, the transapical approach could not be considered as an alternative access for these specific patients. A recent study reported feasibility of TAVI using the Medtronic CoreValve bioprosthesis in patients presenting with pure severe native aortic valve regurgitation [10].
Procedure
The procedure was performed under general anaesthesia by a multidisciplinary team comprising cardiac surgeons, cardiologists and anaesthesiologists in a hybrid operating room. Intraprocedural transoesophageal (TOE) was used in 2 of the 3 patients for guiding and assessing paravalvular regurgitation.
The day before the procedure, 100 mg acetylsalicylic acid was administered to all patients. Additionally, they received an intravenous antibiotic prophylaxis (cefuroxim 750 mg), which was repeated twice after the intervention. Clopidogrel (75 mg daily) was added for 6 months.
A 5F femoral access was used for insertion of a pigtail catheter for dye injection and better orientation during valve deployment. A temporary pacemaker catheter was positioned in the right ventricle via the contralateral jugular vein in 2 patients and via the right femoral vein in one. Rapid or fast pacing was used in all patients. In 1 patient, the intervention was combined with a percutaneous coronary intervention of the right coronary artery.
Surgical cut-down was performed to facilitate access to the left common carotid artery and haemostasis at the end of the procedure. To avoid complete carotid occlusion, an 8 mm woven Dacron prosthesis was sutured to the common carotid artery end-to-side with Prolene 4/0 using a tangential clamp. Next, the 18F delivery sheath (Cook Medical, Bloomington, IN, USA) was introduced through the 8 mm graft.
PATIENTS
In 2013, three patients underwent a transcarotid CoreValve implantation for pure aortic regurgitation due to degeneration of the aortic valve of previously implanted Shelhigh conduits (Shelhigh, Inc., Millburn, NJ, USA) at our institution.
Patient 1
The first patient was a 56-year old male with a past history of autosomal-dominant polycystic kidney disease and unilateral left renal agenesis. At the age of 45 years, he suffered acute type A aortic dissection and underwent emergent replacement of the aortic valve using a mechanical St Jude Medical/Hemashield 27 mm valved graft conduit. After an initial uneventful course, a subacute infective endocarditis with Streptococcus bovis was diagnosed in 2005, 3 years after valve replacement. Despite aggressive antibiotic treatment, the infection led to an abscess formation around the mechanical valve, which required urgent surgery and repeat replacement of the aortic valve and the ascending aorta with a 27 mm stentless Shelhigh valved conduit.
Following progressive decline in renal function, a Cimino fistula was created on the left side in 2010 and the patient was put on haemodialysis. Two years later, the decision was made to implant a cardiac resynchronization therapy defibrillator (CRT-D) for worsening LVEF (30%) accompanied by left bundle branch block. Because of the left forearm Cimino fistula, the generator was implanted into the right pectoral region. After initial improvement of symptoms and LVEF, the patient reported sudden onset of recurrent dyspnoea [New York Heart Association (NYHA) class IV]. On TTE, severe aortic regurgitation was diagnosed (Fig. 1A) and diastolic reverse flow in the descending aorta was observed. The preinterventional evaluations showed extension of the chronic dissection membrane from the origin of the left subclavian artery to both iliac arteries rendering left subclavian and transfemoral access unsuitable due to the risk of complicating the existing dissection through interaction of the delivery catheter with the residual membrane (Fig. 1E). Right subclavian access was restricted by the presence of the CRT-D device, such that the most appropriate vascular access for TAVI was deemed to be the left carotid artery (Fig. 1B–D).
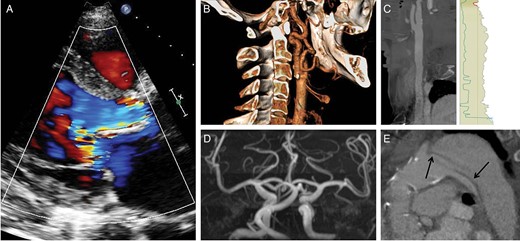
Anatomical characteristics of the first patient. (A) Parasternal long axis view obtained by Doppler TTE showing a wide regurgitation jet completely filling the LVOT during diastole; (B) three-dimensional reconstruction of the left carotid artery obtained by MSCT showing only mild calcification at the level of the bifurcation; (C) stretched reconstruction of the common carotid artery performed with the 3mensio software confirming sufficient diameter to accommodate the 18F sheath; (D) cerebral MR-angiography demonstrating patency of the circle of Willis. (E) MSCT sagittal view showing the residual dissection membrane (arrows) following type A aortic dissection involving the origin of the left subclavian artery and the descending aorta. The stentless Shelhigh conduit is moderately calcified. MSCT: multislice computed tomography.
Patient 2
The second patient (74 years) suffered an acute type A aortic dissection in 2002 with extension of the membrane into the descending aorta. This resulted in the formation of a large perfused false lumen. The patient underwent emergent combined replacement of both the aortic valve and the ascending aorta using a 27 mm stentless Shelhigh valved conduit. Despite multiple ischaemic strokes due to transient cerebral hypoperfusion, he recovered well and could be discharged 14 days after the operation. The long-term postoperative course was uneventful and the patient was followed up periodically at our institution. Eleven years after the operation, he was readmitted for congestive heart failure (NYHA IV). Severe aortic valve regurgitation due to prolapse of the ruptured non-coronary leaflet into the left ventricular outflow tract was diagnosed by TTE and TOE (Fig. 2A). In addition, MSCT showed that an aneurysm of 54 mm had developed at the level of the distal anastomosis between the composite-graft and the native aorta (Fig. 2B and D). Due to the risk of rupture during aortic arch passage, both the transfemoral and the left subclavian accesses were considered impracticable. Because of favourable angulation and absent atherosclerosis, the right carotid access was finally selected (Fig. 2C).
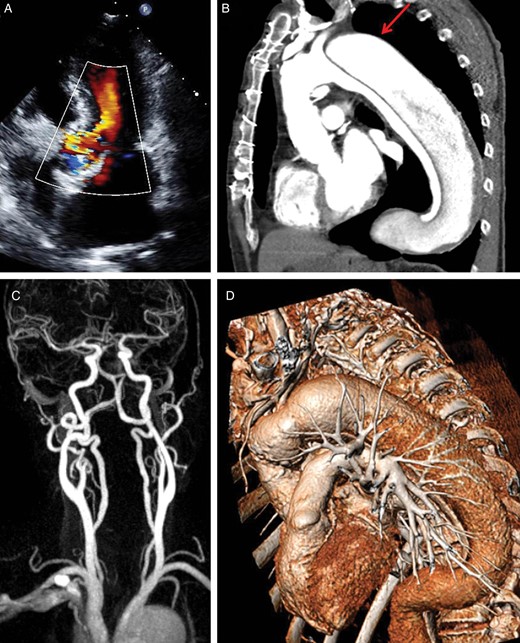
Vascular anatomy of the second patient. (A) Five-chamber view obtained by TTE showing moderate to severe aortic regurgitation; (B) MSCT-angiography enhancing residual findings following type A aortic dissection. The red arrow shows the localization of an aneurysm at the level of the distal conduit anastomosis; (C) MR-angiography of the supra-aortic vessels excluding relevant stenosis of the carotid arteries and confirming patency of the circle of Willis; (D) three-dimensional reconstruction of the thoracic aorta enhancing acute angulation between the conduit and the aortic arch rendering access via the left subclavian and left carotid arteries particularly hazardous.
Patient 3
The third male patient was 84 years old when he underwent elective composite valve-graft conduit replacement of the aortic root, replantation of the coronary arteries and bypass of the right coronary artery in 2006 to treat an aneurysm of the ascending aorta (52 mm). The postoperative course was uneventful. However, 7 years later, he was referred to his cardiologist for evaluation of progressive dyspnoea on exertion leading to marked limitation of daily physical activity (NYHA III). On physical examination, a faint systolic murmur (2/6) and a loud diastolic decrescendo murmur (3/6) were audible. There were no signs of acute cardiac decompensation. Echocardiography revealed severe aortic valve regurgitation (Fig. 3A) and a beginning dilatation of the left ventricle (LVEDD 55 mm). Thus, the patient was referred for TAVI. Vascular imaging revealed bilateral partially thrombosed iliac aneurysms associated with decreased lumen diameter precluding transfemoral access due to the relevant risk of distal embolization (Fig. 3B and D). Furthermore, the left subclavian artery showed unfavourable angulation (Fig. 3C), such that right carotid was finally chosen.
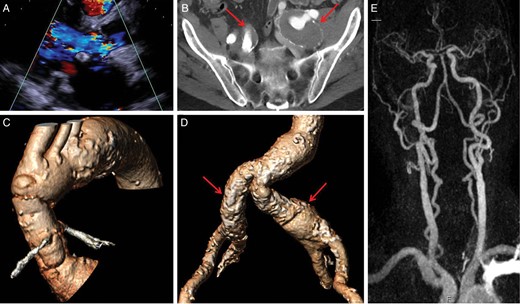
Summary of the pre-interventional diagnostic work-up of the third patient. (A) Parasternal long-axis enabling visualization of the relevant aortic regurgitation jet accompanied by beginning dilatation of the left ventricle; (B) transversal pelvic view (MSCT) showing dilatative arteriopathy of both iliac vessels. The arrows point thrombosed aneurysms of both common iliac arteries precluding transfemoral access; (C) three-dimensional reconstruction of the ascending aorta and the aortic arch enhancing unfavourable angulation of the left subclavian and carotid arteries. The right carotid offers the most direct access route; (D) three-dimensional reconstruction of the iliofemoral vascular axis showing bilateral involvement by dilatative arteriopathy; (E) MR-angiography of the supra-aortic vessels excluding stenoses of the carotid arteries and confirming patency of the circle of Willis.
RESULTS
All three patients underwent unproblematic TAVI and had a favourable post-procedural course without complications and were alive at 6 months of the follow-up. The implantation steps are illustrated in Fig. 4. Post-procedural clinical and echocardiographic results are summarized in Table 1. In the second patient, normalization of left ventricular function was observed 9 months after the intervention. Doppler ultrasonography of the access vessel at 6 months revealed normal flow patterns without stenosis or visible vascular lesion. No adverse events fulfilling the Valve Academic Research Consortium II criteria were recorded during the first 6 months of the follow-up period. TTE 30 days after the intervention confirmed excellent valve function with only mild paravalvular leak in all patients.
| Age, Gender . | STS score . | ES I . | ES II . | Echocardiographic outcome . | NYHA class (before/at 6 months) . |
|---|---|---|---|---|---|
| 56, male | 9.4% | 12/31% | 16% | MG 6 mmHg, mild PVL, EOA 1.8 cm2 | IV/I |
| 74, male | 3.9% | 10/18% | 3.6% | MG 3 mmHg, mild PVL, EOA 2.2 cm2 | III/I |
| 84, male | 8.5% | 12/30% | 14% | MG 11 mmHg, mild PVL, EOA 1.7 cm2 | III/I |
| Age, Gender . | STS score . | ES I . | ES II . | Echocardiographic outcome . | NYHA class (before/at 6 months) . |
|---|---|---|---|---|---|
| 56, male | 9.4% | 12/31% | 16% | MG 6 mmHg, mild PVL, EOA 1.8 cm2 | IV/I |
| 74, male | 3.9% | 10/18% | 3.6% | MG 3 mmHg, mild PVL, EOA 2.2 cm2 | III/I |
| 84, male | 8.5% | 12/30% | 14% | MG 11 mmHg, mild PVL, EOA 1.7 cm2 | III/I |
EOA: effective orifice area; ES: EuroSCORE; MG: mean gradient; NYHA: New York Heart Association; PVL: paravalvular leak.
| Age, Gender . | STS score . | ES I . | ES II . | Echocardiographic outcome . | NYHA class (before/at 6 months) . |
|---|---|---|---|---|---|
| 56, male | 9.4% | 12/31% | 16% | MG 6 mmHg, mild PVL, EOA 1.8 cm2 | IV/I |
| 74, male | 3.9% | 10/18% | 3.6% | MG 3 mmHg, mild PVL, EOA 2.2 cm2 | III/I |
| 84, male | 8.5% | 12/30% | 14% | MG 11 mmHg, mild PVL, EOA 1.7 cm2 | III/I |
| Age, Gender . | STS score . | ES I . | ES II . | Echocardiographic outcome . | NYHA class (before/at 6 months) . |
|---|---|---|---|---|---|
| 56, male | 9.4% | 12/31% | 16% | MG 6 mmHg, mild PVL, EOA 1.8 cm2 | IV/I |
| 74, male | 3.9% | 10/18% | 3.6% | MG 3 mmHg, mild PVL, EOA 2.2 cm2 | III/I |
| 84, male | 8.5% | 12/30% | 14% | MG 11 mmHg, mild PVL, EOA 1.7 cm2 | III/I |
EOA: effective orifice area; ES: EuroSCORE; MG: mean gradient; NYHA: New York Heart Association; PVL: paravalvular leak.
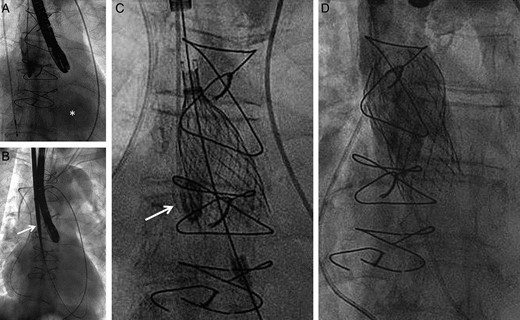
Implantation steps using patient two as an example. (A) Angiography of the ascending aorta with rapid opacification of the left ventricle (asterisk) confirming severe aortic regurgitation; (B) following surgical cut-down, introduction of the 18F sheath (arrow) via the right carotid artery; (C) deployment of the 29 mm Medtronic CoreValve using a pigtail placed in the right coronary cusp (arrow) as an anatomic marker of the annulus plane; (D) final angiography excluding relevant paravalvular regurgitation. Due to the limited diameter of the conduit (27 mm) the upper part of the nitinol frame was not fully deployed.
DISCUSSION
TAVI is currently a widely accepted therapeutic option for patients at high risk for open-heart surgery, particularly in patients already having undergone previous cardiac surgery and in those with relevant comorbidities (especially chronic lung disease, severely diminished LVEF, severe pulmonary hypertension and chronic renal failure). In the PARTNER trials, TAVI was shown to be superior to medical treatment (PARTNER B) [11] and to result in similar mortality compared with cardiac surgery in high-risk patients (PARTNER A) [12]. Recently, TAVI using the Medtronic CoreValve was associated with a significantly lower mortality at 1 year when compared with conventional aortic valve replacement in patients at increased surgical risk [1]. The growing evidence supporting TAVI as an attractive alternative to surgery will inevitably result in an increased penetration of this strategy. Continuous technical improvements have led to reduced sheath profiles, and may even eliminate the use of any introducer sheath to facilitate vascular access and minimize the risk of vascular complications. Thanks to this technical evolution, most patients can now be treated via the transfemoral route, which is generally considered to be the least invasive and thus the preferred route for TAVI. In many centres, the transapical route is established as a second option since its use is supported by broad safety and feasibility data with favourable clinical outcomes [13, 14]. Easy valve insertion and placement due to the short distance between access and deployment sites, as well as a low access-related complication rate are relevant strengths of the transapical approach. Similar to the transfemoral access, both balloon-expandable and self-expanding TAVI systems are available, enabling device selection in consideration of annulus anatomy and calcification level.
However, as the number and complexity of interventions inexorably grows, there is still room for alternative approaches. Recently, successful implantation of both balloon-expandable and self-expanding valves via the transcarotid access were reported by the same group [9, 15]. During this early experience, retrograde dissection of the common carotid artery and the ascending aorta accompanied by transient hemiparesis without long-term sequelae occurred in the first patient [9]. In another study, including 12 patients treated via the transcarotid access, Modine et al. reported one transient ischaemic attack (7%), but no vascular access site complications. Successful valve implantation was achieved in 100% of the patients [16]. More recently, surgical cut-down has been described as being safely performed under local anaesthesia only [17, 18]. Azmoun et al. reported procedural outcomes of 19 patients. In 2 patients, loss of consciousness occurred shortly after cross-clamping of the common carotid artery requiring the use of a femoro-carotid shunt, which finally allowed uneventful valve implantation. No cerebrovascular event and no access site-related complications were observed [18]. So far, the available data seem to confirm the safety and feasibility of TAVI implantation via the transcarotid access.
In the present series, we aim to show that patients with complex vascular anatomy may benefit from an alternative access such as the transcarotid route. Especially among patients presenting with vertical elongation of the ascending aorta, this approach is very attractive and a valuable alternative to the trans-subclavian access, which is not always technically feasible. Due to short distance between the puncture site and the valvular level, the transcarotid route offers a very well-controlled release of a self-expanding TAVI prosthesis comparable with the direct aortic approach. The latter, however, requires a more complex access site preparation using ministernotomy or a right thoracotomy, and carries the risk of cerebrovascular events during manipulation of a diseased ascending aorta. Full surgical control of the access site (carotid artery) minimizes the risk of vascular complications and the use of local anaesthesia considerably simplifies the procedure. However, due to the absence of radio-opaque anatomic landmarks delimiting the landing zone, we chose to use TOE guidance requiring general anaesthesia in these specific situations.
This is the first report of valve-in-valve implantation in stentless aortic root conduits via the transcarotid route. Our experience confirms the excellent safety and feasibility of this procedure. TTE showed only mild paravalvular regurgitation and all patients experienced a dramatic improvement in symptoms.
CONCLUSION
The transcarotid approach can be safely performed for valve-in-valve procedures using the Medtronic CoreValve. From an anatomical point of view, it offers direct access to the implantation plane allowing accurate positioning and deployment of the valve.
Funding
Research grant to the institution (University of Berne) from Medtronic.
Conflict of interest: Christoph Huber has received proctoring and lecture fees from Edwards Life Sciences and Symetis; consultancy for Medtronic. Bernhard Meier received educational and research support to the institution from Abbott, Cordis, Boston Scientific, St Jude and Medtronic and also lecture fees from St Jude. Stephan Windecker has received research grants to the institution from Abbott, Boston Scientific, Biosensors, Cordis, Medtronic and St Jude. Peter Wenaweser has received proctoring and lecture fees from Edwards Lifesciences and Medtronic.
REFERENCES
Author notes
The first two authors contributed equally.
- aorta
- aortic valve insufficiency
- aortic valve stenosis
- echocardiography
- conduit implant
- bioprosthesis
- anesthesia, general
- drug administration routes
- follow-up
- objective (goal)
- surgical procedures, operative
- vomiting
- treatment outcome
- vascular access
- supraaortic valve area
- transcatheter aortic-valve implantation
- paravalvular regurgitation
- prostheses
- transcatheter valve-in-valve implantation
- transcatheter heart valve prosthesis




