-
PDF
- Split View
-
Views
-
Cite
Cite
Henri de Lesquen, Jean-Philippe Avaro, Lucile Gust, Robert Michael Ford, Fabien Beranger, Claudia Natale, Pierre-Mathieu Bonnet, Xavier-Benoît D'Journo, Surgical management for the first 48 h following blunt chest trauma: state of the art (excluding vascular injuries), Interactive CardioVascular and Thoracic Surgery, Volume 20, Issue 3, March 2015, Pages 399–408, https://doi.org/10.1093/icvts/ivu397
Close - Share Icon Share
Abstract
This review aims to answer the most common questions in routine surgical practice during the first 48 h of blunt chest trauma (BCT) management. Two authors identified relevant manuscripts published since January 1994 to January 2014. Using preferred reporting items for systematic reviews and meta-analyses statement, they focused on the surgical management of BCT, excluded both child and vascular injuries and selected 80 studies. Tension pneumothorax should be promptly diagnosed and treated by needle decompression closely followed with chest tube insertion (Grade D). All traumatic pneumothoraces are considered for chest tube insertion. However, observation is possible for selected patients with small unilateral pneumothoraces without respiratory disease or need for positive pressure ventilation (Grade C). Symptomatic traumatic haemothoraces or haemothoraces >500 ml should be treated by chest tube insertion (Grade D). Occult pneumothoraces and occult haemothoraces are managed by observation with daily chest X-rays (Grades B and C). Periprocedural antibiotics are used to prevent chest-tube-related infectious complications (Grade B). No sign of life at the initial assessment and cardiopulmonary resuscitation duration >10 min are considered as contraindications of Emergency Department Thoracotomy (Grade C). Damage Control Thoracotomy is performed for either massive air leakage or refractive shock or ongoing bleeding enhanced by chest tube output >1500 ml initially or >200 ml/h for 3 h (Grade D). In the case of haemodynamically stable patients, early video-assisted thoracic surgery is performed for retained haemothoraces (Grade B). Fixation of flail chest can be considered if mechanical ventilation for 48 h is probably required (Grade B). Fixation of sternal fractures is performed for displaced fractures with overlap or comminution, intractable pain or respiratory insufficiency (Grade D). Lung herniation, traumatic diaphragmatic rupture and pericardial rupture are life-threatening situations requiring prompt diagnosis and surgical advice. (Grades C and D). Tracheobronchial repair is mandatory in cases of tracheal tear >2 cm, oesophageal prolapse, mediastinitis or massive air leakage (Grade C). These evidence-based surgical indications for BCT management should support protocols for chest trauma management.
INTRODUCTION
One-third of patients involved in a road traffic accident have a significant chest injury. Chest trauma is the leading cause of death after brain injury with an associated mortality up to 25%. It is the first cause of preventable death. Less than 10% of blunt chest injuries require operative intervention, such as thoracotomy or thoracoscopy [1–3].
This review aims to answer the most common questions in routine surgical practice during the first 48 h of management of blunt chest trauma (BCT). It will not take into account the vascular injuries, already subjected to recommendations. Every clinician involved in the management of chest trauma could be interested by this review, which deals with chest tube insertion, damage control surgery, chest wall reconstruction and tracheobronchial repair.
METHODS
Two authors (H.D.L. and X.B.D.J.) identified relevant manuscripts in Medline and Sciencedirect published since January 1994 to January 2014 concerning BCT. The Mesh terms used were as follows: ‘Thorax/injuries’, ‘Thorax/surgery’, ‘Thoracoscopy/therapeutic use’, ‘Thoracostomy/therapeutic use’; ‘Thoracotomy/therapeutic use’; ‘Thoracic wall/surgery’; ‘Hemothorax/surgery’; ‘Pneumothorax/surgery’ and ‘Bronchi/injuries’. Keywords used are as follows: ‘blunt chest trauma’; ‘traumatic hemothorax’; ‘traumatic pneumothorax’; ‘emergency department thoracotomy’; ‘urgent thoracotomy’; ‘video-assisted thoracic surgery AND trauma’; ‘flail chest’; ‘lung herniation’; ‘diaphragmatic rupture’; ‘pericardial rupture’ and ‘tracheobronchial injury’. Using the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) statement, we focused on the surgical management of thoracic trauma, excluded both child and vascular injuries and selected only 80 original full text articles for this review (Fig. 1).
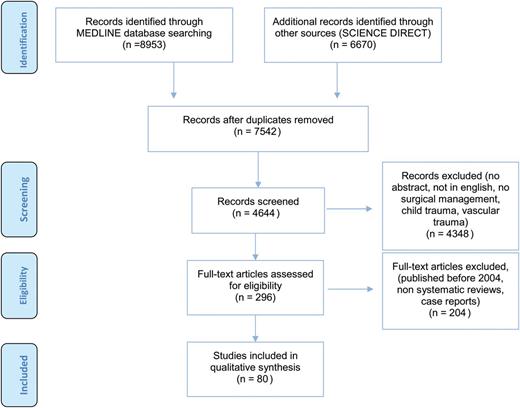
A preferred reporting items for systematic reviews and meta-analyses flow diagram.
Practice guidelines levels of evidence and grades of recommendations used by the National Guideline Clearinghouse (www.guidelines.gov) have been applied.
Levels of evidence are as follows:
IA: evidence from meta-analysis of randomized controlled trial;
IB: evidence from at least one randomized controlled trial;
IIA: evidence from at least one controlled study without randomization;
IIB: evidence from at least one other type of quasiexperimental study;
III: evidence from non-experimental descriptive studies, such as comparative studies, correlation studies and case–control studies;
IV: evidence from expert committee reports or opinions or clinical experience of respected authorities, or both.
Grades of recommendations are as follows:
A: directly based on Level I evidence;
B: directly based on Level II evidence or extrapolated recommendations from Level I evidence;
C: directly based on Level III evidence or extrapolated recommendations from Level I or II evidence;
D: directly based on Level IV evidence or extrapolated recommendations from Level I, II or III evidence.
RESULTS
Chest tube in blunt chest trauma management
Traumatic pneumothorax: is chest tube insertion required?
Almost 90% of chest traumas are managed with a chest tube insertion [4] before any further consideration; this is done to maintain or regain respiratory and haemodynamic stability as recommended by the American College of Surgeons [5] and the British Thoracic Society [6]. The following three situations should be identified as indications of chest tube insertion in the first 48 h.
Tension pneumothorax
Traumatic symptomatic pneumothorax
Secondary worsening occult pneumothorax
Tension pneumothorax
A tension pneumothorax is the leading cause of preventable death in chest trauma [3]. The lack of breath sound, associated with subcutaneous emphysema and desaturation on mechanical ventilation, allows the diagnosis with a sensitivity close to 100% [7]. Neither clinical diagnosis nor treatment should be delayed. It is hardly recommended to perform immediate decompression by inserting a 5-cm large bore needle to the second intercostal space in the mid-clavicular line due to access and carrier reasons [8, 9]. Success of needle decompression (50–95%) depends on thickness of the chest wall. Chest tube insertion should systematically follow, placed safely in the fourth or fifth intercostal space in the anterior axillary line [6, 10].
Tension pneumothorax should be promptly diagnosed and treated by inserting a large bore needle in to the second intercostal space crossing the mid-clavicular line closely followed with a chest tube insertion (Grade D, extrapolated recommendations from Level III evidences).
Traumatic symptomatic pneumothorax
Management with a chest tube is recommended in case of traumatic pneumothorax associated with respiratory distress, shock or impaired vigilance because of the high probability of tension pneumothorax [8, 11]. According to advanced trauma life support (ATLS) courses, chest X-ray and ultrasound, in an extended focused assessment with sonography for trauma process, are sufficient for traumatic pneumothorax assessment [5]. Conservative treatment is possible in selected stable patients: carrying small pneumothoraces (<1.5 cm at the third rib), without underlying respiratory disease or need for positive pressure ventilation [12]. Nearly 10% of pneumothoraces managed conservatively require late chest tube insertion due to progressing pneumothorax without symptoms [11, 12].
All traumatic pneumothoraces should be considered for chest tube insertion. However, observation is possible for selected patients without respiratory disease or need for positive pressure ventilation presenting with small unilateral pneumothoraces (Grade D, Level IV evidences).
Secondary worsening of occult pneumothorax
Occult pneumothorax is, by definition, highlighted by a Computerized Tomography (CT) scan and not diagnosed by chest X-ray. It involves 2–16% of all chest trauma patients and up to 64% of patients in Intensive Care Unit (ICU) [13, 14]. The indication for chest tube insertion in this situation continues to be debated. While some have proposed classification or radiological scores depending on the size of occult pneumothoraces in order to provide a prophylactic drainage [15, 16], none of the three last randomized controlled trials found any difference in terms of length of stay and mortality between tube thoracostomy and observation in occult pneumothoraces management [17–19]. The risk of tension pneumothoraces under mechanical ventilation in observation groups was acceptable without pneumothorax-related death [13–20]. No evidence supports the monitoring of occult pneumothoraces by repeated CT scan [17]. Moore published the largest prospective multicentre study including 569 BCTs with occult pneumothoraces. Twenty-one percent of patients were treated with chest drainage and 79% were monitored. Late drainage concerned 6% of patients (73 patients) due to the progression of pneumothoraces, haemothoraces or respiratory distress; only 14% of them (10 of 73) were under positive pressure ventilation. None presented with tension pneumothorax. Both length of hospital stay and length of ICU stay were longer in cases of late chest tube insertion. There was no difference in mortality between the two groups [20] (Table 1).
| References . | Patients . | Design . | Outcomes . | Key results . | |
|---|---|---|---|---|---|
| Chest tube . | Observation . | ||||
| Brasel et al. (1999) USA [17] | n = 39 | RCT | PPV (%) | 50 | 42.8 |
| Late CT (%) | 0 | 9.5 | |||
| Tension PTX (%) | 0 | 0 | |||
| LOS (days) | 5 | 8 | |||
| Death (%) | 0 | 0 | |||
| Wilson et al. (2009) Canada [21] | n = 68 | Retrospective cohort study | PPV (%) | 82.8 | 46.4 |
| Late CT (%) | 0 | 0 | |||
| Tension PTX (%) | 0 | 0 | |||
| LOS (days) | 17.4 | 10* | |||
| Death (%) | 11.4 | 9.1 | |||
| Ouellet et al. (2009) Canada [18] | n = 24 | RCT | PPV (%) | 100 | 100 |
| Late CT (%) | 0 | 31 | |||
| Tension PTX (%) | 0 | 0 | |||
| LOS (days) | 10 | 16 | |||
| Death (%) | 22 | 15 | |||
| Lee et al. (2010) China [16] | n = 36 | Retrospective cohort study | PPV (%) | 100 | 22 |
| Late CT (%) | 0 | 0 | |||
| Tension PTX (%) | 0 | 0 | |||
| LOS (days) | NA | NA | |||
| Death | 37 | 0 | |||
| Kirpatrick et al. (2013) Canada [19] | n = 90 | RCT | PPV (%) | 100 | 100 |
| Late CT (%) | 0 | 20 | |||
| Tension PTX (%) | 0 | 2 | |||
| LOS (days) | 18 | 16 | |||
| Death (%) | 4 | 4 | |||
| References . | Patients . | Design . | Outcomes . | Key results . | |
|---|---|---|---|---|---|
| Chest tube . | Observation . | ||||
| Brasel et al. (1999) USA [17] | n = 39 | RCT | PPV (%) | 50 | 42.8 |
| Late CT (%) | 0 | 9.5 | |||
| Tension PTX (%) | 0 | 0 | |||
| LOS (days) | 5 | 8 | |||
| Death (%) | 0 | 0 | |||
| Wilson et al. (2009) Canada [21] | n = 68 | Retrospective cohort study | PPV (%) | 82.8 | 46.4 |
| Late CT (%) | 0 | 0 | |||
| Tension PTX (%) | 0 | 0 | |||
| LOS (days) | 17.4 | 10* | |||
| Death (%) | 11.4 | 9.1 | |||
| Ouellet et al. (2009) Canada [18] | n = 24 | RCT | PPV (%) | 100 | 100 |
| Late CT (%) | 0 | 31 | |||
| Tension PTX (%) | 0 | 0 | |||
| LOS (days) | 10 | 16 | |||
| Death (%) | 22 | 15 | |||
| Lee et al. (2010) China [16] | n = 36 | Retrospective cohort study | PPV (%) | 100 | 22 |
| Late CT (%) | 0 | 0 | |||
| Tension PTX (%) | 0 | 0 | |||
| LOS (days) | NA | NA | |||
| Death | 37 | 0 | |||
| Kirpatrick et al. (2013) Canada [19] | n = 90 | RCT | PPV (%) | 100 | 100 |
| Late CT (%) | 0 | 20 | |||
| Tension PTX (%) | 0 | 2 | |||
| LOS (days) | 18 | 16 | |||
| Death (%) | 4 | 4 | |||
LOE: level of evidence; PPV: positive pressure ventilation; CT: chest tube; PTX: pneumothorax; LOS: length of stay; NS: not significant; NA: not available.
*P < 0.05.
| References . | Patients . | Design . | Outcomes . | Key results . | |
|---|---|---|---|---|---|
| Chest tube . | Observation . | ||||
| Brasel et al. (1999) USA [17] | n = 39 | RCT | PPV (%) | 50 | 42.8 |
| Late CT (%) | 0 | 9.5 | |||
| Tension PTX (%) | 0 | 0 | |||
| LOS (days) | 5 | 8 | |||
| Death (%) | 0 | 0 | |||
| Wilson et al. (2009) Canada [21] | n = 68 | Retrospective cohort study | PPV (%) | 82.8 | 46.4 |
| Late CT (%) | 0 | 0 | |||
| Tension PTX (%) | 0 | 0 | |||
| LOS (days) | 17.4 | 10* | |||
| Death (%) | 11.4 | 9.1 | |||
| Ouellet et al. (2009) Canada [18] | n = 24 | RCT | PPV (%) | 100 | 100 |
| Late CT (%) | 0 | 31 | |||
| Tension PTX (%) | 0 | 0 | |||
| LOS (days) | 10 | 16 | |||
| Death (%) | 22 | 15 | |||
| Lee et al. (2010) China [16] | n = 36 | Retrospective cohort study | PPV (%) | 100 | 22 |
| Late CT (%) | 0 | 0 | |||
| Tension PTX (%) | 0 | 0 | |||
| LOS (days) | NA | NA | |||
| Death | 37 | 0 | |||
| Kirpatrick et al. (2013) Canada [19] | n = 90 | RCT | PPV (%) | 100 | 100 |
| Late CT (%) | 0 | 20 | |||
| Tension PTX (%) | 0 | 2 | |||
| LOS (days) | 18 | 16 | |||
| Death (%) | 4 | 4 | |||
| References . | Patients . | Design . | Outcomes . | Key results . | |
|---|---|---|---|---|---|
| Chest tube . | Observation . | ||||
| Brasel et al. (1999) USA [17] | n = 39 | RCT | PPV (%) | 50 | 42.8 |
| Late CT (%) | 0 | 9.5 | |||
| Tension PTX (%) | 0 | 0 | |||
| LOS (days) | 5 | 8 | |||
| Death (%) | 0 | 0 | |||
| Wilson et al. (2009) Canada [21] | n = 68 | Retrospective cohort study | PPV (%) | 82.8 | 46.4 |
| Late CT (%) | 0 | 0 | |||
| Tension PTX (%) | 0 | 0 | |||
| LOS (days) | 17.4 | 10* | |||
| Death (%) | 11.4 | 9.1 | |||
| Ouellet et al. (2009) Canada [18] | n = 24 | RCT | PPV (%) | 100 | 100 |
| Late CT (%) | 0 | 31 | |||
| Tension PTX (%) | 0 | 0 | |||
| LOS (days) | 10 | 16 | |||
| Death (%) | 22 | 15 | |||
| Lee et al. (2010) China [16] | n = 36 | Retrospective cohort study | PPV (%) | 100 | 22 |
| Late CT (%) | 0 | 0 | |||
| Tension PTX (%) | 0 | 0 | |||
| LOS (days) | NA | NA | |||
| Death | 37 | 0 | |||
| Kirpatrick et al. (2013) Canada [19] | n = 90 | RCT | PPV (%) | 100 | 100 |
| Late CT (%) | 0 | 20 | |||
| Tension PTX (%) | 0 | 2 | |||
| LOS (days) | 18 | 16 | |||
| Death (%) | 4 | 4 | |||
LOE: level of evidence; PPV: positive pressure ventilation; CT: chest tube; PTX: pneumothorax; LOS: length of stay; NS: not significant; NA: not available.
*P < 0.05.
Occult pneumothoraces should be managed by observation with daily chest X-rays (Grade B, Level II evidences and extrapolated recommendations from Level I evidences).
Positive pressure ventilation is not an argument or indication for chest tube insertion in case of occult pneumothoraces (Grade C, Level III evidences and extrapolated recommendations from Level I evidences).
Traumatic haemothorax: is a chest tube insertion required?
Traumatic haemothorax
In trauma management, any acute pleural effusion large enough to be detected on chest X-ray should be drained. It should be one to evaluate the volume of blood loss and to reduce the risk of clotted haemothorax, empyema or fibrothorax [5]. It is visible as a meniscus at a volume of 200 ml and as hemi-diaphragm obliteration at a volume of ∼500 ml. According to ATLS courses and our clinical experience, traumatic haemothoraces >500 ml should be managed with a large bore chest tube.
All symptomatic traumatic haemothoraces or haemothoraces >500 ml should be treated by chest tube insertion (Grade D, Level IV evidences).
Occult haemothorax
Occult haemothorax is detected by CT scan and not by chest X-ray examination. It has been reported in 20–30% of severe BCT [22]. Simple X-ray monitoring should be recommended. Chest tube is inserted for increasing haemothorax or respiratory distress [22–24]. However, association of mechanical ventilation and/or occult pneumothorax (50% of cases) should not be considered as arguments for chest tube insertion [24]. The thickness of the haemothorax is an important prognostic factor: patients whose haemothorax thickness is >1.5 cm are four times more likely to undergo chest tube insertion [23, 24].
Occult haemothoraces should be managed with daily chest X-rays (Grade C, Level III evidences).
Chest tube insertion for blunt chest trauma: should antibiotics be used?
Antibiotic prophylaxis before chest tube insertion remains a controversial issue in BCT. Following the Sanabria's work, Bosman et al. [25] published in 2012 a meta-analysis including 11 RCTs encompassing 1241 patients and 1234 chest tubes from 1979 to 2009. Penetrating trauma (69.4%) was more frequent. Antibiotic prophylaxis, using first-generation cephalosporins, decreases the incidence of overall infectious complications [odd ratio (OR): 0.24 (0.12–0.49)] and empyema [OR: 0.32 (0.17–0.61)] after chest tube insertion. In subgroup analysis, antibiotic therapy was effective in patients with penetrating chest trauma [OR: 0.28 (0.14–0.57)] but had no significant effect after BCT [OR: 1.30 (0.46–3.67)]. Moore et al. [26] concluded with lack of data to recommend antibiotic therapy in chest tube insertion for the Eastern Association for the Surgery of Trauma. In 2013, an observational prospective study, using the Post-Traumatic Retained Hemothorax database of the American Association for the Surgery of Trauma, points out three independent risk factors for pneumonia after thoracic drainage for post-traumatic haemothorax and/or pneumothorax:injury severity score (ISS) >25 [OR: 7.1; (3.1; 16.4), P < 0.001], BCT [OR: 3.5; (1.7; 7.2), P = 0.001] and lack of antibiotic prophylaxis during chest tube insertion [OR: 2.6; (1.30; 5.4), P = 0.01] [27]. Thus, the authors suggested a systematic antibiotic prophylaxis for severe BCT. Ideally given in the first 24 h (78), Cefazolin is preferred, in order to target Staphylococcus and Haemophilus influenzae [25, 27, 28].
Periprocedural antibiotics should be used to prevent chest-tube-related infectious complication in both penetrating and BCT (Grade B, Level II evidences and extrapolated recommendations from Level I evidences).
Haemostatic surgery in blunt chest trauma management
The need for surgical haemostasis during the first 48 h of BCT management depends on three levels of emergency:
First, ‘in extremis’ surgical exploration for an agonal patient, represented by Emergency Department Thoracotomy (EDT).
Secondly, urgent surgical exploration in a drained patient with active bleeding or haemodynamic instability. This is damage control thoracotomy (DCT).
Thirdly and finally, surgical exploration within the first 48 h, either to assess intra-thoracic injuries or to evacuate residual haemothoraces. These clinical situations represent the main indications for video-assisted thoracic surgery (VATS) in trauma.
Should emergency department thoracotomy be performed in blunt chest trauma management?
EDT in chest trauma is indicated when the patient presents in cardiac arrest despite resuscitation. EDT allows both diagnosis and treatment of post-traumatic cardiorespiratory arrest. EDT provides a direct access to the heart, lungs and great vessels and should enable effective resuscitation (evacuation of pleural or pericardial effusion, open cardiac massage and cross-clamping the descending aorta) [29]. There are no Level I data on the usefulness of the EDT in BCT. For ethical reasons, it seems unlikely that there will ever be one.
Reviewing 25 years of published data and including more than 4600 patients, Rhee et al. shown that survival rate after EDT were up 8.8% in penetrating chest trauma compared with 1.4% in BCT [30]. Khorsandi et al. specifically evaluated EDT for BCT in a systematic review of 24 studies, 2 systematic reviews and 22 retrospective cohort series. The authors reported a survival rate from 0 to 6%. One study reported a higher survival rate (12.2%) [31], the majority of survivors were in a vegetative state [32]. Several authors agree to not perform EDT if no sign of life is noticed at the initial assessment [30, 33]. Recently, a prospective multicentre study of 18 US centres showed that the EDT appeared as ‘futile’ in three situations of chest trauma: (i) prehospital cardiopulmonary resuscitation exceeded 10 min without response after BCT, (ii) prehospital cardiopulmonary resuscitation exceeded 15 min without response after penetrating chest trauma and (c) persistence of an initial asystole without compressive pericardial effusion [34–36]. Powell et al. [37] suggested that the prehospital cardiopulmonary resuscitation should not exceed 5 min without response after BCT. Some suggest benefit of EDT in BCT with regard to organ retrieval [38].
No signs of life at the initial assessment and cardiopulmonary resuscitation duration >10 min should be considered as contraindications of EDT in BCT management (Grade C, Level III evidences and extrapolated level II evidences).
When should damage control thoracotomy be performed in blunt chest trauma management?
The principle of damage control surgery, first developed in abdominal surgery, is to control during an initial and fast procedure, an ongoing bleeding in the presence of the ‘lethal triad’: acidosis, coagulopathy and hypothermia. Applied to chest trauma, intra-thoracic injuries require simpler, faster and more definitive procedure to stop bleeding or air leak process. Like EDT developed for agonal patients, DCT aims to manage casualties with haemorrhagic shock or massive air leakage with a standardized approach of the heart, lungs or great vessels. Abbreviated thoracotomy performed for life-threatening injury decreases the predicted 59% mortality rate to an actual 36% mortality rate [39]. In BCT management, DCT should be considered for patients with ongoing bleeding, haemodynamic instability or massive air leakage. However, most studies did not specify the mechanism of injury and included both blunt and penetrating trauma. Karmy–Jones et al. [40] stated that the mortality rate increased with drain flow and suggest considering a bleeding of 1500 ml/24 h as an indication for thoracotomy regardless of the mechanism of injury. ATLS courses recommend to choose thoracotomy if chest tube output exceeds 1500 ml/24 h or 200 ml/h over three consecutive hours in case of haemodynamic stability [5]. Thoracotomy for BCT is associated with 3–10 times higher mortality than penetrating chest trauma, primarily due to systemic injuries [41, 42]. Non-therapeutic thoracotomies are more frequent in BCT and should not be considered on the basis of chest tube output alone [41].
In BCT management, DCT should be performed for either massive air leakage or refractive shock or ongoing chest bleeding enhanced by chest tube output >1500 ml initially or >200 ml/h for 3 h (Grade D, Level IV evidences and extrapolated from Level III evidences).
When should video-assisted thoracic surgery be performed in blunt chest trauma management?
Indications of VATS have been extended to the management of chest trauma in the 1990s for diagnostic and therapeutic purposes. Its feasibility has been demonstrated in several prospective and retrospective studies [43–48]. This approach has a number of pros: chest exploration, minimally invasive surgery, chest tube setting and reduced postoperative pain. Trauma surgeons use it for different indications in haemodynamically stable patients: empyema, persistent pneumothorax, retained haemothorax, mediastinal and diaphragmatic exploration. For this review, only persistent pneumothorax, retained haemothorax, diaphragmatic and pleuro-pericardial ruptures are described. Persistent pneumothorax associates persistent air leak and radiological pneumothorax within 72 h after chest tube insertion. Two retrospective studies demonstrated the economic value of early VATS in this indication [49, 50]. A retained haemothorax is a persistent effusion after chest tube insertion on chest X-ray examination. For several authors retained haemothorax over 300 ml should be considered for surgery because 20–30% of these are associated with empyema and/or pneumonia [51–53]. The American Association for the Surgery of Trauma published the most important prospective study concerning the surgical management of retained haemothorax in BCT with a 328-patient cohort [51]. About 33% of the patients underwent VATS, 25% required more than two procedures and 5% over three procedures to obtain a complete washout. Thoracotomy for failed VATS was necessary for 20% of the patients. Independent factors of success were as follows: no diaphragmatic wound, periprocedural antibiotic therapy during chest tube insertion and retained haemothorax less than 900 ml. Two randomized studies considered VATS as beneficial for retained haemothorax [43, 54]. Meyer et al. compared VATS vs a second chest tube insertion, over a period of 4 years, including 39 patients in each group. VATS decreased the length of chest drainage (2.53 vs 4.50 days, P < 0.02), length of stay after randomization (3.60 vs 7.21 days, P < 0.02), overall length of stay (5.40 vs 8.13 days, P < 0.02) and hospital costs ($7689 vs $13,273, P < 0.02). Conversely, the second chest tube insertion was associated with a high failure rate and led to a secondary surgical treatment in ∼40% of cases. VATS should be preferred over second chest tube insertion for management of retained haemothorax [54]. Cobanoglu et al. [43] compared chest tube insertion vs VATS in first line therapy for BCT, over a period of 2 years, randomizing 60 patients. VATS decreased the length of stay and the number of reoperation (chest tube insertion, thoracoscopy and thoracotomy). In the chest tube group, indications of reoperation were clotted haemothorax (23%), empyema (13%), fibrothorax (6%) and ongoing bleeding over 100 ml/h (3%). Furthermore, time to surgery seems to be important: Smith et al. [44] suggested to perform the procedure in the first 5 days, Vassiliu et al. [46] insisted that surgery would be easier during the first 3 days after trauma, while the protocol performed by Fabrucci et al. [45] found a net profit for systematic VATS in the first 48 h for both persistent pneumothorax and retained haemothorax with ongoing bleeding >100 ml/h (Table 2).
| References . | Patients . | Design . | Outcomes . | Key results . | |
|---|---|---|---|---|---|
| CT . | VATS . | ||||
| Meyer et al. (1997) USA [54] | n = 39 | RCT | Reoperation | 42% | 0% |
| BCT = 15% | CT duration | 4.5 | 2.53 | ||
| Indication: RH | LOS | 8.13 | 5.4* | ||
| Schermer et al. (1999) USA [50] | n = 39 | Prospective cohort study | Reoperation | 22.2 | 0a |
| BCT = 70% | CT duration | 11.8 | 8.1* | ||
| Indication: PP | LOS | 16.5 | 8.4* | ||
| Fabbrucci et al. (2008) Italy [45] | n = 81 | Retrospective cohort study | Reoperation | 0 | 0 |
| BCT = 97% | CT duration | 5.7 | 6,3 | ||
| Indication: PP, RH | LOS | 7 | 7 | ||
| DuBose et al. (2011) USA [52] | n = 328 | Prospective cohort study | Reoperation | 35.2 | 30 |
| BCT = 49% | |||||
| Indication: RH | |||||
| Cobanoğlu U et al. (2011) Turkey [43] | n = 60 | RCT | Reoperation | 50 | 0 |
| BCT = 62% | CT duration | 7.19 | 4.84* | ||
| Indication: RH | LOS | 7.19 | 4.84* | ||
| References . | Patients . | Design . | Outcomes . | Key results . | |
|---|---|---|---|---|---|
| CT . | VATS . | ||||
| Meyer et al. (1997) USA [54] | n = 39 | RCT | Reoperation | 42% | 0% |
| BCT = 15% | CT duration | 4.5 | 2.53 | ||
| Indication: RH | LOS | 8.13 | 5.4* | ||
| Schermer et al. (1999) USA [50] | n = 39 | Prospective cohort study | Reoperation | 22.2 | 0a |
| BCT = 70% | CT duration | 11.8 | 8.1* | ||
| Indication: PP | LOS | 16.5 | 8.4* | ||
| Fabbrucci et al. (2008) Italy [45] | n = 81 | Retrospective cohort study | Reoperation | 0 | 0 |
| BCT = 97% | CT duration | 5.7 | 6,3 | ||
| Indication: PP, RH | LOS | 7 | 7 | ||
| DuBose et al. (2011) USA [52] | n = 328 | Prospective cohort study | Reoperation | 35.2 | 30 |
| BCT = 49% | |||||
| Indication: RH | |||||
| Cobanoğlu U et al. (2011) Turkey [43] | n = 60 | RCT | Reoperation | 50 | 0 |
| BCT = 62% | CT duration | 7.19 | 4.84* | ||
| Indication: RH | LOS | 7.19 | 4.84* | ||
RH: retained haemothorax; PP: persistent pneumothorax; BCT: blunt chest trauma.
aEleven patients with persistent air leak were excluded from VATS: 4 due to injuries requiring further ICU stay, 3 due to pneumonia, 2 patients were too small for dual lumen intubation and 2 needed further operations.
*P < 0.05.
| References . | Patients . | Design . | Outcomes . | Key results . | |
|---|---|---|---|---|---|
| CT . | VATS . | ||||
| Meyer et al. (1997) USA [54] | n = 39 | RCT | Reoperation | 42% | 0% |
| BCT = 15% | CT duration | 4.5 | 2.53 | ||
| Indication: RH | LOS | 8.13 | 5.4* | ||
| Schermer et al. (1999) USA [50] | n = 39 | Prospective cohort study | Reoperation | 22.2 | 0a |
| BCT = 70% | CT duration | 11.8 | 8.1* | ||
| Indication: PP | LOS | 16.5 | 8.4* | ||
| Fabbrucci et al. (2008) Italy [45] | n = 81 | Retrospective cohort study | Reoperation | 0 | 0 |
| BCT = 97% | CT duration | 5.7 | 6,3 | ||
| Indication: PP, RH | LOS | 7 | 7 | ||
| DuBose et al. (2011) USA [52] | n = 328 | Prospective cohort study | Reoperation | 35.2 | 30 |
| BCT = 49% | |||||
| Indication: RH | |||||
| Cobanoğlu U et al. (2011) Turkey [43] | n = 60 | RCT | Reoperation | 50 | 0 |
| BCT = 62% | CT duration | 7.19 | 4.84* | ||
| Indication: RH | LOS | 7.19 | 4.84* | ||
| References . | Patients . | Design . | Outcomes . | Key results . | |
|---|---|---|---|---|---|
| CT . | VATS . | ||||
| Meyer et al. (1997) USA [54] | n = 39 | RCT | Reoperation | 42% | 0% |
| BCT = 15% | CT duration | 4.5 | 2.53 | ||
| Indication: RH | LOS | 8.13 | 5.4* | ||
| Schermer et al. (1999) USA [50] | n = 39 | Prospective cohort study | Reoperation | 22.2 | 0a |
| BCT = 70% | CT duration | 11.8 | 8.1* | ||
| Indication: PP | LOS | 16.5 | 8.4* | ||
| Fabbrucci et al. (2008) Italy [45] | n = 81 | Retrospective cohort study | Reoperation | 0 | 0 |
| BCT = 97% | CT duration | 5.7 | 6,3 | ||
| Indication: PP, RH | LOS | 7 | 7 | ||
| DuBose et al. (2011) USA [52] | n = 328 | Prospective cohort study | Reoperation | 35.2 | 30 |
| BCT = 49% | |||||
| Indication: RH | |||||
| Cobanoğlu U et al. (2011) Turkey [43] | n = 60 | RCT | Reoperation | 50 | 0 |
| BCT = 62% | CT duration | 7.19 | 4.84* | ||
| Indication: RH | LOS | 7.19 | 4.84* | ||
RH: retained haemothorax; PP: persistent pneumothorax; BCT: blunt chest trauma.
aEleven patients with persistent air leak were excluded from VATS: 4 due to injuries requiring further ICU stay, 3 due to pneumonia, 2 patients were too small for dual lumen intubation and 2 needed further operations.
*P < 0.05.
In case of haemodynamically stable patients, early VATS should be performed for retained haemothoraces (Grade B, Level II evidences and extrapolated recommendations from Level I evidence).
Chest wall reconstruction in blunt chest trauma management
Ribs and sternal fractures: is open reduction and internal fixation needed?
Flail chest
Flail chest corresponds to three or more consecutive fractured ribs, causing paradoxical respiration in a typical clinical setting. Since the 1970s, surgical fixations have been developed in order to decrease chest pain, length of mechanical ventilation and overall related infectious complications [55–60]. However, there are only three prospective randomized studies comparing medical and surgical strategies [61–63]. Tanaka et al. [61] randomized 37 patients into two groups: surgical stabilization (Judet staples) vs internal stabilization by positive pressure ventilation. All patients had at least six rib fractures and sustained positive pressure ventilation for 5 days. Eighteen patients were randomized in the surgery group and 19 in the internal stabilization group. Surgical treatment decreased significantly the length of mechanical ventilation (10.8 vs 18.3 days), length of stay in ICU (16.5 vs 26.8 days) and the rate of pneumonia (22 vs 90%). Economic outcomes favoured the surgical strategy. In another study performed by Granetzny et al. [62], 40 patients were randomized into two groups: surgical stabilization vs external stabilization with strapping. Patients were randomized within 24 h after admission. Similar clinically significant results were found regarding length of mechanical ventilation (2 vs 12 days), ICU length of stay (9.6 vs 14.6 days) and the rate of pneumonia (10 vs 50%). This study also reported better lung function tests at 2-month follow-up for the group receiving surgical stabilization. In 2013, Slobogean et al. published a meta-analysis of 11 studies comparing surgical treatment vs conservative treatment, published between 1972 and 2009, with 753 patients. Surgery decreased the length of mechanical ventilation (8 days) and the incidence of pneumonia [OR: 0.18; (0.11–0.32)]. Similar results were found by Leinicke et al. [64]. One of the main limits according to this meta-analysis was the mix of several techniques and indications. In fact, there was a huge variance in the indications: thoracotomy performed for another reason, need for positive pressure ventilation, unsuccessful non-operative treatment, bilateral flail chest, chest wall deformity or paradoxical movement. Several surgical techniques were also used such as K wires, sutures, plates, Judet or Adkin struts. Only two cohort studies evaluated chest pain and concluded two non-significant results [65]. At last, Marasco et al. [63] published recently a well-designed controlled trial but only ICU length of stay was significantly reduced in the operative group (11.9 vs 15 days, P = 0.03). They included patients with three or more consecutive rib fractures who were ventilator-dependent for probably >48 h (Table 3).
| References . | Patients . | Design . | Outcomes . | Key results . | |
|---|---|---|---|---|---|
| OT . | NOT . | ||||
| Ahmed et al. (1995) UAE [55] | n = 64 | Retrospective cohort study | DMV (days) | 3.9 | 15 |
| ICULOS (days) | 9 | 21 | |||
| Pneumonia (%) | 15 | 50 | |||
| Mortality (%) | 8 | 29 | |||
| Karev (1997) Ukraine [56] | n = 40 | Retrospective cohort study | DMV (days) | 2.3 | 6.3* |
| ICULOS (days) | NA | NA | |||
| Pneumonia (%) | 15 | 34* | |||
| Mortality (%) | 22.5 | 46† | |||
| Voggenreiter (1998) Germany [57] | N = 20 | Retrospective cohort study | DMV (days) | 6.5 | 27* |
| ICULOS (days) | NA | NA | |||
| Pneumonia (%) | 15 | 39* | |||
| Mortality (%) | NA | NA | |||
| Tanaka et al. (2002) Japan [61] | n = 37 | RCT | DMV (days) | 10.8 | 18* |
| ICULOS (days) | 16.5 | 27* | |||
| Pneumonia (%) | 24 | 77* | |||
| Mortality (%) | NA | NA | |||
| Balci et al. (2004) Turkey [58] | n = 64 | Retrospective cohort study | DMV (days) | 3.1 | 7.2 |
| ICULOS (days) | NA | NA | |||
| Pneumonia (%) | NA | NA | |||
| Mortality (%) | 11 | 27 | |||
| Granetzny et al. (2005) Germany [62] | n = 40 | RCT | DMV (days) | 2 | 12 |
| ICULOS (days) | 9 | 14 | |||
| Pneumonia (%) | 10 | 50 | |||
| Mortality (%) | 10 | 15 | |||
| Nirula et al. (2006) USA [59] | n = 60 | Prospective controlled study | DMV (days) | 6.5 | 11.2* |
| ICULOS (days) | 12.1 | 14.1 | |||
| Pneumonia (%) | NA | NA | |||
| Mortality (%) | NA | NA | |||
| Althausen et al. (2011) USA [60] | n = 50 | Retrospective controlled study | DMV (days) | 4.1 | 9.7* |
| ICULOS (days) | 7.59 | 9.68 | |||
| Pneumonia | 4.55 | 25* | |||
| Mortality | 0 | 0 | |||
| Marasco et al. (2013) USA [63] | n = 46 | RCT | DMV (days) | 6.3 | 7.5 |
| ICULOS (days) | 13.5 | 18.7* | |||
| Pneumonia (%) | 48 | 74 | |||
| Mortality (%) | 0 | 4 | |||
| Slobogean et al. (2013) USA [65] | n = 732; 11 studies | Meta- analysis | DMV (days) | −7.5; 95% CI : −9.9 to −5 | |
| ICULOS (days) | −4.8; 95% CI : −1.6 to −7.9 | ||||
| Pneumonia | OR: 0.18; (0.11–0.32) | ||||
| Mortality | OR: 0.31; (0.20–0.48) | ||||
| Leinicke et al. (2013) USA [64] | n = 538; 9 studies | Meta-analysis | DMV (days) | −4.52; (−5.54 to −3.5) | |
| ICULOS (days) | −3.40; (−6.0 to −0.80) | ||||
| Pneumonia | RR: 0.45; (0.29–0.67) | ||||
| Mortality | RR: 0.43; (0.28–0.69) | ||||
| References . | Patients . | Design . | Outcomes . | Key results . | |
|---|---|---|---|---|---|
| OT . | NOT . | ||||
| Ahmed et al. (1995) UAE [55] | n = 64 | Retrospective cohort study | DMV (days) | 3.9 | 15 |
| ICULOS (days) | 9 | 21 | |||
| Pneumonia (%) | 15 | 50 | |||
| Mortality (%) | 8 | 29 | |||
| Karev (1997) Ukraine [56] | n = 40 | Retrospective cohort study | DMV (days) | 2.3 | 6.3* |
| ICULOS (days) | NA | NA | |||
| Pneumonia (%) | 15 | 34* | |||
| Mortality (%) | 22.5 | 46† | |||
| Voggenreiter (1998) Germany [57] | N = 20 | Retrospective cohort study | DMV (days) | 6.5 | 27* |
| ICULOS (days) | NA | NA | |||
| Pneumonia (%) | 15 | 39* | |||
| Mortality (%) | NA | NA | |||
| Tanaka et al. (2002) Japan [61] | n = 37 | RCT | DMV (days) | 10.8 | 18* |
| ICULOS (days) | 16.5 | 27* | |||
| Pneumonia (%) | 24 | 77* | |||
| Mortality (%) | NA | NA | |||
| Balci et al. (2004) Turkey [58] | n = 64 | Retrospective cohort study | DMV (days) | 3.1 | 7.2 |
| ICULOS (days) | NA | NA | |||
| Pneumonia (%) | NA | NA | |||
| Mortality (%) | 11 | 27 | |||
| Granetzny et al. (2005) Germany [62] | n = 40 | RCT | DMV (days) | 2 | 12 |
| ICULOS (days) | 9 | 14 | |||
| Pneumonia (%) | 10 | 50 | |||
| Mortality (%) | 10 | 15 | |||
| Nirula et al. (2006) USA [59] | n = 60 | Prospective controlled study | DMV (days) | 6.5 | 11.2* |
| ICULOS (days) | 12.1 | 14.1 | |||
| Pneumonia (%) | NA | NA | |||
| Mortality (%) | NA | NA | |||
| Althausen et al. (2011) USA [60] | n = 50 | Retrospective controlled study | DMV (days) | 4.1 | 9.7* |
| ICULOS (days) | 7.59 | 9.68 | |||
| Pneumonia | 4.55 | 25* | |||
| Mortality | 0 | 0 | |||
| Marasco et al. (2013) USA [63] | n = 46 | RCT | DMV (days) | 6.3 | 7.5 |
| ICULOS (days) | 13.5 | 18.7* | |||
| Pneumonia (%) | 48 | 74 | |||
| Mortality (%) | 0 | 4 | |||
| Slobogean et al. (2013) USA [65] | n = 732; 11 studies | Meta- analysis | DMV (days) | −7.5; 95% CI : −9.9 to −5 | |
| ICULOS (days) | −4.8; 95% CI : −1.6 to −7.9 | ||||
| Pneumonia | OR: 0.18; (0.11–0.32) | ||||
| Mortality | OR: 0.31; (0.20–0.48) | ||||
| Leinicke et al. (2013) USA [64] | n = 538; 9 studies | Meta-analysis | DMV (days) | −4.52; (−5.54 to −3.5) | |
| ICULOS (days) | −3.40; (−6.0 to −0.80) | ||||
| Pneumonia | RR: 0.45; (0.29–0.67) | ||||
| Mortality | RR: 0.43; (0.28–0.69) | ||||
DMV: duration of mechanical ventilation (days); ICULOS: intensive care unit length of stay (days); RR: relative risk.
*P < 0.05.
| References . | Patients . | Design . | Outcomes . | Key results . | |
|---|---|---|---|---|---|
| OT . | NOT . | ||||
| Ahmed et al. (1995) UAE [55] | n = 64 | Retrospective cohort study | DMV (days) | 3.9 | 15 |
| ICULOS (days) | 9 | 21 | |||
| Pneumonia (%) | 15 | 50 | |||
| Mortality (%) | 8 | 29 | |||
| Karev (1997) Ukraine [56] | n = 40 | Retrospective cohort study | DMV (days) | 2.3 | 6.3* |
| ICULOS (days) | NA | NA | |||
| Pneumonia (%) | 15 | 34* | |||
| Mortality (%) | 22.5 | 46† | |||
| Voggenreiter (1998) Germany [57] | N = 20 | Retrospective cohort study | DMV (days) | 6.5 | 27* |
| ICULOS (days) | NA | NA | |||
| Pneumonia (%) | 15 | 39* | |||
| Mortality (%) | NA | NA | |||
| Tanaka et al. (2002) Japan [61] | n = 37 | RCT | DMV (days) | 10.8 | 18* |
| ICULOS (days) | 16.5 | 27* | |||
| Pneumonia (%) | 24 | 77* | |||
| Mortality (%) | NA | NA | |||
| Balci et al. (2004) Turkey [58] | n = 64 | Retrospective cohort study | DMV (days) | 3.1 | 7.2 |
| ICULOS (days) | NA | NA | |||
| Pneumonia (%) | NA | NA | |||
| Mortality (%) | 11 | 27 | |||
| Granetzny et al. (2005) Germany [62] | n = 40 | RCT | DMV (days) | 2 | 12 |
| ICULOS (days) | 9 | 14 | |||
| Pneumonia (%) | 10 | 50 | |||
| Mortality (%) | 10 | 15 | |||
| Nirula et al. (2006) USA [59] | n = 60 | Prospective controlled study | DMV (days) | 6.5 | 11.2* |
| ICULOS (days) | 12.1 | 14.1 | |||
| Pneumonia (%) | NA | NA | |||
| Mortality (%) | NA | NA | |||
| Althausen et al. (2011) USA [60] | n = 50 | Retrospective controlled study | DMV (days) | 4.1 | 9.7* |
| ICULOS (days) | 7.59 | 9.68 | |||
| Pneumonia | 4.55 | 25* | |||
| Mortality | 0 | 0 | |||
| Marasco et al. (2013) USA [63] | n = 46 | RCT | DMV (days) | 6.3 | 7.5 |
| ICULOS (days) | 13.5 | 18.7* | |||
| Pneumonia (%) | 48 | 74 | |||
| Mortality (%) | 0 | 4 | |||
| Slobogean et al. (2013) USA [65] | n = 732; 11 studies | Meta- analysis | DMV (days) | −7.5; 95% CI : −9.9 to −5 | |
| ICULOS (days) | −4.8; 95% CI : −1.6 to −7.9 | ||||
| Pneumonia | OR: 0.18; (0.11–0.32) | ||||
| Mortality | OR: 0.31; (0.20–0.48) | ||||
| Leinicke et al. (2013) USA [64] | n = 538; 9 studies | Meta-analysis | DMV (days) | −4.52; (−5.54 to −3.5) | |
| ICULOS (days) | −3.40; (−6.0 to −0.80) | ||||
| Pneumonia | RR: 0.45; (0.29–0.67) | ||||
| Mortality | RR: 0.43; (0.28–0.69) | ||||
| References . | Patients . | Design . | Outcomes . | Key results . | |
|---|---|---|---|---|---|
| OT . | NOT . | ||||
| Ahmed et al. (1995) UAE [55] | n = 64 | Retrospective cohort study | DMV (days) | 3.9 | 15 |
| ICULOS (days) | 9 | 21 | |||
| Pneumonia (%) | 15 | 50 | |||
| Mortality (%) | 8 | 29 | |||
| Karev (1997) Ukraine [56] | n = 40 | Retrospective cohort study | DMV (days) | 2.3 | 6.3* |
| ICULOS (days) | NA | NA | |||
| Pneumonia (%) | 15 | 34* | |||
| Mortality (%) | 22.5 | 46† | |||
| Voggenreiter (1998) Germany [57] | N = 20 | Retrospective cohort study | DMV (days) | 6.5 | 27* |
| ICULOS (days) | NA | NA | |||
| Pneumonia (%) | 15 | 39* | |||
| Mortality (%) | NA | NA | |||
| Tanaka et al. (2002) Japan [61] | n = 37 | RCT | DMV (days) | 10.8 | 18* |
| ICULOS (days) | 16.5 | 27* | |||
| Pneumonia (%) | 24 | 77* | |||
| Mortality (%) | NA | NA | |||
| Balci et al. (2004) Turkey [58] | n = 64 | Retrospective cohort study | DMV (days) | 3.1 | 7.2 |
| ICULOS (days) | NA | NA | |||
| Pneumonia (%) | NA | NA | |||
| Mortality (%) | 11 | 27 | |||
| Granetzny et al. (2005) Germany [62] | n = 40 | RCT | DMV (days) | 2 | 12 |
| ICULOS (days) | 9 | 14 | |||
| Pneumonia (%) | 10 | 50 | |||
| Mortality (%) | 10 | 15 | |||
| Nirula et al. (2006) USA [59] | n = 60 | Prospective controlled study | DMV (days) | 6.5 | 11.2* |
| ICULOS (days) | 12.1 | 14.1 | |||
| Pneumonia (%) | NA | NA | |||
| Mortality (%) | NA | NA | |||
| Althausen et al. (2011) USA [60] | n = 50 | Retrospective controlled study | DMV (days) | 4.1 | 9.7* |
| ICULOS (days) | 7.59 | 9.68 | |||
| Pneumonia | 4.55 | 25* | |||
| Mortality | 0 | 0 | |||
| Marasco et al. (2013) USA [63] | n = 46 | RCT | DMV (days) | 6.3 | 7.5 |
| ICULOS (days) | 13.5 | 18.7* | |||
| Pneumonia (%) | 48 | 74 | |||
| Mortality (%) | 0 | 4 | |||
| Slobogean et al. (2013) USA [65] | n = 732; 11 studies | Meta- analysis | DMV (days) | −7.5; 95% CI : −9.9 to −5 | |
| ICULOS (days) | −4.8; 95% CI : −1.6 to −7.9 | ||||
| Pneumonia | OR: 0.18; (0.11–0.32) | ||||
| Mortality | OR: 0.31; (0.20–0.48) | ||||
| Leinicke et al. (2013) USA [64] | n = 538; 9 studies | Meta-analysis | DMV (days) | −4.52; (−5.54 to −3.5) | |
| ICULOS (days) | −3.40; (−6.0 to −0.80) | ||||
| Pneumonia | RR: 0.45; (0.29–0.67) | ||||
| Mortality | RR: 0.43; (0.28–0.69) | ||||
DMV: duration of mechanical ventilation (days); ICULOS: intensive care unit length of stay (days); RR: relative risk.
*P < 0.05.
For flail chest, early surgical stabilization can be considered in patients who would require mechanical ventilation for >48 h (Grade B, extrapolated recommendations from Level I evidences).
Isolated sternal fracture
Included in seat belt syndrome, sternal fractures are a common injury of motorized vehicle accidents. Two patterns are described with a difference in terms of morbidity and mortality: the isolated sternal fracture (ISF), often described as a benign entity, and the non-isolated sternal fracture (NISF), whose morbidity is related to associated injuries. A review of US National Trauma Database highlighted that sternal fractures are representative of severe trauma (56% with ISS >15) associated with a high mortality rate (7.9%) related to associated injuries: myocardial contusion (4%), rib fractures (58%), lung contusions (3%), pneumothorax (22%), thoracic or lumbar vertebrae fractures (22 and 16%) [66]. To assess sternal fractures, it is mandatory to look for associated injuries by chest X-ray examination, standard 12-lead electrocardiogram and cardiac troponin I assay. In a systematic review published in 2003, Sybrandy et al. showed that patients with normal electrocardiogram and normal troponin assay can be safely discharged [67]. Reasons for admission for ISFs are pain control (50%), dyspnoea (3%) or abnormal electrocardiogram or cardiac enzymes (40%), requiring complementary exploration by transthoracic ultrasound or CT scan. Control of pain and control of respiratory involvement are the goals of sternal fractures management. In most cases, conservative treatment is sufficient and only selected patients should undergo an intervention (2%) [68]. Most surgeons consider that sternal fractures fixation is relevant for selected patients who present with non-union fracture (over 6 weeks) or lung herniation [69]. However, surgical indications in the first 48 h are unstable, comminuted and/or displaced (overlap) fractures, causing intractable pain or dyspnoea. Uncomplicated pericardial effusion is not associated with an adverse outcome and is not an indication for surgery [70].
Early open reduction and internal fixation of sternal fractures should be performed when displaced sternal fractures with overlap or when comminution, intractable pain or respiratory insufficiency is present. (Grade D, extrapolated recommendations from Level III evidences).
Should traumatic lung herniation be repaired?
Incarcerated lung herniation is a life-threatening situation. It can be a consequence of parietal defects often related to seat belt syndrome. Clinical findings associate chest tenderness, respiratory distress and parasternal hernias. Delayed diagnosis is possible in obese people and CT scan contributes to the diagnosis. In these complex and rare cases, the level of evidence is based only on reported cases (300 cases reported to date). Non-operative treatment seems feasible [71, 72], but an operative strategy is preferred for incarcerated or large lung herniations. Sometimes, parietal reconstruction using prosthetic material is the only option to restore respiratory mechanics in large chest wall deformity. Costal cartilages and/or fractured ends should be brought together over the mesh by absorbable sutures. VATS could be used for less extended injuries.
Early surgery should be considered for lung herniation based upon size, incarceration and respiratory distress (Grade D, Level IV evidence).
What is the best surgical strategy for diaphragmatic ruptures?
Traumatic diaphragmatic rupture (TDR) is present in 0.2–4% of admissions for chest or abdominal trauma. Nearly 70% of TDR sits on the left side. Approximately, 10–20% of TDRs are diagnosed after 48 h [73]. For diaphragmatic wounds, the sensibility of conventional radiological examinations is low: 30% of TDR diagnoses are missed by chest X- ray [74]. CT scan remains a gold standard to detect TDR in BCT [75]. Whatever the side, TDR is a formal indication for surgical repair. Progression leads to hollow organs herniation. Time to surgical repair depends on respiratory and haemodynamic status and associated injuries of the patient. Once diagnosed, the repair should be performed as soon as the patient's status allows it. Right and left TDR are similar in terms of morbidity and mortality [76].
The choice of surgical approach remains controversial: abdominal or thoracic approach? It is closely related to patient's haemodynamic status, associated injuries and surgeon's experience. There is no Level I evidence supporting an approach to another. Williams et al. [76] in a retrospective study with 732 patients with TDR, showed that need for thoracotomy is an important and significant predictor of mortality. Several authors argue to perform laparotomy first in order to repair all TDRs, sequels of abdominal organ entrapment, and associated abdominal injury, most commonly splenic rupture [77]. The choice of technique, conventional surgery or minimally invasive approach, depends only on the experience of the surgeon. Feasibility studies were performed but they remain insufficient to make any recommendation.
Based on retrospectives studies, three patterns can be described for surgical indications in the first 48 h:
For TDR in unstable patients and/or abdominal associated injury: laparotomy is mandatory.
For TDR in stable patients without abdominal and/or thoracic associated injuries: thoracotomy should be performed. Or, as an alternative, mini invasive approach such as VATS could be proposed.
When TDR is suspected in a stable patient without thoracic or abdominal injury, VATS or laparoscopy is required for diagnosis and treatment.
TDR is a life-threatening situation requiring both early diagnosis and surgical intervention (Grade C, Level III evidences).
Should traumatic pericardial rupture be repaired?
VATS has proved its usefulness in BCT management for pericardial rupture as well as diaphragmatic rupture. Pericardial rupture is associated with a high mortality. Radiological diagnosis is rare and difficult. Only 20% of pericardial ruptures are diagnosed preoperatively while VATS allows both diagnosis and treatment. The level of evidence is based only on case reports.
Urgent surgery should be performed in suspected pericardial rupture (Grade D, Level IV evidences).
Tracheobronchial repair in blunt chest trauma management
Is surgical repair needed for tracheobronchial injury?
Tracheobronchial injuries (TBIs) are involved in 0.8–2.8% of deaths secondary to road traffic accidents. The TBI-related pre-hospital mortality is ∼80%, whereas hospital mortality is <10%. In 80% of cases, TBIs are located on an area covering two inches above and below the carina, whereas 20% are located at the laryngotracheal junction. These two points of attachment of the trachea are subjected to either a widening of their diameter in a transverse abrupt compression of the rib cage, an anteroposterior mobilization of the lungs during abrupt deceleration or a closed glottis related barotraumatism. Clinical findings of TBI are subcutaneous emphysema, respiratory distress, dysphonia or haemoptysis. The typical pattern is a complete lack of lung re-expansion after chest tube drainage for pneumothorax. Need for surgical repair evaluated on retrospective series, is based on the risk of airway obstruction, massive air leak and mediastinitis [78–80]. Overall, surgery is performed after bronchoscopic evaluation: tracheal tear >2 cm and/or prolapse of oesophageal wall or pericardial fat and/or mediastinitis, combined with massive air leak (persistent pneumothorax, acute bilateral pneumothorax, increasing pneumomediastinum or extensive subcutaneous emphysema). In the case of both tracheobronchial and oesophageal injuries, surgery is mandatory.
Bronchoscopy is the first step in blunt TBI management. It allows correct airway control and indicates surgical repair in case of tracheal tear >2 cm, oesophageal prolapse and mediastinitis. Massive air leak (increasing pneumomediastinum or subcutaneous emphysema or persistent pneumothorax despite adequate drainage) indicates for prompt surgical repair (Grade C, Level III evidences).
CONCLUSION
Like in other surgical fields, technological progress changes the art and craft of trauma surgery. Concerning BCT, even if EDT could never be supported by level I evidence for ethical reasons, it should be considered for patients with signs of life at initial assessment in the first 10 min of cardiopulmonary resuscitation. Recent studies demonstrate some benefits of old surgical practices like flail chest fixation. VATS is a new surgical approach in trauma management and should be a useful tool for trauma surgeons.
These evidence-based surgical indications for early BCT management, excluding vascular injuries, should support protocols for chest trauma management.
Conflict of interest: none declared.





Comments
© The Author 2014. Published by Oxford University Press on behalf of the European Association for Cardio-Thoracic Surgery. All rights reserved
I read the article of De Lesquen et al. [1] and thank them for their systematically designed study. I think this well designed study can be used as a reference article.
I want to add a comment on the occult pneumothorax. Occult pneumothorax is the collapse of the lung in the sagittal plane (anteroposterior collapse). If the lung does not collapse in the transverse plane, pneumothorax cannot be recognized in anteroposterior roentgenograms. Contact of the lung tissue to the lateral chest wall determines the noticeability in plain graphs. Even large pneumothoraces can be overlooked, if the lung was not collapsed from the lateral region. I agree with the authors about the conservative treatment in minimal pneumothorax cases. But even in stable patients, large occult pneumothoraces (pneumothorax constituting half of the hemithorax in axial CT) should be evacuated in order not to worsen the clinical status. Also, if the patient is not able to cough and clean the secretions, especially in intensive care unit, pneumothorax can be complicated. Pleural thickening occurs day by day and this limits the lung expansion. As a result, in occult pneumothoraces, even if it is diagnosed in subsequent days; I recommend aspirating the air via a small pleural catheter from the second intercostal space on the midclavicular line.
In blunt chest trauma patients, situations that require chest tube is generally associated with rib fractures or pulmonary contusion. Taking into consideration this realization, I think that using prophylactic antibiotic is appropriate in chest tube insertion. Although the statement of insignificant effect of prophylactic antibiotherapy in blunt chest trauma declared by the authors [2], there can be legal issues if prolonged hospital stay occurs or the patient progresses to empyema. As a result, I think that if an intervention is performed to a patient, prophylactic antibiotherapy should be given.
I agree with the authors about the management of diaphragmatic ruptures. I want to point out the Bochdalek's hernia. This hernia can be misdiagnosed as diaphragmatic rupture in trauma patients [3]. So, the clinician should have a suspicion of a hernia when he/she faces a diaphragmatic defect in the posterolateral region in a trauma patient.
References
[1] De Lesquen H, Avaro JP, Gust L, Ford RM, Berangera F, Natale C, et al. Surgical management for the first 48 h following blunt chest trauma: state of the art (excluding vascular injuries). Interact CardioVasc Thorac Surg (2014) 1-10. doi:10.1093/icvts/ivu397
[2] Bosman A, De Jong MB, Debeij J, Van den Broek PJ, Schipper IB. Systematic review and meta-analysis of antibiotic prophylaxis to prevent infections from chest drains in blunt and penetrating thoracic injuries. Br J Surg 2012;99:506-13.
[3] Edwin F. A practical approach for imaging of diaphragmatic injury. Interact CardioVasc Thorac Surg 2009;9:49.
Conflict of interest: none declared