-
PDF
- Split View
-
Views
-
Cite
Cite
Enrico Ferrari, Giuseppe Siniscalchi, Sotirios Marinakis, Denis Berdajs, Ludwig von Segesser, ‘Fast-implantable’ aortic valve implantation and concomitant mitral procedures, Interactive CardioVascular and Thoracic Surgery, Volume 19, Issue 4, October 2014, Pages 682–684, https://doi.org/10.1093/icvts/ivu204
Close - Share Icon Share
Abstract
Concomitant aortic and mitral valve replacement or concomitant aortic valve replacement and mitral repair can be a challenge for the cardiac surgeon: in particular, because of their structure and design, two bioprosthetic heart valves or an aortic valve prosthesis and a rigid mitral ring can interfere at the level of the mitroaortic junction. Therefore, when a mitral bioprosthesis or a rigid mitral ring is already in place and a surgical aortic valve replacement becomes necessary, or when older high-risk patients require concomitant mitral and aortic procedures, the new ‘fast-implantable’ aortic valve system (Intuity™ valve, Edwards Lifesciences, Irvine, CA, USA) can represent a smart alternative to standard aortic bioprosthesis. Unfortunately, this is still controversial (risk of interference). However, transcatheter aortic valve replacements have been performed in patients with previously implanted mitral valves or mitral rings. Interestingly, we learned that there is no interference (or not significant interference) among the standard valve and the stent valve. Consequently, we can assume that a fast-implantable valve can also be safely placed next to a biological mitral valve or next to a rigid mitral ring without risks of distortion, malpositioning, high gradient or paravalvular leak. This paper describes two cases: a concomitant Intuity™ aortic valve and bioprosthetic mitral valve implantation and a concomitant Intuity™ aortic valve and mitral ring implantation.
INTRODUCTION
Concomitant aortic and mitral procedures represent a challenge for the surgeon, in particular when older high-risk patients are concerned. Two bioprosthetic heart valves can interfere because of their design (the same in all available stented bioprostheses) but there is also a risk of interference in the case of aortic valve replacement and mitral ring implantation [1].
Concerning patients with mitral bioprosthesis or mitral rings who require an aortic valve replacement, or high-risk patients with double valve disease, the use of a ‘fast-implantable’ aortic bioprosthesis represents an advantage, but this is still controversial.
The Intuity™ valve (Edwards Lifesciences, Irvine, CA, USA) is a fast-implantable bioprosthesis with a stent below the annulus (Fig. 1A) the assumption is that a mitral valve or ring in place or implanted during the same procedure can interfere with the stent. However, we already know that transcatheter valves can be implanted next to the mitral prosthesis or rings without interferences [2, 3] (Fig. 1B).

(A) The Intuity™ aortic valve (Edwards Lifesciences, Irvine, CA, USA). (B) Fluoroscopic view of a transcatheter Sapien™ valve implanted in a patient with a previously implanted mechanical mitral prosthesis: absence of interference between the two valves. (C) An explanted pig heart showing the relationship between the Intuity™ valve and a standard mitral bio-prosthesis.
Following this assumption, we demonstrated, in an animal model, that the Intuity™ does not interferes with a mitral valve already in place (Fig. 1C) and we describe two cases: a double valve replacement with an Intuity™ and a standard mitral prosthesis, and an Intuity™ implantation plus coronary revascularization and mitral repair.
PROCEDURAL DETAILS
Tests on pig hearts showed that a mitral valve allows the placement of an Intuity™ valve, with safe stent deployment and valve fixation (Fig. 1C). After this preliminary experience, and after personal surgical experience (Author: Enrico Ferrari) of more than 25 Intuity™ valves implanted in ‘standard’ patients, we performed two cases where the use of the Intuity™ system had added value in terms of ease and speed of implantation. Patients signed their informed consent.
Case 1: concomitant aortic and mitral valve replacement
The first case was a female patient aged 82 with symptomatic aortic and mitral regurgitation who had already been operated on for ascending aortic aneurysm with a 30-mm vascular graft, with no comorbidities and good health status. The calculated EuroSCORE II was 16%. We decided to replace both valves to save surgical time (impaired left ventricular function and redo). Transcatheter aortic valve replacement (TAVR) options were not discussed because the patient had pure aortic regurgitation without calcifications.
The mitral valve was replaced with a 29-mm Perimount™ bio-prosthesis (Edwards Lifesciences), while a 21-mm Intuity™ was placed through the vascular graft in an aortic position (implanting time: 9 min) (Fig. 2A). Aortic cross-clamp time was 130 min (the mitral valve in this redo case was deep and far, and time consuming). The rest of the procedure and the postoperative recovery were uneventful, and the patient left the hospital on postoperative day 10. The echocardiographic control revealed a good haemodynamic status: mean aortic gradient of 12 mmHg and absence of paravalvular leak; mean mitral gradient of 6 mmHg and absence of paravalvular leak. No interference showed on chest radiogram (Fig. 2B and C).
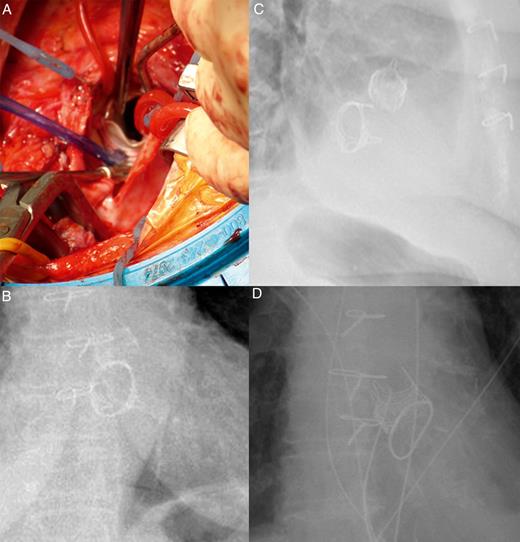
(A) Surgical view of the Intuity™ aortic valve (Edwards Lifesciences, Irvine, CA, USA) implanted after mitral valve replacement with a 29-mm Edwards Perimount™ valve (Edwards Lifesciences). (B and C) Thorax radiograms showing the simultaneous implant of the Intuity™ valve and the Perimount™ mitral valve. (D) A thorax radiogram showing the simultaneous implant of the Intuity™ valve and the Physio™ II mitral ring.
Case 2: concomitant aortic valve replacement and mitral repair (+ myocardial revascularization)
A 78-year old female patient suffering from multiple coronary disease, severe mitral regurgitation and aortic valve stenosis with impaired left ventricular function (ejection fraction: 25%). The calculated EuroSCORE II was 8%. We opted for a fast valve treatment to save surgical and ischaemic time (high-risk profile). We did not discuss TAVR options because of the concomitant mitral and coronary disease not treatable with percutaneous approaches.
We performed two venous grafts, we placed a 28-mm mitral Physio™ II ring (Edwards Lifesciences) and we implanted a 23-mm Intuity™ following the standard protocol and without interference with the ring (implanting time: 8 min). Finally, we placed the mammary artery on the anterior descending coronary artery. Aortic cross-clamp time was 116 min. The rest of the procedure and the postoperative recovery were uneventful and the patient left the hospital 9 days later.
The echocardiographic control revealed good haemodynamic status: mean aortic gradient of 3 mmHg and absence of paravalvular leak; mean mitral gradient of 3 mmHg. The radiogram did not show interference between the valve and the ring (Fig. 2D).
DISCUSSION
The Intuity™ valve is a ‘fast-implantable’ aortic bioprosthesis allowing faster and easier aortic valve replacement when compared with the standard bioprosthesis, which requires multiple sutures or stitches for the fixation [4, 5].
However, in multiple valve disease, there could be a risk of interference when a mitral bioprosthesis or a ring is already in place or is implanted during the same surgical procedure (impeded stent deployment or stent deformation with risk of high gradients or paravalvular leak). Nevertheless, several reports have confirmed that transcatheter aortic procedures are feasible in patients with the mitral prosthesis in place, without an increased risk of high gradient, leak or valve distortion [2, 3].
After this surgical experience, we confirm that the fast-implantable Intuity™ valve can also be implanted next to a mitral ring or a mitral prosthesis without increased risk of valve displacement, distortion or paravalvular leak: the technique is safe, standard and reproducible and does not require special skills or knowledge (apart from a certain experience with the placement of Intuity™ valves in standard cases).
In conclusion, we know that the patient's outcome can be affected by a long procedural and ischaemic time, in particular when concomitant multiple procedures or redo cases are concerned: the new fast-implantable valve requires 8–10 min for fixation and plays a role in reducing cross-clamp time and alleviating the ischaemic cardiac arrest.
Conflict of interest: none declared.
REFERENCES
- aorta
- aortic valve
- stents
- mitral valve repair
- cardiac surgery procedures
- mitral valve
- aortic valve replacement
- mitral valve replacement surgery
- bioprosthesis
- muscle rigidity
- surgical procedures, operative
- heart
- heart valve prosthesis, biologic
- thoracic aortic procedures
- transcatheter aortic-valve implantation
- prostheses
- aortic valve prosthesis




