-
PDF
- Split View
-
Views
-
Cite
Cite
Weston Andrews, Subroto Paul, Navneet Narula, Nasser K. Altorki, Localized mesothelioma tumour arising synchronously with a primary contralateral lung cancer, Interactive CardioVascular and Thoracic Surgery, Volume 17, Issue 6, December 2013, Pages 1061–1062, https://doi.org/10.1093/icvts/ivt365
Close - Share Icon Share
Abstract
Mesothelioma is a malignant growth of mesothelial cells found in the serosal membrane of pleural, peritoneal and pericardial surfaces as a result of prolonged exposure to asbestos. Malignant pleural mesothelioma (MPM) typically presents itself in a diffuse pattern of growth over the pleura of the lung or in more rare cases as a localized focus (LMPM). We present the first reported case of a synchronous LMPM and non-small adenocarcinoma of the lung treated by sequential resections.
CASE PRESENTATION
An 82-year old male former smoker with a past medical history significant for hypertension, type II diabetes mellitus and emphysema presented to his primary care physician with left-sided back pain and was sent for a chest radiograph. This demonstrated a left lateral-posterior seventh rib fracture and a left lower lobe mass. Subsequently, a computed tomography (CT) scan (Fig. 1A and B) was completed that showed a 7.2 cm left lower lobe mass with direct invasion into a posterior portion of the seventh rib along with a 5.4 cm right lower lobe sub-solid opacity widely abutting the pleural surface.
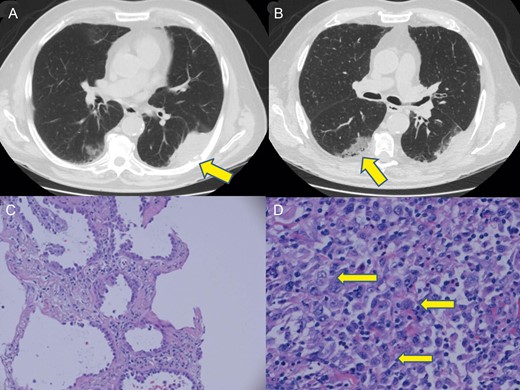
(A) Chest CT demonstrates a 7.2 cm left lower lobe pleural based mass with direct extension into the seventh rib denoted (arrow). (B) Chest CT image of the biopsy-proven adenocarcinoma arising in the right lower lobe denoted (arrow). (C) Haematoxylin and eosin staining of right lower lobe biopsy demonstrates a distinct lepidic pattern of malignant growth confirming adenocarcinoma. (D) Haematoxylin and eosin staining of left lower lobe pleural based tumour demonstrates epithelioid cells with vesicular nuclei and prominent nucleoli denoted (arrows). Numerous neutrophils are admixed with the tumour cells.
CT-guided biopsy of the left lower lobe lesion demonstrated poorly differentiated carcinoma with extensive necrosis. Subsequently, the right lower lobe lesion was biopsied with CT guidance and showed a well to moderately differentiated adenocarcinoma (Fig. 1C). The two lesions were clinically thought to be synchronous primary lung cancers. Pulmonary function tests showed a forced expiratory volume in one second (FEV1) percent predicted of 89%, a forced vital capacity (FVC) percent predicted of 71% and a DLCO percent predicted of 89%, which were adequate for the patient to tolerate serial bilateral resections. Positron Emission Tomography (PET)-CT and brain magnetic resonance imaging (MRI) did not reveal any evidence of metastatic disease. The left-sided lesion was approached first as the lesion was larger with chest-wall extension. The patient underwent a mediastinoscopy, which demonstrated no evidence of lymph node metastasis. The patient underwent video-assisted thoracoscopic resection of the left lower lobe followed by a limited thoracotomy with resection of the sixth, seventh and eighth ribs and chest-wall reconstruction with a soft-tissue Gore-Tex patch.
Pathology revealed the tumour to be composed of epithelioid cells with vesicular nuclei and prominent nucleoli (Fig. 1D), consistent with a localized malignant mesothelioma, epithelioid type, of the left lung pleura. This diagnosis was confirmed with positive immunohistochemical stains for Pan Cytokeratin, Cam5.2, calretinin and WT-1. The tumour was negative for thyroid transcription factor-1 (TTF-1), Napsin, CD15, carcinoembryonic antigen and D240. This immunohistochemical profile is consistent with malignant mesothelioma. All the resection margins and lymph nodes were negative for tumour.
The patient had an uneventful recovery. He returned in 8 weeks for evaluation for resection of his right-sided lesion. He appeared well and had a room air oxygen saturation of 95%. Spirometry revealed a FEV1 percent predicted of 54% and a FVC percent predicted of 53%, which was sufficient for him to tolerate a sublobar resection. He then underwent a right video-assisted thoracoscopic surgical resection of the superior segment of his right lower lobe. Pathology revealed this to be a T2a, N0, M0 stage IB 5.0 cm moderately differentiated adenocarcinoma based on immunohistochemistry (TTF-1/cytokeratin-7+, - calretinin -). His hospital course was uncomplicated. The patient received six cycles of adjuvant pemetrexed and remains free of recurrent disease 10 months after his sequential resections.
DISCUSSION
There have only been 52 published cases of LMPM in the English-language literature [1]. Although there has been a single report of a LMPM arising simultaneously with multiple adenocarcinomas, a LMPM arising synchronously with a non-small-cell adenocarcinoma of the contralateral lung as in our case has never been reported in the literature [2]. Diffuse mesothelioma has a poor clinical outcome with patients typically dying within 2 years of diagnosis [3]. Excision of a localized mesothelioma in the absence of metastasis has better overall survival. Surgical resection with negative margins can be considered a curative treatment for LMPM [1].
LMPM and MPM are histologically, immunohistochemically and ultrastructurally indistinguishable [3]. As a result, it is difficult to pathologically distinguish the two variants. The diagnosis of MPM, diffuse or localized, is confirmed by positive vimentin and cytokeratin stains as well as positive podoplanin, calretinin, CK 5/6, WT-1 protein, thrombomodulin and mesothelin and is classified as epithelial, sarcomatous or mixed type [4, 5].
Our case illustrates the difficulties encountered in distinguishing mesothelioma from lung adenocarcinoma using only limited tissue from core biopsies. In our case, the LMPM diagnosis was made only after resection. Had a diagnosis of MPM been suspected preoperatively, our management would have been altered. We would initially try to confirm the pathology and determine whether the MPM was the more common diffuse phenotype. A thoracoscopic biopsy of the mass as well as surrounding pleura would have been performed. If the pleural biopsies revealed diffuse type MPM with chest-wall invasion on frozen section, no further surgery would be performed and the patient would be offered chemotherapy and radiation. Given the poor prognosis associated with diffuse MPM, the right-sided lung cancer would have been managed non-operatively. If the pleural biopsies were negative for MPM, resection would have proceeded on the left as the patient has resectable disease whether it is LMPM or a chest-wall-invading adenocarcinoma. Given the rarity of a diagnosis of LMPM, the diagnosis would be questioned even with thoracoscopic biopsies of the mass as long as the pleural biopsies remained negative for MPM.
Our case also illustrates the difficulties in dealing with large bilateral lung lesions. The right-sided lesion was resected with a segmentectomy despite its size. The margin was negative with a tumour/margin ratio of <1, which is not ideal. Yet, this case represents the best possible surgical option to spare parenchyma. Non-operative therapies such as stereotactic radiosurgery would also be suboptimal, given the tumour's size, and could be considered if the patient could not tolerate resection.
In summary, we present the first reported case of LMPM arising synchronously with a non-small-cell adenocarcinoma of the contralateral lung that was treated by sequential surgical resection.
Conflict of interest: none declared.




