-
PDF
- Split View
-
Views
-
Cite
Cite
Henriette Brinks, Fabian Nietlispach, Volkhard Göber, Lars Englberger, Peter Wenaweser, Bernhard Meier, Thierry Carrel, Christoph Huber, Transapical access closure: the TA PLUG device, Interactive CardioVascular and Thoracic Surgery, Volume 17, Issue 5, November 2013, Pages 806–810, https://doi.org/10.1093/icvts/ivt309
Close - Share Icon Share
Abstract
Percutaneous closure of the transapical (TA) access site for large-calibre devices is an unsolved issue. We report the first experimental data on the TA PLUG device for true-percutaneous closure following large apical access for transcatheter aortic valve implantation.
The TA PLUG, a self-sealing full-core closure device, was implanted in an acute animal study in six pigs (60.2 ± 0.7 kg). All the pigs received 100 IU/kg of heparin. The targeted activated clotting time was left to normalize spontaneously. After accessing the left ventricular apex with a 39 French introducer, the closure plug device was delivered with a 33 French over-the-wire system under fluoroscopic guidance into the apex. Time to full haemostasis as well as rate of bleeding was recorded. Self-anchoring properties were assessed by haemodynamic push stress under adrenalin challenge. An additional feasibility study was conducted in four pigs (58.4 ± 1.1 kg) with full surgical exposure of the apex, and assessed device anchoring by pull-force measurements with 0.5 Newton (N) increments. All the animals were electively sacrified. Post-mortem analysis of the heart was performed and the renal embolic index assessed.
Of six apical closure devices, five were correctly inserted and fully deployed at the first attempt. One became blocked in the delivery system and was placed successfully at the second attempt. In all the animals, complete haemostasis was immediate and no leak was recorded during the 5-h observation period. Neither leak nor any device dislodgement was observed under haemodynamic push stress with repeated left ventricular peak pressure of up to 220 mmHg. In the feasibility study assessing pull-stressing, device migration occurred at a force of 3.3 ± 0.5 N corresponding to 247.5 mmHg. Post-mortem analyses confirmed full expansion of all devices at the intended target. No macroscopic damage was identified at the surrounding myocardium. The renal embolic index was zero.
True-percutaneous left ventricular apex closure following large access is feasible with the self-sealing TA PLUG. The device allows for immediate haemostasis and a reliable anchoring in the acute animal setting. This is the first report of a true-percutaneous closure for large-calibre transcatheter aortic valve implantation access.
INTRODUCTION
Transapical transcatheter aortic valve implantation (TA TAVI) has gained wide popularity [1, 2] because of many advantages over non-antegrade access methods [3, 4]. The short and antegrade approach allows for a precise device delivery. TA TAVI is not dependent on good vascular access and the wire crossing of the severely stenosed aortic valve is eased and directed by the native blood flow of the ejecting heart. No crossing of the aortic arch by the valve delivery systems is required and steady wire guidance is supported by wire placement in the descending aorta. The TA access is not only beneficial for TAVI, but it might also be the most versatile access for other interventions in structural heart disease [5–8] and specially well suited to access the heart for transcatheter mitral valve repair and replacement [9].
Despite all the very favourable evidence, the enthusiasm for the TA access is hampered by the mere fact that this approach requires a surgical cut-down and handling of the left ventricle (LV).
Reliable and safe true-percutaneous entry and closure of the TA access site are essential to further increase acceptance in the clinical routine. Similarly, suture-mediated and suture-less access closure has become an established routine for many femoral transcatheter techniques [10].
Access closure of the apex is technically feasible. First experimental data have been reported and first clinical results are expected soon [11]. Nevertheless, reported data do only present device-based apex closure after an open surgical cut-down with active hooks or suture-based anchoring relying on the myocardial quality.
Data on true-percutaneous large calibre access closure without active hook or suture-mediated anchoring have not been reported so far.
This is the first experimental study reporting successful sealing of large-calibre TA access with the TA PLUG device in a true-percutaneous method.
MATERIALS AND METHODS
The sutureless TA PLUG closure device is a self-expanding and full-core device made from biocompatible cellulose. Expanding from a crimped diameter of 27 French (F) to a fully expanded diameter of 66F, the device is constructed to be self-anchoring and self-sealing. The outer surface is lined with a porous polyethylene/polypropylene membrane. Integrated proximal and distal radio opaque markers define the device area to be seated in the myocardial wall. The 1.75-cm long closure device is delivered over the wire with a 33F delivery system (Fig. 1).

Over-the-wire apical closure TA PLUG loaded into the 33F delivery system, ready for implantation.
Study design and ethics
The study was preformed in compliance with the European Convention on Animal Care and with permission of the national and cantonal veterinary agency including permanent on-site veterinary supervision. Experiments were performed in six adult pigs (mean weight 60.2 ± 0.7 kg, range 58–62 kg). The pigs were intubated and mechanically ventilated under general anaesthesia. A 4F pigtail catheter was inserted via the right carotid artery into the LV for contrast injection. Invasive haemodynamic monitoring and blood sampling were performed through the left common carotid artery and a venous infusion catheter inserted into the left brachiocephalic vein. Continuous electrocardiogram monitoring was recorded. All the animals were electively sacrified after a 5-h observation period.
Method
True-percutaneous access to the apex of the LV was gained under fluoroscopic guidance with a long biopsy needle inserted in the subxyphoid region. After wire exchange, a 0.035-in. extra-stiff wire was placed in the ascending aorta. A cutaneous stab incision of 0.5 mm was made to overcome the porcine skin resistance. Intravenous heparin (100 U/kg) was administered only once before the procedure targeting an activated clotting time at 200 s. Heparin was not antagonized with protamine and the activated clotting time (ACT) left to normalize spontaneously. A 39F endoscopic port access device of a 12-cm length was subsequently inserted into the apex to create a standardized large-calibre myocardial defect.
In the next step, the 33F delivery system was advanced over the wire through the port access into the LV. Then, the port access device was removed and the TA PLUG device placed into the created myocardial defect, aiming to align the apex wall within the radio opaque markers. Finally, the closure device was released and fully deployed. After successful positioning and sealing, the wire was partially pulled to reach the pericardial space and a modified pigtail catheter inserted into the pericardium to measure bleeding. To confirm stable anchoring, throughout the observation period of 5 h, the device was visualized by repeat percutaneous contrast medium injection into the device and into the LV (Fig. 2).
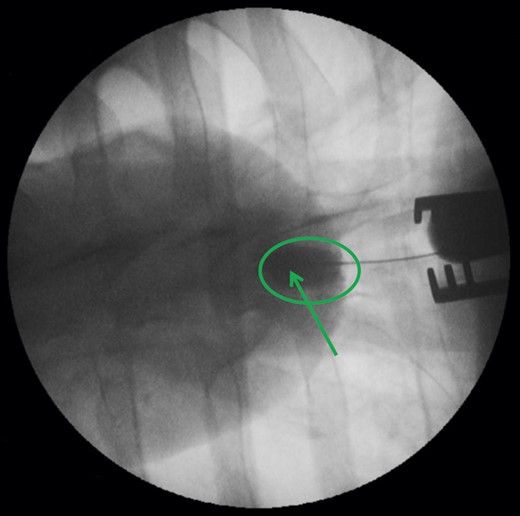
Angiographic view of the deployed TA PLUG after contrast medium injection into the device within the green circle. The green arrow points at the ventricular contrast markers of the device.
Haemodynamic stress testing with adrenalin challenges was used to simulate increased left ventricular pressure and provoke potential device dislodgement. Adrenalin administration was titrated to reach a systolic blood pressure of 200–220 mmHg for at least 2 min. The adrenalin challenges were repeated every 30 min throughout.
Further feasibility experiments were conducted in four additional pigs with surgical cut-down for apex exposure and access. After a 2-h observation period, additional pull-force measurements at 0.5 Newton (N) increments were performed to measure the pull-force necessary for device dislodgement. These pull-force tests were not performed in the six study pigs with true-percutaneous access and closure for technical reasons.
All the animals were electively sacrified and the hearts underwent post-mortem analysis. The explanted specimens were prepared at the site of closure device implantation and inspected for macroscopic myocardial damage or clot formation at the device or the surrounding tissue.
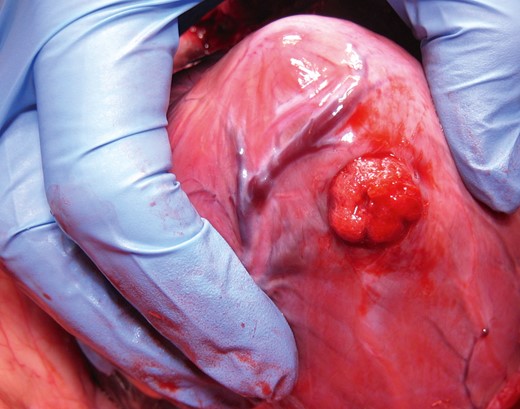
Intraoperative view of the pig heart. The TA PLUG device is precisely deployed and fully expanded within the myocardial wall of the left ventricle.
The embolic load was assessed by means of the macroscopic embolic counts in both renal cortices.
RESULTS
Of six true-percutaneous TA PLUG closure devices, five were correctly inserted and fully deployed at the first attempt. Malfunction of the first-generation delivery device resulted in device obstruction in the second animal. The delivery system was exchanged and a second device was loaded and delivered successfully at the second attempt. Haemostasis during the exchange of the delivery system was achieved by temporary reinsertion of the 39F port access. Device success was 100% (Fig. 3).
All implantations were performed without rapid pacing and all the animals reached the 5-h observation timeline. No major haemodynamic changes or arrhythmias were observed.
Left ventricular contrast medium injection and continuous pericardial effusion measurements revealed immediate sealing of the apex with complete haemostasis in all the animals after device deployment. Because of accumulated pericardial effusion from exchanging the delivery system in the second animal, 80 ml of blood was drained from this animal immediately after device insertion.
No device migration was observed and all the devices remained safely anchored and fully expanded during the 5-h observation period despite repeated adrenalin challenges generating prolonged periods of systolic arterial hypertension (pressures up to 220 mmHg).
In vivo pull-stress testing was performed in four additional animals with open surgical access to the apex. Device migration occurred at a pull force of 3.3 ± 0.5 N corresponding to a left ventricular systolic pressure of 247.5 mmHg.
All the devices were firmly anchored at the intended target location and fully expanded. No macroscopic damage of the myocardium surrounding the closure device was identified. Specifically neither clot formation nor haemorrhagic changes were identified and no pericardial effusion shown. The bilateral renal cortices showed zero macroscopic emboli at post-mortem.
DISCUSSION
TA access to the aortic valve and to other intracardiac structures is the most direct and most promising route for transcatheter treatment of structural heart disease or even coronary heart disease [12]. For TAVI, the TA access together with the transfemoral access has become the most popular access choices. Both techniques have shortcomings.
The transfemoral technique is generally contraindicated in patients with peripheral artery disease and has been repeatedly reported to have an important local complication rate [13, 14]. Stroke rate is reported to be higher [15] in transfemoral than in TA access TAVI patients. Despite well-engineered delivery systems with increasing miniaturization, the procedure remains retrograde and as such requires a more challenging aortic valve crossing and generates increased shear stress in the aortic arch. The longer working distance from the femoral puncture site to the target area negatively affects the precision of placement and the delivery process and might be responsible for the significant number of patients with paravalvular regurgitation [16, 17].
A stable guiding rail is missing as the guide wire can only be advanced into the LV in a dead-end fashion and therefore cannot provide a safe and firm support. Further, left ventricular guide wire perforations are repeatedly observed and reported [18]. Overall, the transfemoral approach often represents a trade-off in many respects including the mandatory lower crimping profile with increased shear forces on the device.
The main limitation of the TA access is seen in the surgical cut-down and the direct access through the left ventricular myocardium. Despite local complications being reported to be <1% [1, 19], special care is needed when handling the apical myocardium. Proper surgical techniques, including placing deep intramural stiches with Teflon felt reinforcements and avoiding excessive tightening of the apical purse string sutures, do decrease procedural myocardial trauma. Access localization via TTE and meticulous assessment of apical myocardial thickness with CT are essential elements of the TA access strategy and increase procedural safety. Lack of experience or wrong decision-making when faced with increased myocardial frailty can transform the apex from a friendly access option into a hostile environment.
For the above-mentioned reasons, safe and reliable percutaneous apex access and device-based closure might become essential tools for further methodical adoption and widespread use of the TA TAVI procedure.
This study is the first experimental animal trial with the TA PLUG device to demonstrate that true-percutaneous TA TAVI becomes a reproducible reality that will challenge other TAVI access routes in the near future.
A large calibre access of 39F was repeatedly created in the LV to simulate TA aortic valve implantation. All percutaneous apex closures were successful. All the animals reached the targeted 5-h observation period. Only in the second animal the closure device remained blocked in the delivery system. A second device was loaded and successfully implanted. After modification of the delivery system with a more controlled pusher-aid, no system malfunctions were noted in the remaining experiments.
With the exception of animal 2 (80 ml), no measurable blood loss was recorded throughout. No device migration was observed despite pharmacological intervention to increase intraventricular pressure on the device up to 220 mmHg.
Results from a previously unreported feasibility study of four additional animals with open access to the apex confirmed the present results. In the same study, the devices underwent pull-stress testing. Dislodgement was noted only above a pull force of 3.3 N corresponding to a left ventricular pressure equivalent of >275 mmHg.
The precise deployment at the planned target location confirms the technical feasibility and ease of the device set-up. The absence of myocardial damage or clots indicates good tissue tolerance and low risk of arterial embolism in the acute setting. The low thrombogenicity of the device is also demonstrated by the absence of emboli in the renal cortices.
In contrast to previous studies [11, 20, 21], the present closure device mechanism is free of any active stent-like structures or anchoring hooks. The sealing is immediate and does not relay on haemostasis.
Given the feasibility character of the study, there are numerous limitations. The early technical nature of the device requires further improvements, for example, a more precise delivery mechanism. Given the fact that heparin was not antagonized, the question of thrombogenicity remains of central importance to be addressed in a chronic animal study. The issue of long-term biotolerance and inflammatory response caused by the device, as well as long-term anchoring, will also have to be addressed in further experiments.
A true-percutaneous TA TAVI would provide many patient' benefits including device durability, procedural handling and decreased complication profile resulting in improved overall outcomes.
The encouraging results of this relatively simple experimental set-up indicate that apical closure devices are on the edge of becoming a clinical reality. TA TAVI may become the primary true-percutaneous access for structural heart disease in the coming years. This approach will challenge all other TAVI routes.
Funding
This work was fully supported by the Swiss National Grant (3200B-113437).
Conflict of interest: Christoph Huber is Proctor for Edwards Lifesciences and for Symetis, and Consultant for Medtronic.
REFERENCES
APPENDIX. CONFERENCE DISCUSSION
Dr S. Bleiziffer(Munich, Germany): This report presents a new concept for a percutaneous closure device. I have two questions for you, one about percutaneous transapical closure in general, and one specifically about the device. How do you think your device would compare to the other concepts that are already being tested now in clinical settings? What are the advantages over other concepts? Secondly, when we think about achieving true percutaneous access through the apex, I think we would need more imaging to precisely reach the area of interest. Do you have any thoughts or ideas on that, how not to damage coronaries and really access the correct area of interest?
Dr Huber: First of all, how does it differ from the other devices? I am not aware of any device that can close a 39F hole percutaneously, but it does totally differ in that there is no stent-like structure. There is nothing that goes into the myocardium in order to close the hole, no stent material in order to have a self-sustaining frame. This is modified full core cellulose that expands and does swell when it is in contact with blood, and, surprisingly enough, this is good enough to anchor itself in place. There is no other means. This is a totally different concept compared to all the other devices. And even though the other devices are magnificently engineered, it is very impressive to see that this very basic concept is valid. So there is a huge difference. It can go into much bigger holes.
I also think friability of the apex is not an issue, because it expands within the access hole and is very atraumatic. There will be no tearing of the surrounding tissue. It is just going to plug. It is the way you plug a hole in your boat. That is why I call it a plug device.
In regards to your second question, obviously true percutaneous means in and out. I think "out" we can handle and "in" I think we will handle as well. There are systems, for example, ECG needles, that now are echo controlled in order to make sure that they will point more or less exactly to the apex and are fired at the time of the ECG cycle that you think is adequate for insertion. Usually coronaries do not go over the apex; the LAD might wrap around the apex but always stays above the septum. I don't really see that it will be difficult to get a safe true percutaneous access.
Dr Bleiziffer: Maybe one final question. Do you think the apex will be re-accessible if you want to do a second procedure later?
Dr Huber: We know from other studies that after three months the apex has already healed again. So if you have a closure device that is flush with the inside myocardial border, the amazing mother nature is just going to heal that hole by itself. That means you can take the plug out or whatever you have stuck inside and the thing is good. So let's think about absorbable systems, and the apex will be fully recyclable.
Author notes
Presented at the 26th Annual Meeting of the European Association for Cardio-Thoracic Surgery, Barcelona, Spain, 27–31 October 2012.
Both authors contributed equally.




