-
PDF
- Split View
-
Views
-
Cite
Cite
Filippo Lococo, Alfredo Cesario, Fabia Attili, Marco Chiappetta, Giovanni Leuzzi, Guido Costamagna, Pierluigi Granone, Alberto Larghi, Transoesophageal endoscopic ultrasound-guided fine-needle aspiration of pleural effusion for the staging of non-small cell lung cancer, Interactive CardioVascular and Thoracic Surgery, Volume 17, Issue 2, August 2013, Pages 237–241, https://doi.org/10.1093/icvts/ivt166
Close - Share Icon Share
Abstract
The efficacy of endoscopic ultrasound (EUS) for evaluating mediastinal adenopathy in lung cancer is nowadays proven. However, its accuracy for detection of malignant pleural effusion per se has not been yet investigated. Herein we report our experience with EUS for detecting pleural effusion during the staging procedure of non-small cell lung cancer (NSCLC) patients.
Between January 2009 and December 2011, we performed endoscopic ultrasound-guided fine needle aspiration (EUS-FNA) on 92 selected NSCLC patients to evaluate the T and N factors and to acquire bioptic material and when this was detected, to sample the pleural effusion.
In 10 patients (8 males and 2 females, mean age 66.9 ± 9.2 years) a pleural effusion was detected and sampled. In 7 out of the 10 cases, the cytological examination of the fluid obtained by EUS-FNA tested positive for malignant cells, thereby upgrading the case to Stage IV, irrespective of T and N statuses. In 3 cases the cytology on the EUS-FNA material was proven to be negative for malignancy thereby allowing patients to be treated with curative intent without further delay.
EUS-FNA of the pleural fluid is a safe and simple procedure. Our data, albeit stemming from a limited study population, show that it can be efficient in selected NSCLC cases for obtaining useful material and information with significant impact on the staging and, therefore, on the planning of the optimum therapeutic strategy.
INTRODUCTION
Endoscopic ultrasound-guided fine needle aspiration (EUS-FNA) and endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) stand as one of the major breakthroughs in the procedures linked to the diagnosis and staging of non-small-cell lung cancer (NSCLC). These procedures are highly accurate in the assessment of mediastinal lymph nodes involvement and have been proven to be safe and cost-effective for the assessment of the presence of posterior and anterior mediastinal lymph node involvement [1, 2].
A revised staging system for NSCLCs has recently been proposed [3], where the presence of malignant pleural effusion (PE) has been upgraded from T4 to M1 (invariably Stage IV). Thus the search for PE and—when present—obtaining a sample for cytological examination to verify or exclude the presence of malignant cells have become two very important steps in the staging of these neoplasms [4].
In this regard, EUS is able to detect small-size PEs (irrespective of the side explored) and represents a safe and feasible sampling technique for reaching a definitive diagnosis and staging definition [4]. Initially, we evaluate and discuss the impact of EUS in the clinical setting as a clinical decision support system, by retrospectively analysing the evidence stemming from our direct experience.
MATERIALS AND METHODS
We performed a retrospective review of all 92 patients with NSCLC, who underwent EUS-FNA for diagnostic and staging purposes at our institution in the period between January 2009 and December 2011. Prior to EUS examination, all patients routinely underwent chest CT and PET-CT scan in order to guide tissue acquisition during EUS procedures.
Indication for endoscopic ultrasound procedures
The data on all patients observed and treated in the declared time-period, with a central tumour adjacent to the oesophagus or with a PET/CT scan positive for mediastinal lymph nodes or for an adrenal lesion, who underwent EUS-FNA for diagnostic or staging purposes, have been included in this study. In particular, among these 92 patients, the EUS examination was carried out to directly sample the pulmonary mass (tumour, T-factor) or to achieve a tissue acquisition of the mediastinal lymph nodes (MLN, N-factor) or of the adrenal gland lesions (AL, M-factor) that were suspected to be malignant at PET/CT (see Table 1). During the EUS examination, the presence of pleural effusion (PE) was evaluated and, when present, an EUS-FNA was systematically attempted (see Table 1).
EUS procedures performed to achieve a diagnosis or a staging in patients with NSCLC
| Sampling EUS procedures . | n . |
|---|---|
| Tumour only | 5 |
| MLN only | 49 |
| AL only | 10 |
| Tumour + MLN | 14 |
| Tumour + AL | 3 |
| Tumour + PE | 2 |
| MLN + AL | 0 |
| MLN + PE | 8 |
| AL + PE | 0 |
| Tumour + MLN + AL | 1 |
| Sampling EUS procedures . | n . |
|---|---|
| Tumour only | 5 |
| MLN only | 49 |
| AL only | 10 |
| Tumour + MLN | 14 |
| Tumour + AL | 3 |
| Tumour + PE | 2 |
| MLN + AL | 0 |
| MLN + PE | 8 |
| AL + PE | 0 |
| Tumour + MLN + AL | 1 |
*MLN: mediastinal lymph node; Tumour: lung cancer; AL: adrenal lesion; PE: pleural effusion.
EUS procedures performed to achieve a diagnosis or a staging in patients with NSCLC
| Sampling EUS procedures . | n . |
|---|---|
| Tumour only | 5 |
| MLN only | 49 |
| AL only | 10 |
| Tumour + MLN | 14 |
| Tumour + AL | 3 |
| Tumour + PE | 2 |
| MLN + AL | 0 |
| MLN + PE | 8 |
| AL + PE | 0 |
| Tumour + MLN + AL | 1 |
| Sampling EUS procedures . | n . |
|---|---|
| Tumour only | 5 |
| MLN only | 49 |
| AL only | 10 |
| Tumour + MLN | 14 |
| Tumour + AL | 3 |
| Tumour + PE | 2 |
| MLN + AL | 0 |
| MLN + PE | 8 |
| AL + PE | 0 |
| Tumour + MLN + AL | 1 |
*MLN: mediastinal lymph node; Tumour: lung cancer; AL: adrenal lesion; PE: pleural effusion.
Endoscopic technique
All EUS examinations were performed by an experienced endosonographer (A.L.), using a conventional linear echo-endoscope. When PE was detected, EUS-FNA was performed after administration of prophylactic antibiotics, using a 22- or a 19-gauge needle (Echotip Ultra: Cook Medical Inc., Winston-Salem, NC, USA), depending on the amount of the effusion. A single-needle pass was done and the collected specimen was immediately placed into a preservative solution (ThinPrep® CytoLyt® solution: Cytyc Corp., Boxborough, MA, USA) for later evaluation.
RESULTS
In 10 patients (8 males and 2 females, mean age 66.9 ± 9.2 years) a PE was detected and sampled; mediastinal lymph node sampling was associated in eight cases. We did not observe any morbidity related to the endoscopic procedure, except in three patients who complained about non-specific mild chest discomfort that was quickly resolved with mild medication.
The main clinical and radiological characteristics and the EUS-findings of the case series are summarized in Table 2. In all patients but one, the PE had already been detected at a previously-performed CT scan within the context of the routine staging process (Fig. 1).
Main clinical and radiological characteristics of the case series, with EUS-findings.
| Case no. . | Age, sex . | Radiological findings . | Radiological clinical staging . | Strategy of care before EUS-FNA . | EUS-FNA findings . | Definitive staging . | Strategy of care after EUS-FNA . |
|---|---|---|---|---|---|---|---|
| 1 | 64, M | Small right PE without pleural lesions | T2N2Mx (Stage IIIa) | Induction RT-CHT | MLN metastasis and malignant PE | T2N2M1a (Stage IV) | CHT |
| 2 | 71, M | Large right PE with diffuse pleural thicknesses | T4N2M1 (Stage IV) | CHT | MLN metastasis and malignant PE | T4N2M1 (Stage IV) | CHT |
| 3 | 62, M | Small left PE without pleural nodules | T2N2Mx (Stage IIIa) | Induction RT-CHT | MLN metastasis and benign PE | T2N2M0 (Stage IIIa) | Induction RT-CHT |
| 4 | 68, F | Small left PE without pleural lesions | T2N2Mx (Stage IIIa) | Induction RT-CHT | MLN metastasis and malignant PE | T2N2M1a (Stage IV) | CHT |
| 5 | 59, M | No detection of PE (left NSCLC)a | T3N1Mx | Surgery | Malignant PE | T3N1M1a | CHT |
| 6 | 49, M | Small PE without pleural lesions (left NSCLC)a | T2N1Mx (Stage IIb) | Surgery | Benign PE | T2N1M0 (Stage IIb) | Surgery |
| 7 | 75, M | Small left PE with pleural lesions | T1N2Mx (Stage IIIa) | Induction RT-CHT | MLN metastasis and malignant PE | T1N2M1a (Stage IV) | CHT |
| 8 | 79, F | Large right PE with pleural thicknesses | T3N3M1 (Stage IV) | CHT | MLN metastasis and benign PE | T3N3M1 [liver] (Stage IV) | CHT |
| 9 | 73, M | Small right PE without pleural lesions | T4N2Mx (Stage IIIb) | CHT or Induction RT-CHT | MLN metastasis and malignant PE | T4N2M1 (Stage IV) | CHT |
| 10 | 69, M | Small PE without pleural lesions (left NSCLC)a | T2N1Mx (Stage IIb) | Surgery | MLN metastasis and malignant PE | T2N2M1 (Stage IV) | CHT |
| Case no. . | Age, sex . | Radiological findings . | Radiological clinical staging . | Strategy of care before EUS-FNA . | EUS-FNA findings . | Definitive staging . | Strategy of care after EUS-FNA . |
|---|---|---|---|---|---|---|---|
| 1 | 64, M | Small right PE without pleural lesions | T2N2Mx (Stage IIIa) | Induction RT-CHT | MLN metastasis and malignant PE | T2N2M1a (Stage IV) | CHT |
| 2 | 71, M | Large right PE with diffuse pleural thicknesses | T4N2M1 (Stage IV) | CHT | MLN metastasis and malignant PE | T4N2M1 (Stage IV) | CHT |
| 3 | 62, M | Small left PE without pleural nodules | T2N2Mx (Stage IIIa) | Induction RT-CHT | MLN metastasis and benign PE | T2N2M0 (Stage IIIa) | Induction RT-CHT |
| 4 | 68, F | Small left PE without pleural lesions | T2N2Mx (Stage IIIa) | Induction RT-CHT | MLN metastasis and malignant PE | T2N2M1a (Stage IV) | CHT |
| 5 | 59, M | No detection of PE (left NSCLC)a | T3N1Mx | Surgery | Malignant PE | T3N1M1a | CHT |
| 6 | 49, M | Small PE without pleural lesions (left NSCLC)a | T2N1Mx (Stage IIb) | Surgery | Benign PE | T2N1M0 (Stage IIb) | Surgery |
| 7 | 75, M | Small left PE with pleural lesions | T1N2Mx (Stage IIIa) | Induction RT-CHT | MLN metastasis and malignant PE | T1N2M1a (Stage IV) | CHT |
| 8 | 79, F | Large right PE with pleural thicknesses | T3N3M1 (Stage IV) | CHT | MLN metastasis and benign PE | T3N3M1 [liver] (Stage IV) | CHT |
| 9 | 73, M | Small right PE without pleural lesions | T4N2Mx (Stage IIIb) | CHT or Induction RT-CHT | MLN metastasis and malignant PE | T4N2M1 (Stage IV) | CHT |
| 10 | 69, M | Small PE without pleural lesions (left NSCLC)a | T2N1Mx (Stage IIb) | Surgery | MLN metastasis and malignant PE | T2N2M1 (Stage IV) | CHT |
MLN: mediastinal lymph nodal; PE: pleural effusion; RT-CHT: radiochemotherapy.
aEUS initially scheduled for sampling the pulmonary lesion adjacent to the oesophagus.
Main clinical and radiological characteristics of the case series, with EUS-findings.
| Case no. . | Age, sex . | Radiological findings . | Radiological clinical staging . | Strategy of care before EUS-FNA . | EUS-FNA findings . | Definitive staging . | Strategy of care after EUS-FNA . |
|---|---|---|---|---|---|---|---|
| 1 | 64, M | Small right PE without pleural lesions | T2N2Mx (Stage IIIa) | Induction RT-CHT | MLN metastasis and malignant PE | T2N2M1a (Stage IV) | CHT |
| 2 | 71, M | Large right PE with diffuse pleural thicknesses | T4N2M1 (Stage IV) | CHT | MLN metastasis and malignant PE | T4N2M1 (Stage IV) | CHT |
| 3 | 62, M | Small left PE without pleural nodules | T2N2Mx (Stage IIIa) | Induction RT-CHT | MLN metastasis and benign PE | T2N2M0 (Stage IIIa) | Induction RT-CHT |
| 4 | 68, F | Small left PE without pleural lesions | T2N2Mx (Stage IIIa) | Induction RT-CHT | MLN metastasis and malignant PE | T2N2M1a (Stage IV) | CHT |
| 5 | 59, M | No detection of PE (left NSCLC)a | T3N1Mx | Surgery | Malignant PE | T3N1M1a | CHT |
| 6 | 49, M | Small PE without pleural lesions (left NSCLC)a | T2N1Mx (Stage IIb) | Surgery | Benign PE | T2N1M0 (Stage IIb) | Surgery |
| 7 | 75, M | Small left PE with pleural lesions | T1N2Mx (Stage IIIa) | Induction RT-CHT | MLN metastasis and malignant PE | T1N2M1a (Stage IV) | CHT |
| 8 | 79, F | Large right PE with pleural thicknesses | T3N3M1 (Stage IV) | CHT | MLN metastasis and benign PE | T3N3M1 [liver] (Stage IV) | CHT |
| 9 | 73, M | Small right PE without pleural lesions | T4N2Mx (Stage IIIb) | CHT or Induction RT-CHT | MLN metastasis and malignant PE | T4N2M1 (Stage IV) | CHT |
| 10 | 69, M | Small PE without pleural lesions (left NSCLC)a | T2N1Mx (Stage IIb) | Surgery | MLN metastasis and malignant PE | T2N2M1 (Stage IV) | CHT |
| Case no. . | Age, sex . | Radiological findings . | Radiological clinical staging . | Strategy of care before EUS-FNA . | EUS-FNA findings . | Definitive staging . | Strategy of care after EUS-FNA . |
|---|---|---|---|---|---|---|---|
| 1 | 64, M | Small right PE without pleural lesions | T2N2Mx (Stage IIIa) | Induction RT-CHT | MLN metastasis and malignant PE | T2N2M1a (Stage IV) | CHT |
| 2 | 71, M | Large right PE with diffuse pleural thicknesses | T4N2M1 (Stage IV) | CHT | MLN metastasis and malignant PE | T4N2M1 (Stage IV) | CHT |
| 3 | 62, M | Small left PE without pleural nodules | T2N2Mx (Stage IIIa) | Induction RT-CHT | MLN metastasis and benign PE | T2N2M0 (Stage IIIa) | Induction RT-CHT |
| 4 | 68, F | Small left PE without pleural lesions | T2N2Mx (Stage IIIa) | Induction RT-CHT | MLN metastasis and malignant PE | T2N2M1a (Stage IV) | CHT |
| 5 | 59, M | No detection of PE (left NSCLC)a | T3N1Mx | Surgery | Malignant PE | T3N1M1a | CHT |
| 6 | 49, M | Small PE without pleural lesions (left NSCLC)a | T2N1Mx (Stage IIb) | Surgery | Benign PE | T2N1M0 (Stage IIb) | Surgery |
| 7 | 75, M | Small left PE with pleural lesions | T1N2Mx (Stage IIIa) | Induction RT-CHT | MLN metastasis and malignant PE | T1N2M1a (Stage IV) | CHT |
| 8 | 79, F | Large right PE with pleural thicknesses | T3N3M1 (Stage IV) | CHT | MLN metastasis and benign PE | T3N3M1 [liver] (Stage IV) | CHT |
| 9 | 73, M | Small right PE without pleural lesions | T4N2Mx (Stage IIIb) | CHT or Induction RT-CHT | MLN metastasis and malignant PE | T4N2M1 (Stage IV) | CHT |
| 10 | 69, M | Small PE without pleural lesions (left NSCLC)a | T2N1Mx (Stage IIb) | Surgery | MLN metastasis and malignant PE | T2N2M1 (Stage IV) | CHT |
MLN: mediastinal lymph nodal; PE: pleural effusion; RT-CHT: radiochemotherapy.
aEUS initially scheduled for sampling the pulmonary lesion adjacent to the oesophagus.
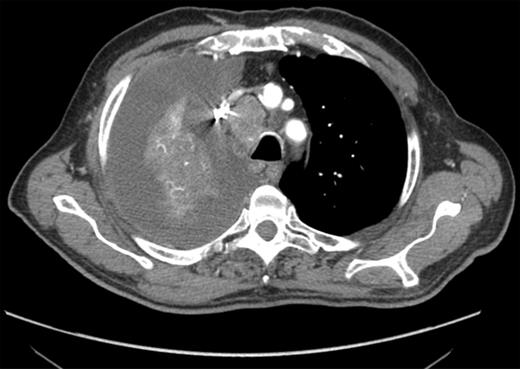
CT-scan findings: a moderate right PE, associated with large and necrotic mediastinal lymph nodes, was detected and EUS-FNA indicated.
In 7 out of the sampled 10 cases, the cytological examination of the fluid obtained by EUS-FNA (Fig. 2) tested positive for malignant cells (Stage IV) (Fig. 3). This represented crucial information (see Table 2); in fact, while in two of these seven cases (numbers 2 and 8) the M1-status had already been determined by other parameters detected in the standard staging process, in the remaining five cases (numbers 1, 4, 5, 7 and 10) the malignant nature of the PE sampled via EUS-FNA changed the further planning of therapy in each of the patients (Table 2). In particular, in the absence of the information provided by the EUS-FNA procedure (and consequent cytology) these patients would have been given local treatment such as surgery (cases 5 and 10) and concurrent radio-chemotherapy (cases 1, 4 and 7). Thanks to the EUS-FNA/cytology findings, patients were up-staged to Stage IV and this prompted the re-thinking of the therapeutic strategy, with systemic control taking priority with respect to the local one.
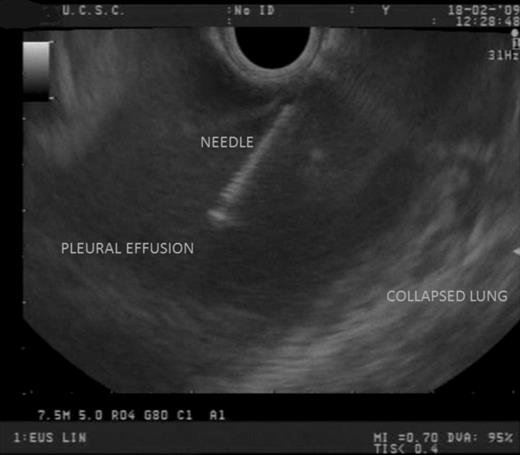
EUS findings: the right PE was easily sampled with a 22-gauge needle, without complications.
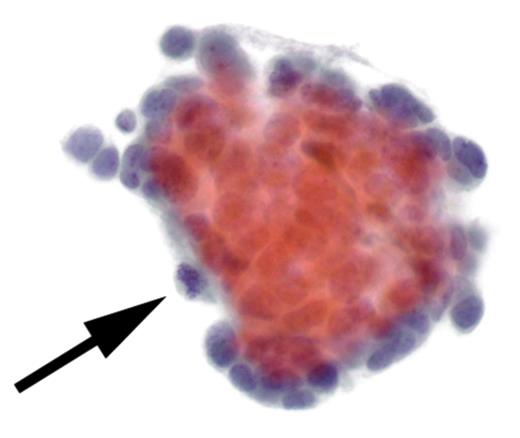
Cytological findings: the examination of the pleural fluid demonstrated the presence of malignant neoplastic cells (see arrow).
In three cases the cytology of the EUS-FNA material was proven to be negative for malignancy, thereby allowing patients to be treated without further delay, with curative intent. In accordance with the cytological findings obtained by EUS-FNA procedure, in none of the nine patients with concurrent evidence of PE by CT and EUS evaluation was a thoracentesis performed. At surgery, no patient was found to have a malignant PE that had been missed by the EUS procedure.
DISCUSSION
The EUS-guided FNA is an accurate, safe and cost-effective tool to screen patients with NSCLC for mediastinal metastases [2]. Although several studies on the role of EUS in NSCLC had included the staging of tumour or lymph node mediastinal invasion [2, 4], to our knowledge, the accuracy of the EUS for evaluating PE and, therefore, its potential impact in the clinical decision-making process has never been detailed in a focussed analysis.
In the English literature there are limited data on the utility of EUS-guided thoracentesis [5, 6]. Chang et al. [5] performed this procedure in two patients with gastric carcinoma to diagnose malignant ascites and malignant PEs not detected by standard radiological examinations. De Witt et al. [6], in a retrospective single-centre study, identified nine consecutive cancer patients who underwent EUS-guided thoracentesis: in two cases the pleural sampling provided the diagnosis of a malignant effusion while, in the remaining seven cases, pleural fluid cytology was negative.
In the present study, we analysed the efficacy and usefulness of this procedure in a subset of NSCLC patients, who underwent EUS to sample only mediastinal lymph-nodes (more rarely to sample the pulmonary lesions or adrenal lesions) and in whom a PE was also detected and, therefore, sampled.
Although limited by the small number of patients (with retrospective data analysis), our experience confirms that the EUS–FNA of pleural fluid is technically feasible and safe and that it may provide elements for a precise diagnosis of a malignant PE (Stage IV). The positivity of pleural fluid cytology (obtained in 7 patients out of the 10 sampled) has influenced the strategy of care; in particular, in 5 out of 10 patients, the indication for a predominantly local treatment (based on surgical resection or radio-chemotherapy within a neoadjuvant setting) was modified—thus advocating a systemic treatment—according to the EUS-FNA findings of the PE sampling (see Table 2). On the other hand, in three cases, the cytology on the EUS-FNA material was proven to be negative for malignancy, thereby allowing patients to be treated without further delay. Of course, the same information (on positivity or negativity of the PE and, thus, the confirmation of local or disseminated stages of disease) may be achieved by classical techniques, such as intercostal needle sampling, which represents the ‘gold standard’.
Concerning the remaining 82 patients in our series, we did not observe any PE detected at CT scan and PET/CT scan but missed during EUS: on the contrary, in one case (no. 5) a small PE (unknown at standard radiological evaluation) was detected during EUS. Although it is not possible to define the sensitivity/sensibility of our procedure with data drawn from such a small cohort, some preliminary evidence reported by McLoud and co-workers suggest EUS to be comparable with (if not superior to) standard chest CT scan for characterizing the PE, especially in a malignant effusion [7]. In this setting, further studies are needed to investigate the accuracy of EUS in diagnosing a PE in patients with NSCLC (or other tumours).
In the literature, multiple studies have evaluated the sensitivity of cytological diagnosis of pleural fluid from thoracentesis fluid sample, compared with needle biopsy and thoracoscopy [8]. However, very few studies exist to determine the optimal volume of pleural fluid withdrawn during a thoracentesis to diagnose malignancy. In a recent prospective study, in which 23 patients were diagnosed with pleural malignancy by thoracentesis, there was no difference shown between 50 ml of pleural fluid and larger volumes of fluid for the cytological diagnosis of malignancy [9]. Moreover, as hypothesized by Baumann [10], “Pleural fluid cells may not be homogeneously distributed within the pleural fluid. Cells may settle in a gravity-dependent gradient that creates an environment, whereby a larger volume will more likely recover malignant cells”. In particular, this evidence may partly explain the high rate of positive pleural fluid cytology obtained in our series, being the fluid sampled via EUS-FNA localized (for gravity) in the cardio-phrenic recess and, thus, more ‘adequate for diagnosis’ (in accordance with the theory just reported).
Finally, in this scenario, some authors [11, 12] have recently reported their results with the use of FDG-PET/CT in distinguishing benign from malignant PE. Erasmus et al. [12] examined 25 patients with NSCLC. Of 22 patients with positive findings on FDG-PET, 21 had pleural metastases and, of three patients with negative findings, one had pleural metastases. Although these preliminary results could be rewarding, these studies should be confirmed by information stemming from large, prospective analyses.
In the debate on what is the best option for pleural fluid evaluation, our data stress the importance of a correct staging when NSCLC is associated with PE. Considering the ease, efficacy and directness of EUS in sampling pulmonary lesions (T-status assessment) and lymph nodes involved (N-status assessment) [1, 4], we believe that EUS may play a significant role in the decision-making process of NSCLC management. In fact, in selected cases (posterior effusion or patients with comorbidities who could not withstand a thoracentesis procedure or, generally, when thoracentesis may not be feasible), EUS-FNA of PE (M-status assessment) may represent a very useful tool in the NSCLC clinical staging. Considering the small number of patients of our series and the background of substantially absent information on this procedure in the English literature, our preliminary findings warrant further confirmation by prospective, large-series studies.
In conclusion, when EUS-FNA is performed during pre-operative diagnosis and/or staging and a concurrent PE is identified, we strongly advocate, in selected patients, the detection and eventual sampling of the PE, considering the safety and accuracy of this technique, documented in this report.
Conflict of interest: none declared




