-
PDF
- Split View
-
Views
-
Cite
Cite
Karen McRae, Yaron Shargall, Martin Ma, John Thenganatt, Peter Slinger, John T. Granton, Marc de Perrot, Feasibility of blood conservation strategies in pulmonary endarterectomy for chronic thromboembolic pulmonary hypertension, Interactive CardioVascular and Thoracic Surgery, Volume 13, Issue 1, July 2011, Pages 35–39, https://doi.org/10.1510/icvts.2010.252767
Close - Share Icon Share
Abstract
Blood transfusion requirements were reviewed for a consecutive series of 25 patients undergoing elective pulmonary endarterectomy (PEA) between August 2005 and March 2009 in our institution. Patients were divided into two groups based on the implementation of a conservative blood transfusion algorithm that combined antifibrinolytic therapy, intraoperative blood sequestration, blood salvage and lack of correction of coagulation parameters in the absence of ongoing bleeding. Despite similar perioperative coagulation profiles in the two groups, the introduction of a conservative blood transfusion algorithm was associated with a significant increase in the number of patients receiving no homologous blood products. Of 16 patients who underwent surgery after the introduction of the algorithm, nine (56%) required no homologous blood products and five (31%) required one or two units of homologous red blood cells only. The international normalized ratio normalized within six to 12 hours after discontinuation of cardiopulmonary bypass without transfusion of fresh frozen plasma or platelets in 13 of the 16 patients. In conclusion, a conservative blood transfusion strategy allows PEA to be safely performed with no or minimal blood product transfusions in a majority of patients despite deep hypothermic circulatory arrest.
1. Introduction
Pulmonary endarterectomy (PEA) is a potentially curative option for patients with chronic thromboembolic pulmonary hypertension (CTEPH). The technical aspects of the procedure have been refined over the past 20 years [1, 2]. The surgery is performed on cardiopulmonary bypass (CPB) with periods of deep hypothermic circulatory arrest (DHCA) to stop back bleeding from the bronchial circulation. Although DHCA can be associated with significant blood loss and coagulopathy, no study has yet investigated homologous blood transfusion requirements in patients undergoing PEA. We therefore reviewed our experience with particular emphasis on the feasibility of conservative blood transfusion strategies.
2. Material and methods
Blood transfusion requirements were reviewed for a consecutive series of 25 patients undergoing elective PEA at the Toronto General Hospital between August 2005 and March 2009 (Table 1 ). Two patients who underwent emergency surgery after cardiovascular collapse and two others requiring concomitant valve replacement for severe mitral regurgitation and for infective endocarditis of the tricuspid valve during the same operation were not included in the study to have an homogenous group of patients.
| Age (years) | |
| Median | 61 |
| Range | 25–82 |
| Gender (n) | |
| Male | 14 |
| Female | 11 |
| Blood group (n) | |
| A | 14 |
| B | 2 |
| AB | 5 |
| O | 4 |
| Body mass index (kg/m2) | |
| Median | 28 |
| Range | 21–45 |
| Thrombophilic abnormality (n) | 6 |
| Factor V Leyden deficiency | 3 |
| Protein S deficiency | 1 |
| Antiphospholipid antibody | 1 |
| Prothrombin mutation | 1 |
| TPR (dynes/s/cm5) | |
| Median | 1005 |
| Range | 438–2545 |
| Age (years) | |
| Median | 61 |
| Range | 25–82 |
| Gender (n) | |
| Male | 14 |
| Female | 11 |
| Blood group (n) | |
| A | 14 |
| B | 2 |
| AB | 5 |
| O | 4 |
| Body mass index (kg/m2) | |
| Median | 28 |
| Range | 21–45 |
| Thrombophilic abnormality (n) | 6 |
| Factor V Leyden deficiency | 3 |
| Protein S deficiency | 1 |
| Antiphospholipid antibody | 1 |
| Prothrombin mutation | 1 |
| TPR (dynes/s/cm5) | |
| Median | 1005 |
| Range | 438–2545 |
PEA, pulmonary endarterectomy; TPR, total pulmonary vascular resistance.
| Age (years) | |
| Median | 61 |
| Range | 25–82 |
| Gender (n) | |
| Male | 14 |
| Female | 11 |
| Blood group (n) | |
| A | 14 |
| B | 2 |
| AB | 5 |
| O | 4 |
| Body mass index (kg/m2) | |
| Median | 28 |
| Range | 21–45 |
| Thrombophilic abnormality (n) | 6 |
| Factor V Leyden deficiency | 3 |
| Protein S deficiency | 1 |
| Antiphospholipid antibody | 1 |
| Prothrombin mutation | 1 |
| TPR (dynes/s/cm5) | |
| Median | 1005 |
| Range | 438–2545 |
| Age (years) | |
| Median | 61 |
| Range | 25–82 |
| Gender (n) | |
| Male | 14 |
| Female | 11 |
| Blood group (n) | |
| A | 14 |
| B | 2 |
| AB | 5 |
| O | 4 |
| Body mass index (kg/m2) | |
| Median | 28 |
| Range | 21–45 |
| Thrombophilic abnormality (n) | 6 |
| Factor V Leyden deficiency | 3 |
| Protein S deficiency | 1 |
| Antiphospholipid antibody | 1 |
| Prothrombin mutation | 1 |
| TPR (dynes/s/cm5) | |
| Median | 1005 |
| Range | 438–2545 |
PEA, pulmonary endarterectomy; TPR, total pulmonary vascular resistance.
Data were prospectively entered into a database and retrospectively reviewed after approval by the Institutional Review Board. The Institutional Review Board approval waived the need for patient consent to perform the study. Patients were divided into two groups based on the introduction of a conservative blood transfusion algorithm in January 2007.
2.1. Surgery
PEA was performed according to the standardized technique previously described with a period of DHCA for the right and the left side [1–3]. The circulatory arrest period was limited to 20 min with restoration of flow between each arrest. A hematocrit of 24–28% was targeted during the cooling period and 28–30% during the rewarming period.
2.2. Management of cardiopulmonary bypass (CPB)
A CPB circuit with a centrifugal pump was used. The priming volume ranged between 1250 ml and 1500 ml and included cristalloid (Ringer’s Lactate, Baxter, Mississauga, Canada), 25 g of mannitol, 100 ml of 8.4% sodium bicarbonate and heparin 5000 U. Once CPB was established, up to 1 l of normal saline was used to maintain circuit volume and to achieve the desired hemodilution during the cooling period. Albumin was used, if additional volume was required. If copious urine output was not observed during the rewarming phase, a small-dose of furosemide was given (5 mg). Washed concentrated cells from the cell saver were used to maintain CPB volume and raise hematocrit prior to separation from CPB. At the end of surgery, the residual volume from the CPB circuit was washed in the cell saver and included in the total volume of cells saved.
2.3. Management of anticoagulation
Platelet count, international normalized ratio (INR) and baseline activated coagulation time (ACT) were performed before surgery. ACT was used intraoperatively to monitor heparinization and the reversal of heparin anticoagulation with protamine. ACT of 480 and greater was targeted on CPB. Once patients were weaned from CPB and protamine reversal was given, platelet count, INR and fibrinogen level were used to monitor coagulopathy.
2.4. Blood conservation algorithm
Initially in our experience, transfusion was left to the discretion of the anesthesiologist and was based on the American Society of Anesthesiologists (ASA) guidelines for the transfusion of blood products (http://www.asahq.org/publicationsandservices/transfusion.pdf). Threshold for plasma transfusion was an INR >2, threshold for platelet transfusion was 50–100×109 platelets/l, and threshold for red blood cell (RBC) transfusion was hemoglobin of 80–90 g/l. After observing that patients with CTEPH often have minimal bleeding after coming-off CPB despite the presence of abnormal coagulation parameters, we introduced a specific conservative blood transfusion algorithm for the management of these patients.
Since January 2007, an INR >2 was not treated in the absence of ongoing clinical bleeding but was allowed to drift down spontaneously and an intravenous infusion of unfractionated heparin was started four to six hours postoperatively or once the INR decreased to <2. Unfractionated heparin level was then progressively increased to therapeutic range within 24 hours. Fresh frozen plasma (FFP) was administered (10–15 ml/kg) only if there was ongoing bleeding associated with an abnormal INR. Platelets were transfused only if there was persistent bleeding despite the administration of FFP or if the patient had known underlying platelet disorder. Homologous RBC were transfused only if the hemoglobin dropped below 80 g/l or the hematocrit below 25% postoperatively.
Since January 2007, heparinized autologous blood (500–1000 ml) was also sequestered intraoperatively from the venous cannulas immediately before going on CPB in patients with a preoperative hemoglobin >130 g/l. The blood was stored at room temperature in a sterile bag, agitated every 10 min and reinfused at the end of the surgery before heparin reversal with protamine. This strategy allowed the preservation of intact clotting factors and functional platelets in the sequestered blood. Sequestration of RBC also helped to achieve the desired hemodilution during the cooling phase.
Antifibrinolytic therapy and a cell-saver system were used for all patients. Aprotinin was used until October 2007 and was then switched to tranexamic acid after the release of the preliminary results of the ‘Blood Conservation Using Antifibrinolytics in a Randomized Trial’ (BART) [4], and the resulting FDA recommendation of the suspension of the distribution of aprotinin. Our patients received the dose regimens used in the BART trial for both antifibrinolytics.
2.5. Homologous blood transfusion
Homologous blood transfusion included all homologous RBC, FFP, and platelet transfusion during the hospital stay. Albumin was not included in the homologous blood transfusion. Cryoglobulin, factor VIIa and prothrombin complex concentrates were not administered.
2.6. Postoperative course
The patients were kept relatively hypovolemic after the surgery to reduce the risk of reperfusion pulmonary edema. If additional volume was needed, normal saline up to 500 ml was used as a first choice and then colloid solution, such as albumin was used preferentially. Operative mortality was defined as death occurring within 30 days of surgery or during the postoperative hospital stay. All patients were followed postoperatively at one, three, six and 12 months and then on a yearly basis. Follow-up was complete for all patients until June 2010.
2.7. Statistical analysis
Data are expressed as mean±standard deviation (S.D.) or as median and range. Unpaired t-test was used for normally distributed data and the Mann–Whitney test was used otherwise. Fisher’s exact test was used for categorical variables. Statview V (Abacus Concept, Berkeley, CA, USA) was used for all analyses. P-value <0.05 was considered significant.
3. Results
Total pulmonary vascular resistance decreased from 1074±489 dynes/s/cm5 preoperatively to 377±159 dynes/s/cm5 postoperatively. A total of 19 patients (73%) were extubated within two days from surgery, discharged from intensive care unit within four days and discharged from hospital within 21 days. One patient died postoperatively from right ventricular dysfunction for an overall operative mortality of 4%. Other complications occurred in six patients and included pneumonia (n=2), pulmonary edema (n=1), transient acute renal failure (n=1), transient liver dysfunction (n=1), and sternal dehiscence requiring rewiring (n=1).
The overall in-hospital requirement of homologous RBC, FFP, and platelet transfusions before and after the introduction of the conservative blood transfusion algorithm is presented in Fig. 1 . Despite similar perioperative parameters between the two groups (Table 2 ), the introduction of a conservative blood transfusion algorithm was associated with a significant increase in the number of patients requiring no homologous blood products (Table 3 ). Of 16 patients who underwent surgery after the introduction of the algorithm, nine (56%) received no homologous blood transfusion, and five (31%) required one or two units of homologous RBC transfusion only. The INR normalized within six to 12 hours after discontinuation of CPB without transfusion of FFP or platelets in 13 of 16 patients. The rate of normalization of the INR was similar whether patients received transfusions of FFP or not (Fig. 2 ). The chest tube drainage was not affected by the introduction of the conservative blood transfusion algorithm (Fig. 3 ). The time to start of postoperative heparin infusion (7±6 h vs. 5±2 h, P=0.2), time to therapeutic anticoagulation (13±7 h vs. 11±6 h, P=0.5), hemoglobin level at 24 hours (104±15 g/l vs. 105±13 g/l, P=0.9) and hemoglobin level at seven days (97±15 g/l vs. 97±12 g/l, P=0.9) were also not affected by the introduction of the algorithm.
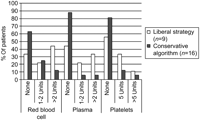
Overall blood transfusion requirement in patients undergoing pulmonary endarterectomy before (liberal strategy) and after the introduction of a conservative blood transfusion algorithm.
| Conservative blood transfusion algorithm | P-value | ||
| No (n=9) | Yes (n=16) | ||
| Preoperative hemoglobin (g/l) | 147±21 | 142±20 | 0.6 |
| Preoperative platelet count (×109/l) | 269±64 | 237±96 | 0.4 |
| Preoperative INR | 1.4±0.2 | 1.3±0.4 | 0.5 |
| Baseline ACT | 145±18 | 157±39 | 0.4 |
| Cardiopulmonary bypass time (min) | 271±45 | 248±37 | 0.2 |
| Aortic cross-clamp (min) | 150±27 | 130±28 | 0.1 |
| Total circulatory arrest (min) | 35±9 | 36±13 | 0.8 |
| Estimated net blood loss (l) | 1.6±0.6 | 1.6±1.1 | 0.8 |
| Intraoperative fluid balance (l) | 2.9±1.7 | 2.4±1.2 | 0.4 |
| Volume of shed blood into cell saver (l) | 4.1±2.4 | 4.1±2.2 | 0.9 |
| Volume of washed cells returned (l) | 1.9±1.3 | 2.1±1.1 | 0.7 |
| Nadir temperature (°C) | 18±1 | 18±1 | 1.0 |
| Lowest hematocrit (%) | 24±3 | 25±3 | 0.4 |
| Maximum ACT | 819±166 | 906±111 | 0.1 |
| Total dose of heparin (×1000) | 49±17 | 67±29 | 0.1 |
| Total protamine dose given | 475±63 | 463±122 | 0.8 |
| ACT after administration of protamin | 140±48 | 150±24 | 0.5 |
| Initial INR postCPB | 2.3±0.4 | 2.6±0.8 | 0.3 |
| Fibrinogen level postCPB | 1.8±0.5 | 1.7±0.4 | 0.6 |
| Platelet count postCPB (×109/l) | 122±41 | 125±57 | 0.9 |
| Conservative blood transfusion algorithm | P-value | ||
| No (n=9) | Yes (n=16) | ||
| Preoperative hemoglobin (g/l) | 147±21 | 142±20 | 0.6 |
| Preoperative platelet count (×109/l) | 269±64 | 237±96 | 0.4 |
| Preoperative INR | 1.4±0.2 | 1.3±0.4 | 0.5 |
| Baseline ACT | 145±18 | 157±39 | 0.4 |
| Cardiopulmonary bypass time (min) | 271±45 | 248±37 | 0.2 |
| Aortic cross-clamp (min) | 150±27 | 130±28 | 0.1 |
| Total circulatory arrest (min) | 35±9 | 36±13 | 0.8 |
| Estimated net blood loss (l) | 1.6±0.6 | 1.6±1.1 | 0.8 |
| Intraoperative fluid balance (l) | 2.9±1.7 | 2.4±1.2 | 0.4 |
| Volume of shed blood into cell saver (l) | 4.1±2.4 | 4.1±2.2 | 0.9 |
| Volume of washed cells returned (l) | 1.9±1.3 | 2.1±1.1 | 0.7 |
| Nadir temperature (°C) | 18±1 | 18±1 | 1.0 |
| Lowest hematocrit (%) | 24±3 | 25±3 | 0.4 |
| Maximum ACT | 819±166 | 906±111 | 0.1 |
| Total dose of heparin (×1000) | 49±17 | 67±29 | 0.1 |
| Total protamine dose given | 475±63 | 463±122 | 0.8 |
| ACT after administration of protamin | 140±48 | 150±24 | 0.5 |
| Initial INR postCPB | 2.3±0.4 | 2.6±0.8 | 0.3 |
| Fibrinogen level postCPB | 1.8±0.5 | 1.7±0.4 | 0.6 |
| Platelet count postCPB (×109/l) | 122±41 | 125±57 | 0.9 |
INR, international normalized ratio; ACT, activated coagulation time; CPB, cardiopulmonary bypass.
| Conservative blood transfusion algorithm | P-value | ||
| No (n=9) | Yes (n=16) | ||
| Preoperative hemoglobin (g/l) | 147±21 | 142±20 | 0.6 |
| Preoperative platelet count (×109/l) | 269±64 | 237±96 | 0.4 |
| Preoperative INR | 1.4±0.2 | 1.3±0.4 | 0.5 |
| Baseline ACT | 145±18 | 157±39 | 0.4 |
| Cardiopulmonary bypass time (min) | 271±45 | 248±37 | 0.2 |
| Aortic cross-clamp (min) | 150±27 | 130±28 | 0.1 |
| Total circulatory arrest (min) | 35±9 | 36±13 | 0.8 |
| Estimated net blood loss (l) | 1.6±0.6 | 1.6±1.1 | 0.8 |
| Intraoperative fluid balance (l) | 2.9±1.7 | 2.4±1.2 | 0.4 |
| Volume of shed blood into cell saver (l) | 4.1±2.4 | 4.1±2.2 | 0.9 |
| Volume of washed cells returned (l) | 1.9±1.3 | 2.1±1.1 | 0.7 |
| Nadir temperature (°C) | 18±1 | 18±1 | 1.0 |
| Lowest hematocrit (%) | 24±3 | 25±3 | 0.4 |
| Maximum ACT | 819±166 | 906±111 | 0.1 |
| Total dose of heparin (×1000) | 49±17 | 67±29 | 0.1 |
| Total protamine dose given | 475±63 | 463±122 | 0.8 |
| ACT after administration of protamin | 140±48 | 150±24 | 0.5 |
| Initial INR postCPB | 2.3±0.4 | 2.6±0.8 | 0.3 |
| Fibrinogen level postCPB | 1.8±0.5 | 1.7±0.4 | 0.6 |
| Platelet count postCPB (×109/l) | 122±41 | 125±57 | 0.9 |
| Conservative blood transfusion algorithm | P-value | ||
| No (n=9) | Yes (n=16) | ||
| Preoperative hemoglobin (g/l) | 147±21 | 142±20 | 0.6 |
| Preoperative platelet count (×109/l) | 269±64 | 237±96 | 0.4 |
| Preoperative INR | 1.4±0.2 | 1.3±0.4 | 0.5 |
| Baseline ACT | 145±18 | 157±39 | 0.4 |
| Cardiopulmonary bypass time (min) | 271±45 | 248±37 | 0.2 |
| Aortic cross-clamp (min) | 150±27 | 130±28 | 0.1 |
| Total circulatory arrest (min) | 35±9 | 36±13 | 0.8 |
| Estimated net blood loss (l) | 1.6±0.6 | 1.6±1.1 | 0.8 |
| Intraoperative fluid balance (l) | 2.9±1.7 | 2.4±1.2 | 0.4 |
| Volume of shed blood into cell saver (l) | 4.1±2.4 | 4.1±2.2 | 0.9 |
| Volume of washed cells returned (l) | 1.9±1.3 | 2.1±1.1 | 0.7 |
| Nadir temperature (°C) | 18±1 | 18±1 | 1.0 |
| Lowest hematocrit (%) | 24±3 | 25±3 | 0.4 |
| Maximum ACT | 819±166 | 906±111 | 0.1 |
| Total dose of heparin (×1000) | 49±17 | 67±29 | 0.1 |
| Total protamine dose given | 475±63 | 463±122 | 0.8 |
| ACT after administration of protamin | 140±48 | 150±24 | 0.5 |
| Initial INR postCPB | 2.3±0.4 | 2.6±0.8 | 0.3 |
| Fibrinogen level postCPB | 1.8±0.5 | 1.7±0.4 | 0.6 |
| Platelet count postCPB (×109/l) | 122±41 | 125±57 | 0.9 |
INR, international normalized ratio; ACT, activated coagulation time; CPB, cardiopulmonary bypass.
| Variable | Conservative blood transfusion algorithm | P-value | |
| No (n=9) | Yes (n=16) | ||
| Patients requiring no transfusion (n) | 1 (11%) | 9 (56%) | 0.04 |
| Patients requiring no RBC (n) | 3 (33%) | 10 (63%) | 0.2 |
| Patients requiring no FFP (n) | 4 (44%) | 14 (88%) | 0.06 |
| Patients requiring no platelets (n) | 5 (56%) | 13 (81%) | 0.2 |
| Variable | Conservative blood transfusion algorithm | P-value | |
| No (n=9) | Yes (n=16) | ||
| Patients requiring no transfusion (n) | 1 (11%) | 9 (56%) | 0.04 |
| Patients requiring no RBC (n) | 3 (33%) | 10 (63%) | 0.2 |
| Patients requiring no FFP (n) | 4 (44%) | 14 (88%) | 0.06 |
| Patients requiring no platelets (n) | 5 (56%) | 13 (81%) | 0.2 |
RBC, red blood cells; FFP, fresh frozen plasma.
| Variable | Conservative blood transfusion algorithm | P-value | |
| No (n=9) | Yes (n=16) | ||
| Patients requiring no transfusion (n) | 1 (11%) | 9 (56%) | 0.04 |
| Patients requiring no RBC (n) | 3 (33%) | 10 (63%) | 0.2 |
| Patients requiring no FFP (n) | 4 (44%) | 14 (88%) | 0.06 |
| Patients requiring no platelets (n) | 5 (56%) | 13 (81%) | 0.2 |
| Variable | Conservative blood transfusion algorithm | P-value | |
| No (n=9) | Yes (n=16) | ||
| Patients requiring no transfusion (n) | 1 (11%) | 9 (56%) | 0.04 |
| Patients requiring no RBC (n) | 3 (33%) | 10 (63%) | 0.2 |
| Patients requiring no FFP (n) | 4 (44%) | 14 (88%) | 0.06 |
| Patients requiring no platelets (n) | 5 (56%) | 13 (81%) | 0.2 |
RBC, red blood cells; FFP, fresh frozen plasma.
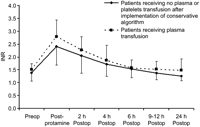
Time course of INR after cardiopulmonary bypass in 13 patients treated without any fresh frozen plasma or platelets after implementation of the conservative algorithm (solid line). The rate of normalization of the INR was similar to patients receiving plasma transfusion (dashed line). INR, international normalized ratio.
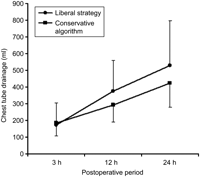
Chest tube drainage before (liberal strategy) and after the introduction of a conservative blood transfusion algorithm.
After a median follow-up of 24 months since surgery (range, 3–47 months), all but one patient have remained in the New York Heart Association (NYHA) class I (n=15) or II (n=9) with a treatment of anti-coagulation only. One patient developed recurrent exercise limitation 18 months after surgery and was started on endothelin receptor antagonist therapy for progression of pulmonary hypertension. No patients experienced recurrent pulmonary emboli during follow-up.
4. Discussion
Elective PEA has become a safe procedure in experienced centers with an operative mortality of <5% [1]. Much of this improvement is related to the current quality of the surgery and better perioperative management of patients with pulmonary hypertension. Animal experiments supported by clinical observations have shown that several periods of circulatory arrest at 18 °C does not alter the brain metabolism as long as the period of each circulatory arrest is limited to <20–25 min [5, 6]. Since most postoperative complications after PEA are related to the persistence of pulmonary hypertension, the primary goal of the surgery must be the normalization of the pulmonary artery pressures rather than limiting the total length of circulatory arrest.
Patients with CTEPH typically develop a hypertrophied bronchial circulation due to the chronic occlusion of the pulmonary artery circulation [7]. Hence, a period of circulatory arrest is usually used to ensure optimal visibility during the endarterectomy at the level of the segmental and subsegmental branches. Although DHCA is often considered to be associated with a high risk of perioperative bleeding due to significant coagulopathy, we observed that PEA can be performed with limited transfusion in patients with CTEPH.
The combination of blood conservation measures with intra-operative blood salvage, blood sequestration immediately before going on CPB, the administration of tranexamic acid and the lack of correction of coagulation parameters in the absence of ongoing bleeding allowed us to do the surgery with no homologous blood transfusion in 56% of the patients and with transfusion of one or two units of RBC only in 31% of the patients. The introduction of a conservative blood transfusion algorithm was associated with a reduction in the number of patients requiring FFP from 56% to 12%. The INR normalized within six to 12 h after discontinuation of CPB without any transfusion of FFP or platelets in almost all patients. The rate of normalization of the INR was very similar whether the patients received FFP or not. The lack of bleeding despite abnormal coagulation parameters in patients with CTEPH is potentially related to an increased in the pro-coagulation drive that may have led to the development of the disease in the first place [8, 9]. Hence, there is a relatively poor correlation between bleeding and the peripheral indices of coagulation in these patients.
This analysis has limitation related to the retrospective nature of the study and the relatively small number of patients. The comparison of two different cohorts of patients over time is also limited by a change in the antifibrinolytic therapy from aprotinin to tranexamic acid and by increasing experience from the surgical and anesthetic teams over time. This study, however, demonstrates that PEA can be safely performed with no or minimal blood product transfusion in the majority of patients. As PEA has become successful with limited operative mortality and excellent long-term outcome, the number of patients that are candidates for this surgery is rapidly expanding and blood conservation strategies are warranted in this patient population in the future.
In conclusion, PEA is the standard of care for patients with CTEPH. PEA has become a safe procedure in experienced centers. Blood conservative strategies allow PEA to be performed with no or minimal blood product transfusion in the majority of patients despite DHCA. Abnormal coagulation parameters do not correlate with postoperative bleeding in patients with CTEPH and should not necessarily be corrected unless there is evidence of ongoing bleeding.
References
- cardiopulmonary bypass
- thromboembolic pulmonary hypertension
- hemorrhage
- blood platelets
- erythrocytes
- antifibrinolytic agents
- blood coagulation
- blood transfusion
- international normalized ratio
- intraoperative care
- surgical procedures, operative
- surgery specialty
- fresh-frozen plasma transfusion
- blood products
- coagulation process
- deep hypothermic circulatory arrest
- pulmonary artery endarterectomy




