-
PDF
- Split View
-
Views
-
Cite
Cite
Luca Deorsola, Enrico Chiappa, Gabriella Agnoletti, Pietro Angelo Abbruzzese, Rescue mitral valve repair one hour after birth, Interactive CardioVascular and Thoracic Surgery, Volume 11, Issue 5, November 2010, Pages 716–718, https://doi.org/10.1510/icvts.2010.241828
Close - Share Icon Share
Abstract
Congenital mitral regurgitation is rare and usually part of complex cardiac anomalies. When needed, early surgery represents a great challenge. In small babies avoiding valve replacement is desirable, but valve repair may be extremely complex. We describe an isolated congenital mitral regurgitation, successfully treated with conservative surgery about 1 h after birth. In a 30-year-old pregnant woman, fetal echocardiography revealed mitral annular dilatation with massive regurgitation, functional aortic atresia and a very small patent foramen ovale. Realizing that the baby had a poor chance of survival after birth, a cesarean section was scheduled at 37 weeks of pregnancy. The procedure was performed in the operating room next to the cardiac surgery theatre, where the newborn was urgently transferred. After an unsuccessful attempt of percutaneous atrial septostomy, a rescue surgical mitral repair was performed. To avoid mitral replacement, moderate residual regurgitation was accepted. Postoperative hospital stay was 57 days and the baby was discharged in good clinical condition. Residual mitral regurgitation was moderate at discharge and decreased thereafter. During a five-year follow-up the child remained asymptomatic with normal growth. Preserved ventricular function and progressive volume reduction of left heart chambers were observed.
1. Introduction
Congenital mitral regurgitation is rare (0.2–0.4% of mitral regurgitations). In 60% of cases, it is associated with other cardiac anomalies [1]. We describe a case of congenitally malformed mitral valve in a newborn, which was surgically treated only 1 h after delivery. Expecting the baby would not survive after birth, because of a restrictive patent foramen ovale (PFO), an emergency intervention was planned and performed after a preterm cesarean section.
2. Materials and methods
A 30-year-old woman was referred to our centre at 32 weeks of pregnancy, because of fetal cardiomegaly detected at routine prenatal ultrasound.
Fetal echocardiography showed marked dilatation of the left ventricle (LV) and a giant left atrium (LA). The interatrial septum was displaced rightwards, with a restrictive PFO. The mitral valve was dilated, with central loss of coaptation and severe regurgitation. Left ventricular function was impaired. Aortic valve showed neither cusp movement nor antegrade flow. Ascending aorta (AAO) and aortic arch were perfused retrogradely through a wide patent ductus arteriosus (PDA) (Fig. 1 ).

Fetal echocardiogram at 35 weeks' gestational age. Cross-sectional view along the four-chamber plane showing a markedly increased cardio-thoracic ratio mainly due to a massive dilation of the left atrium. On the top panel, the white arrowheads indicate the interatrial septum bulging into the right atrium, with the foramen ovale apparently sealed. On the bottom panel, a systolic frame, with color flow mapping showing massive mitral regurgitation. The white arrowhead indicates the negligible forward flow into the ascending aorta. AAO, ascending aorta; LA, left atrium; LV, left ventricle; RA, right atrium; RV, right ventricle.
These findings led to the wrong diagnosis of critical aortic valve stenosis.
At the last fetal echocardiogram (36 weeks), the mitral valve appeared highly malformed, whereas the aortic valve looked anatomically normal. Therefore, massive mitral regurgitation with functional aortic atresia was diagnosed. PFO was confirmed restrictive and of major concern for postnatal survival. Mild pericardial and pleural effusions were observed and judged worrisome signs of congestive heart failure. Anticipating birth and performing an immediate intervention (balloon atrial septostomy or mitral valve repair) was considered mandatory.
A cesarean section was scheduled at 37 weeks and performed in the operating room next to the cardiac surgery theatre. A male was born (approximately 3 kg) and immediately transferred into the adjacent cardiac surgery room, with rapidly deteriorating hemodynamics: systemic hypotension, tachycardia, and important peripheral desaturation were present and kept on worsening even after intubation. Blood gas could be obtained only during the interventional procedure and revealed severe lactic acidosis, hypoxia and hypercarbia despite 100% FiO2 ventilation.
After an unsuccessful balloon atrial septostomy (a Rashkind catheter could not enter the PFO because of bulging of the interatrial septum), surgery was undertaken. As the pericardium was entered, the heart grossly protruded. After aortic and bicaval cannulation, hypothermic (26 °C) extracorporeal circulation (ECC) was started. The aorta was cross-clamped for 38 min and the heart protected with cold crystalloid cardioplegia. A left atriotomy was used, parallel to the interatrial septum. Both mitral leaflets were dysplasic and pulled downwards by short chordae, causing a 5-mm loss of coaptation. Mitral annulus measured 19 mm.
To reduce the annular diameter to 11 mm, a posterior annuloplasty was performed, using a 22-mm bovine pericardial band. The valve was checked with cold saline, the LA closed and its appendage ligated. ECC was discontinued after 80 min, with moderate doses of inotropes.
Because of heart protrusion, the sternum was stented, the wound closed with a silicone patch and the child transferred into the cardiac intensive care unit (CICU).
Echocardiograms, electrocardiograms, chest radiograms and clinical examinations were obtained regularly during the hospital stay and the subsequent five-year follow-up.
3. Results
Postoperative decrease of heart size was extremely slow. Sternal closure was obtained 16 days postoperatively, eight progressive steps being necessary to avoid heart compression. Mechanical ventilation was maintained for 25 days and the baby was discharged from the CICU on day 46.
Hemodynamics remained stable and the clinical condition gradually improved. No arrhythmias occurred.
Postoperative echocardiograms showed good biventricular function, with the left chambers slowly returning to normal size. The mitral valve still presented mild-to-moderate regurgitation, with a 3-mm central loss of coaptation. Pericardial effusion was never observed.
Hospital discharge occurred 57 days postoperatively, with low-dose diuretics and angiotensin-converting enzyme (ACE)-inhibitors.
During follow-up the patient showed normal growth, good clinical condition and stable hemodynamics. Chest radiograms revealed a gradual reduction of cardiac dimensions.
Echocardiography showed good biventricular function and progressive normalization of left heart chambers. Mitral function continued to improve, regurgitation decreased to mild and stabilized after the first year. Central loss of coaptation almost disappeared, without mitral stenosis (Fig. 2 ).
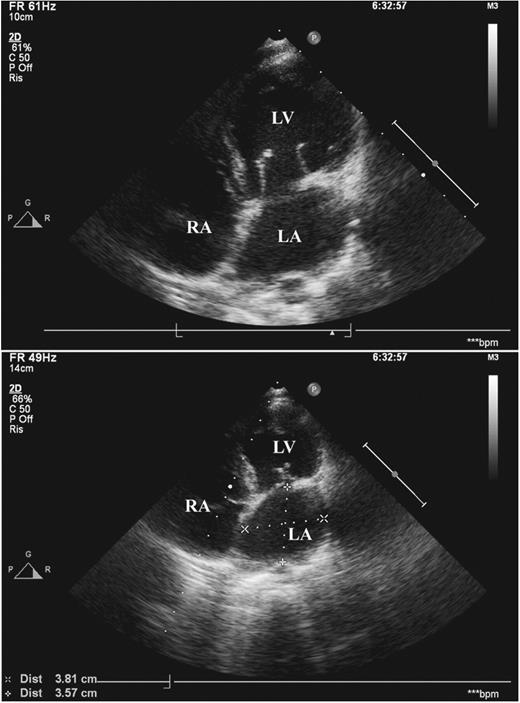
Echocardiogram at five-year follow-up. Cross-sectional view along the four-chamber plane. On the top panel, a diastolic frame showing normal cusp opening of the mitral valve with no annular enlargement. On the bottom panel, a systolic frame of mitral anatomy, with good coaptation depth and height. Both the left ventricle and left atrium show normal dimensions (see measurements of left atrium on the bottom panel). LA, left atrium; LV, left ventricle; RA, right atrium.
Diuretics were discontinued one year postoperatively and ACE-inhibitors six months later.
4. Comment
To the best of our knowledge, this might be the first report of mitral repair in a newborn.
In small children mitral surgery is challenging. Valve replacement, when feasible, requires subsequent reoperations and/or lifelong anticoagulation. Supra-annular implantation is frequently necessary, with altered hemodynamics and controversial results [2, 3]. Valve repair can be extremely difficult: small dimensions, tissue fragility and anatomical complexity pose cumbersome problems [4]. Therefore, surgery for congenital mitral valve disease is delayed if possible, to wait for annular growth and tissue maturation [5, 6].
Prenatally, our patient presented with massive mitral regurgitation, functional aortic atresia and restrictive PFO. Additionally, initial signs of congestive heart failure were detected. This pathophysiological complex exposed the patient to the risk of death at birth, similarly to other cardiac conditions [7, 8]. Thus, accurate planning of delivery and immediate intervention became necessary. Monitoring of fetal development allowed us to schedule a cesarean section at 37 weeks of pregnancy, when fetal maturity was reached.
Two adjacent operating theatres permitted prompt treatment of the rapidly deteriorating newborn.
Balloon atrial septostomy, the least invasive and most effective intervention, was initially attempted. Failure of this procedure prompted immediate surgery. The simplest valve repair was performed, foreseeing and accepting a residual regurgitation.
In our patient, planning of delivery and treatment options were directed to minimize the risk and to obtain the best result, using the least invasive technique.
Accordingly, an initial percutaneous procedure was attempted and, when surgery became unavoidable, a ‘partial’ mitral repair was chosen, to provide the best chance of survival.
Considering that atrioventricular valves continue developing after birth, particularly with leaflet elongation [6], a suboptimal surgical result was accepted, even though it is a predictor for reoperation [4]. Rewardingly, the short- and long-term results were satisfactory: remodeling of the left chambers and the mitral valve led to almost complete disappearance of residual mitral regurgitation.
References
- pregnancy
- mitral valve insufficiency
- congenital insufficiency of mitral valve
- mitral valve repair
- blalock hanlon operation
- ventricular function
- cardiac surgery procedures
- patent foramen ovale
- cesarean section
- child
- dilatation, pathologic
- follow-up
- infant
- newborn
- operating room
- surgical procedures, operative
- vomiting
- heart
- fetal echocardiography
- normal growth
- left side of heart
- congenital atresia of aorta




