-
PDF
- Split View
-
Views
-
Cite
Cite
Eduardo Keller Saadi, Luiz Henrique Dussin, Leandro Moura, André Severo Machado, The axillary artery – a new approach for endovascular treatment of thoracic aortic diseases, Interactive CardioVascular and Thoracic Surgery, Volume 11, Issue 5, November 2010, Pages 617–619, https://doi.org/10.1510/icvts.2010.245274
Close - Share Icon Share
Abstract
Endovascular procedures are increasing in number for the treatment of thoracic aortic diseases (TAD). Retrograde approach through the femoral artery is the preferred vascular access. Despite the improvements in the devices, femoral artery complications still occurs and some times this access is not possible because of the small size of the vessels, obstruction, calcification, dissection or extreme tortuosity. An axillary approach could be an alternative. We present a series of five patients and describe the technique we used in the axillary artery approach to treat TAD. There were two ascending aortas and three descending aortic aneurysms treated. The left axillary artery was used in three patients and the right in two. There were no local or neurological complications. In this preliminary approach, both axillary arteries were a good alternative access for endovascular graft insertion to treat aortic diseases when femoral access was not possible or was suboptimal.
1. Introduction
Endovascular approaches are being increasingly utilized to treat a variety of thoracic aortic diseases (TAD), including aneurysms, pseudoaneurysms, dissections, penetrating aortic ulcers, traumatic aortic rupture and coarctation [1]. The procedure is usually done through the femoral arteries [1–3]. Sometimes this access is impossible or not recommended because of the small size of the vessels, obstruction, calcification, dissection or extreme tortuosity. We present a series of five cases and describe the technique in which the axillary artery was used to deliver the endograft for treatment of different TAD.
2. Case reports and technique
Between April 2008 and June 2010, we treated five patients with TAD in which transfemoral access was contraindicated because of occlusive disease, small vessels or extreme calcification.
The demographic characteristics of the patients and the contraindications to use the femoral artery are presented in Table 1 .
| Patient | Age (years) | Disease | Access problem | Axillary | Prosthesis |
| 1 | 72 | AA | Aorto iliac occlusion | Right | TAG 40×100 |
| 2 | 83 | DA | Small size femoral arteries | Left | TAG 37×200 |
| <7 mm | |||||
| 3 | 49 | PA | Delivery system too short | Right | Two AE 32×4.5 |
| 4 | 65 | DA | Small size femoral arteries | Left | TAG 37×150 |
| <6 mm | |||||
| 5 | 76 | DA | Stenosis and calcification | Left | TAG 37×200 |
| of the femoral arteries |
| Patient | Age (years) | Disease | Access problem | Axillary | Prosthesis |
| 1 | 72 | AA | Aorto iliac occlusion | Right | TAG 40×100 |
| 2 | 83 | DA | Small size femoral arteries | Left | TAG 37×200 |
| <7 mm | |||||
| 3 | 49 | PA | Delivery system too short | Right | Two AE 32×4.5 |
| 4 | 65 | DA | Small size femoral arteries | Left | TAG 37×150 |
| <6 mm | |||||
| 5 | 76 | DA | Stenosis and calcification | Left | TAG 37×200 |
| of the femoral arteries |
AA, ascending aorta aneurysm; TAG, Gore-Tex thoracic aorta self expandable endoprosthesis; DA, descending aorta aneurysm; PA, pseudoaneurysm of ascending aorta; AE, aortic extender cuff for proximal abdominal aorta.
| Patient | Age (years) | Disease | Access problem | Axillary | Prosthesis |
| 1 | 72 | AA | Aorto iliac occlusion | Right | TAG 40×100 |
| 2 | 83 | DA | Small size femoral arteries | Left | TAG 37×200 |
| <7 mm | |||||
| 3 | 49 | PA | Delivery system too short | Right | Two AE 32×4.5 |
| 4 | 65 | DA | Small size femoral arteries | Left | TAG 37×150 |
| <6 mm | |||||
| 5 | 76 | DA | Stenosis and calcification | Left | TAG 37×200 |
| of the femoral arteries |
| Patient | Age (years) | Disease | Access problem | Axillary | Prosthesis |
| 1 | 72 | AA | Aorto iliac occlusion | Right | TAG 40×100 |
| 2 | 83 | DA | Small size femoral arteries | Left | TAG 37×200 |
| <7 mm | |||||
| 3 | 49 | PA | Delivery system too short | Right | Two AE 32×4.5 |
| 4 | 65 | DA | Small size femoral arteries | Left | TAG 37×150 |
| <6 mm | |||||
| 5 | 76 | DA | Stenosis and calcification | Left | TAG 37×200 |
| of the femoral arteries |
AA, ascending aorta aneurysm; TAG, Gore-Tex thoracic aorta self expandable endoprosthesis; DA, descending aorta aneurysm; PA, pseudoaneurysm of ascending aorta; AE, aortic extender cuff for proximal abdominal aorta.
In two cases, the right axillary artery was used and in the other three we used the left axillary artery.
The axillary artery was exposed through an incision in the deltopeitoral groove. The pectoralis major muscle was divided in the direction of its fibers and the insertion of the pectoralis minor was divided. The axillary artery was easily seen superior to the axillary vein and was dissected. A side-biting clamp was applied and an anastomosis was constructed with an 8 mm Dacron tube with a 6-0 prolene suture. The tube was moved to new small stab incision and left long and parallel to the artery (Fig. 1a ). A 7-French sheath was attached to the Dacron tube to avoid bleeding and facilitate the manipulation of wires, catheters and the insertion of the device (Fig. 1b).
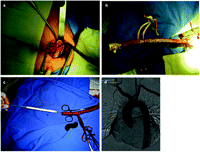
(a) An 8-mm Dacron tube anastomosed to the right axillary artery and brought through another small stab incision; (b) a long tube with a 7-French sheath inserted and snared allows the introduction of wires, catheters and the device without bleeding; (c) a Gore-Tex (TAG) thoracic stent graft inserted through a rigid guide wire and the Dacron tube; (d) final aortography of two aortic extender cuffs deployed in the ascending aorta through the right axillary artery to treat a pseudoaneurysm.
An extra stiff Landerquist 260 cm long guide wire was inserted and the endoprosthesis was introduced without the sheath through the Dacron tube (Fig. 1c). An aortography was done with a pigtail introduced from the same graft, the contralateral arm or from the groin as a diagnostic landmark and before opening the device. Final aortography was performed to check for endoleaks (Fig. 1d). The wires were removed and the Dacron tube was ligated with a large clip 1 cm away from the axillary artery and reinforced with a running suture of 5-0 prolene. There was technical success in all five patients with no hospital mortality or vascular complications.
3. Discussion
The feasibility of endovascular surgery depends on many anatomical factors, including the diameter and the disease state of the access vessels [4]. Stenosis, calcifications, tortuosity, small sizes or dissection of both femoral and iliac arteries can make introduction of large sheath hazardous or impossible. A conduit anastomosed to the iliac artery is an option [5]. An alternative to groin access is through the axillary artery. The exposure of the axillary artery through a small infraclavicular incision is as familiar to cardiovascular surgeons as the arterial return in cardiopulmonary bypass is in aortic arch surgery [6]. They are often good-sized vessels and are usually free from atherosclerosis, even in patients with extensive aorto-iliac occlusive disease.
Both axillary arteries can be used. The decision as to which side to use is made based on anatomical details and the angle is detected in the preoperative multislice computed tomography scan with 3D reconstruction. If both arteries are of good size and the angle in relation to the aortic arch is favorable we prefer to use the left artery. We believe there is less chance of debris embolization to the carotid artery if we avoid the brachiocephalic trunk. If the left subclavian artery has been involved in the dissection or aneurysm we use the right artery.
In four cases, we used a Gore-Tex (TAG) thoracic endoprosthesis. In these patients, we introduced the graft without a sheath through the Dacron tube and delivered it in an antegrade approach. The advantage of this graft for antegrade insertion is that it is designed exactly with the same configuration and the same radial force in both extremities. This does not occur with other commercially available stent grafts.
In one case of pseudoaneurysm of the ascending aorta we used two extension cuffs (aortic extender) through the right axillary artery. The length of the delivery system, designed for the abdominal aorta, is not long enough to reach the ascending aorta through the groin.
The infraclavicular incision to expose the axillary artery is very well tolerated with minimal pain or discomfort. The use of a side graft has been shown to reduce morbidity associated with axillary cannulation [6]. The direct cannulation can cause damage to the artery that can be difficult to repair. The stab incision provides a smooth angle of entry that facilitates the introduction of large devices [5]. There are only a few case reports in the literature where the axillary artery has been used to deploy endografts in the aorta [7]. If there is no other alternative the artery can be ligated without major consequences to the arm perfusion [8]. In this small series, we did not have any complications related to access vessels or neurological problems.
Major vascular injury remains a frequent complication of endovascular procedures in the aorta. We recently published a series on 255 patients who underwent endovascular treatment of TAD through the femoral artery with 3.1% of major vascular access complications [9].
4. Conclusion
In this initial small series, we were able to implant stent grafts in all five patients with TAD without complications. Endovascular aortic procedures through the axillary artery is an attractive alternative in patients where a transfemoral approach is contraindicated. Further studies with more patients and longer follow-up periods are needed to validate this method.




