-
PDF
- Split View
-
Views
-
Cite
Cite
Thomas R. Wyss, Marc Bigler, Mario Stalder, Lars Englberger, Thierry Aymard, Alexander Kadner, Thierry P. Carrel, Absence of prosthesis–patient mismatch with the new generation of Edwards stented aortic bioprosthesis, Interactive CardioVascular and Thoracic Surgery, Volume 10, Issue 6, June 2010, Pages 884–888, https://doi.org/10.1510/icvts.2009.224915
Close - Share Icon Share
Abstract
Prosthesis–patient mismatch (PPM) remains a controversial issue with the most recent stented biological valves. We analyzed the incidence of PPM after implantation of the Carpentier–Edwards Perimount Magna Ease® aortic valve (PMEAV) bioprosthesis and assessed the early clinical outcome. Two hundred and seventy consecutive patients who received a PMEAV bioprosthesis between January 2007 and July 2008 were analyzed. Pre-, peri- and postoperative data were assessed and echocardiographic as well as clinical follow-up was performed. Mean age was 72±9 years, 168 (62.2%) were males. Fifty-seven patients (21.1%) were below 65 years of age. Absence of PPM, corresponding to an indexed effective orifice area >0.85 cm2/m2, was 99.5%. Observed in-hospital mortality was 2.2% (six patients), with a predicted mortality according to the additive EuroSCORE of 7.6±3.1%. At echocardiographic assessment after a mean follow-up period of 150±91 days, mean transvalvular gradient was 11.8±4.8 mmHg (all valve sizes). No paravalvular leakage was seen. Nine patients died during follow-up. The Carpentier–Edwards PMEAV bioprosthesis shows excellent hemodynamic performance. This valve can be implanted in all sizes with an incidence of severe PPM below 0.5%.
1. Introduction
During aortic valve replacement (AVR), and particularly when a stented bioprosthesis is used, one of the most important goals is to implant a valve size corresponding to the patient's need. Prosthesis–patient mismatch (PPM) refers to the presence of a relatively small valve according to the body size, which generates higher transvalvular gradients [1]. PPM is commonly expressed by the size of the effective orifice area (EOA) divided by the body surface area. A severe mismatch is defined as a quotient <0.65 cm2/m2. This condition is associated with lower postoperative survival, increased mid-term and late mortality and more frequent cardiac events following AVR [2, 3]. Therefore, it is essential that AVR ensures sufficient valvular size to avoid mismatch; this can be performed successfully at the time of operation [4].
Increasing demand for biological valves has led to continuous development of the devices. Irrespective of the data provided by the industry, every new product has to be assessed on the basis of echocardiographic and clinical data. Several reports have demonstrated improved hemodynamic characteristics and significantly reduced incidence of PPM following implantation of the Carpentier–Edwards Perimount Magna aortic bioprosthesis compared to the standard Perimount valve and other bioprostheses [5–8]. The Perimount Magna Ease® aortic valve (PMEAV) is the most recent stented, completely supra-annular, aortic valve bioprosthesis developed by Edwards Lifesciences. The major change – compared to the previous generation – has been the lower valve profile and the anatomical design of the sewing ring to facilitate implantability, especially in challenging anatomies. Since there is no clinical report with this specific valve so far, we analyzed the clinical and echocardiographic outcome of patients who received the PMEAV with special attention to the incidence of PPM.
2. Materials and methods
The PMEAV was introduced in our institution in December 2006. It is the last generation of the Edwards Lifesciences' stented bovine pericardial prosthesis. The new characteristics of this valve are the lower profile and cusp height and a scalloped and compliant sewing ring. These modifications are supposed to facilitate implantation, especially in small aortic roots.
It has exactly the same internal diameter and external sewing ring diameter as the Perimount Magna bioprosthesis, its predecessor. The Perimount Magna Ease® series includes bovine pericardial leaflets treated with the ThermaFix process.
Between January 2007 and July 2008, a total of 270 consecutive patients received the PMEAV during AVR performed either as an isolated or a combined procedure. The choice of the valve is left to the discretion of the operator in our institution; the 270 patients represent ∼60% of the overall number of AVRs performed during the same time period. In cases of scheduled aortic root replacement with a biological valve, the PMEAV was sutured to a Gelweave Valsalva® prosthesis to construct a composite graft. Elective and emergency cases were included. Surgery was performed through a median sternotomy using mild hypothermic cardiopulmonary bypass and antegrade cold blood cardioplegia. A sizer was used to determine the largest prosthesis size which would comfortably fit into a supra-annular position. Oversizing was not attempted. After decalcification of the aortic annulus, the valve was sewn in a supra-annular position using non-everted pledget reinforced mattress sutures.
We analyzed preoperative, intraoperative and postoperative clinical data as well as transthoracic Doppler echocardiography performed during follow-up.
PPM was characterized by the indexed effective orifice area (EOAI), i.e. EOA of the valve divided by the patient's body surface area [9]. The EOA of the valve was based on reference echocardiographic measurements published in the literature [6–8], which is a valid method for the prediction of PPM prior to AVR [4, 10]. Threshold values of EOAI for the recognition and quantification of PPM were: <0.65 cm2/m2 for severe and ≤0.85 cm2/m2 for moderate, >0.85 cm2/m2 for absence of PPM [9]. Descriptive statistics were performed using EXCEL. Patients gave written consent for clinical studies using anonymous personal data.
3. Results
Mean age was 72±9 years. One hundred and sixty-eight (62.2%) were males. Fifty-seven (21.1%) were below 65 years of age. The detailed baseline characteristics of the 270 patients are described in Table 1 . The main indication for AVR was aortic stenosis (67.4%). Operative data show a mean aortic cross-clamping time (ACC) of 53.6 min (±18.8) for isolated AVR (Table 2 ). Observed in-hospital mortality was 2.2% (six patients), with a predicted mortality according to the additive EuroSCORE of 7.6±3.1%. The PPM based on different echocardiographic studies with the Perimount Magna valve, is displayed in Table 3[6–8]. No severe mismatch was present in any patient, in any of the calculations. A low incidence of moderate mismatch was present in all groups: in the two series by Dalmau mainly with 19 mm valves and in the series of Botzenhardt with nine 21 mm valves.
Preoperative variables of 270 patients undergoing aortic valve replacement with a Perimount Magna Ease® bioprosthesis
| Baseline characteristics | |
| Age (years) | 71.7 (9.4) |
| Male gender | 168 (62.2) |
| Diabetes mellitus | 71 (26.3) |
| Arterial hypertension | 215 (79.6) |
| Smoking | 109 (40.4) |
| Dyslipidemia | 150 (55.6) |
| Positive family history | 43 (15.9) |
| NYHA class | 2.4 (1.1) |
| Ejection fraction | 56.3 (13.7) |
| EuroSCORE | 7.6 (3.1) |
| Aortic valve pathology | |
| Stenosis | 182 (67.4) |
| Insufficiency | 32 (11.9) |
| Mixed | 40 (14.9) |
| Aortic valve etiology | |
| Degenerative | 234 (86.7) |
| Annuloaortic ectasia | 14 (5.2) |
| Infective | 12 (4.4) |
| Baseline characteristics | |
| Age (years) | 71.7 (9.4) |
| Male gender | 168 (62.2) |
| Diabetes mellitus | 71 (26.3) |
| Arterial hypertension | 215 (79.6) |
| Smoking | 109 (40.4) |
| Dyslipidemia | 150 (55.6) |
| Positive family history | 43 (15.9) |
| NYHA class | 2.4 (1.1) |
| Ejection fraction | 56.3 (13.7) |
| EuroSCORE | 7.6 (3.1) |
| Aortic valve pathology | |
| Stenosis | 182 (67.4) |
| Insufficiency | 32 (11.9) |
| Mixed | 40 (14.9) |
| Aortic valve etiology | |
| Degenerative | 234 (86.7) |
| Annuloaortic ectasia | 14 (5.2) |
| Infective | 12 (4.4) |
All continuous variables are given as mean (standard deviation). All categorical variables are given as numbers (%). Total do not always add up to 270 due to occasional missing values.
Preoperative variables of 270 patients undergoing aortic valve replacement with a Perimount Magna Ease® bioprosthesis
| Baseline characteristics | |
| Age (years) | 71.7 (9.4) |
| Male gender | 168 (62.2) |
| Diabetes mellitus | 71 (26.3) |
| Arterial hypertension | 215 (79.6) |
| Smoking | 109 (40.4) |
| Dyslipidemia | 150 (55.6) |
| Positive family history | 43 (15.9) |
| NYHA class | 2.4 (1.1) |
| Ejection fraction | 56.3 (13.7) |
| EuroSCORE | 7.6 (3.1) |
| Aortic valve pathology | |
| Stenosis | 182 (67.4) |
| Insufficiency | 32 (11.9) |
| Mixed | 40 (14.9) |
| Aortic valve etiology | |
| Degenerative | 234 (86.7) |
| Annuloaortic ectasia | 14 (5.2) |
| Infective | 12 (4.4) |
| Baseline characteristics | |
| Age (years) | 71.7 (9.4) |
| Male gender | 168 (62.2) |
| Diabetes mellitus | 71 (26.3) |
| Arterial hypertension | 215 (79.6) |
| Smoking | 109 (40.4) |
| Dyslipidemia | 150 (55.6) |
| Positive family history | 43 (15.9) |
| NYHA class | 2.4 (1.1) |
| Ejection fraction | 56.3 (13.7) |
| EuroSCORE | 7.6 (3.1) |
| Aortic valve pathology | |
| Stenosis | 182 (67.4) |
| Insufficiency | 32 (11.9) |
| Mixed | 40 (14.9) |
| Aortic valve etiology | |
| Degenerative | 234 (86.7) |
| Annuloaortic ectasia | 14 (5.2) |
| Infective | 12 (4.4) |
All continuous variables are given as mean (standard deviation). All categorical variables are given as numbers (%). Total do not always add up to 270 due to occasional missing values.
| Procedure group | |
| Isolated aortic valve replacement | 81 (30.0) |
| Aortocoronary bypass grafting | 145 (53.7) |
| Composite grafts | 26 (9.6) |
| Mitral valve | 17 (6.3) |
| Annular enlargement | 9 (3.3) |
| Valve size | |
| EOAI | 1.1 (0.1) |
| EOAI ≥0.85 (of n=222 based on | 221 (99.5) |
| Dalmau et al., 2006 measurements) | |
| Times for isolated aortic valve replacements | |
| CPB (min) | 74.8 (31.9) |
| ACC (min) | 53.6 (18.8) |
| Postoperative | |
| Tamponade revision | 13 (4.8) |
| Stroke* | 12 (4.4) |
| Renal failure** | 17 (6.3) |
| Atrial fibrillation/flutter | 80 (29.6) |
| Pacemaker implantation | 12 (4.4) |
| Myocardial infarction | 4 (1.5) |
| In-hospital mortality | 6 (2.2) |
| Procedure group | |
| Isolated aortic valve replacement | 81 (30.0) |
| Aortocoronary bypass grafting | 145 (53.7) |
| Composite grafts | 26 (9.6) |
| Mitral valve | 17 (6.3) |
| Annular enlargement | 9 (3.3) |
| Valve size | |
| EOAI | 1.1 (0.1) |
| EOAI ≥0.85 (of n=222 based on | 221 (99.5) |
| Dalmau et al., 2006 measurements) | |
| Times for isolated aortic valve replacements | |
| CPB (min) | 74.8 (31.9) |
| ACC (min) | 53.6 (18.8) |
| Postoperative | |
| Tamponade revision | 13 (4.8) |
| Stroke* | 12 (4.4) |
| Renal failure** | 17 (6.3) |
| Atrial fibrillation/flutter | 80 (29.6) |
| Pacemaker implantation | 12 (4.4) |
| Myocardial infarction | 4 (1.5) |
| In-hospital mortality | 6 (2.2) |
All continuous variables are given as mean (standard deviation). All categorical variables are given as numbers (%). EOAI, effective opening area index; CPB, cardiopulmonary bypass time; ACC, aortic cross-clamping time.
*Permanent neurological loss of function.
**Dialysis needed.
| Procedure group | |
| Isolated aortic valve replacement | 81 (30.0) |
| Aortocoronary bypass grafting | 145 (53.7) |
| Composite grafts | 26 (9.6) |
| Mitral valve | 17 (6.3) |
| Annular enlargement | 9 (3.3) |
| Valve size | |
| EOAI | 1.1 (0.1) |
| EOAI ≥0.85 (of n=222 based on | 221 (99.5) |
| Dalmau et al., 2006 measurements) | |
| Times for isolated aortic valve replacements | |
| CPB (min) | 74.8 (31.9) |
| ACC (min) | 53.6 (18.8) |
| Postoperative | |
| Tamponade revision | 13 (4.8) |
| Stroke* | 12 (4.4) |
| Renal failure** | 17 (6.3) |
| Atrial fibrillation/flutter | 80 (29.6) |
| Pacemaker implantation | 12 (4.4) |
| Myocardial infarction | 4 (1.5) |
| In-hospital mortality | 6 (2.2) |
| Procedure group | |
| Isolated aortic valve replacement | 81 (30.0) |
| Aortocoronary bypass grafting | 145 (53.7) |
| Composite grafts | 26 (9.6) |
| Mitral valve | 17 (6.3) |
| Annular enlargement | 9 (3.3) |
| Valve size | |
| EOAI | 1.1 (0.1) |
| EOAI ≥0.85 (of n=222 based on | 221 (99.5) |
| Dalmau et al., 2006 measurements) | |
| Times for isolated aortic valve replacements | |
| CPB (min) | 74.8 (31.9) |
| ACC (min) | 53.6 (18.8) |
| Postoperative | |
| Tamponade revision | 13 (4.8) |
| Stroke* | 12 (4.4) |
| Renal failure** | 17 (6.3) |
| Atrial fibrillation/flutter | 80 (29.6) |
| Pacemaker implantation | 12 (4.4) |
| Myocardial infarction | 4 (1.5) |
| In-hospital mortality | 6 (2.2) |
All continuous variables are given as mean (standard deviation). All categorical variables are given as numbers (%). EOAI, effective opening area index; CPB, cardiopulmonary bypass time; ACC, aortic cross-clamping time.
*Permanent neurological loss of function.
**Dialysis needed.
| Valve size | Dalmau et al. 2006 | Dalmau et al. 2007 | Botzenhardt et al. 2005 |
| 11 months postoperative | 12 months postoperative | at discharge | |
| 19 mm (12) | 1.58 | 1.35 | 1.65 |
| 21 mm (66) | 1.90 | 1.75 | 1.66 |
| 23 mm (91) | 2.07 | 2.19 | 2.54 |
| 25 mm (65) | 2.33 | 2.35 | 2.32 |
| 27 mm (29) | – | 2.03 | – |
| 29 mm (7) | – | – | – |
| Moderate PPM | 0.5% | 3.6% | 4.1% |
| (EOAI ≤0.85 cm2/m2) | (1 of 222) | (9 of 251) | (9 of 222) |
| Valve size | Dalmau et al. 2006 | Dalmau et al. 2007 | Botzenhardt et al. 2005 |
| 11 months postoperative | 12 months postoperative | at discharge | |
| 19 mm (12) | 1.58 | 1.35 | 1.65 |
| 21 mm (66) | 1.90 | 1.75 | 1.66 |
| 23 mm (91) | 2.07 | 2.19 | 2.54 |
| 25 mm (65) | 2.33 | 2.35 | 2.32 |
| 27 mm (29) | – | 2.03 | – |
| 29 mm (7) | – | – | – |
| Moderate PPM | 0.5% | 3.6% | 4.1% |
| (EOAI ≤0.85 cm2/m2) | (1 of 222) | (9 of 251) | (9 of 222) |
Effective orifice areas in cm2. EOAI, effective orifice area index; PPM, prosthesis–patient mismatch. Numbers in brackets represent group size.
| Valve size | Dalmau et al. 2006 | Dalmau et al. 2007 | Botzenhardt et al. 2005 |
| 11 months postoperative | 12 months postoperative | at discharge | |
| 19 mm (12) | 1.58 | 1.35 | 1.65 |
| 21 mm (66) | 1.90 | 1.75 | 1.66 |
| 23 mm (91) | 2.07 | 2.19 | 2.54 |
| 25 mm (65) | 2.33 | 2.35 | 2.32 |
| 27 mm (29) | – | 2.03 | – |
| 29 mm (7) | – | – | – |
| Moderate PPM | 0.5% | 3.6% | 4.1% |
| (EOAI ≤0.85 cm2/m2) | (1 of 222) | (9 of 251) | (9 of 222) |
| Valve size | Dalmau et al. 2006 | Dalmau et al. 2007 | Botzenhardt et al. 2005 |
| 11 months postoperative | 12 months postoperative | at discharge | |
| 19 mm (12) | 1.58 | 1.35 | 1.65 |
| 21 mm (66) | 1.90 | 1.75 | 1.66 |
| 23 mm (91) | 2.07 | 2.19 | 2.54 |
| 25 mm (65) | 2.33 | 2.35 | 2.32 |
| 27 mm (29) | – | 2.03 | – |
| 29 mm (7) | – | – | – |
| Moderate PPM | 0.5% | 3.6% | 4.1% |
| (EOAI ≤0.85 cm2/m2) | (1 of 222) | (9 of 251) | (9 of 222) |
Effective orifice areas in cm2. EOAI, effective orifice area index; PPM, prosthesis–patient mismatch. Numbers in brackets represent group size.
Only one patient who needed redo-AVR with a 19 mm valve because of endocarditis, was below the threshold of 0.85 cm2/m2 in the first group [6]. The actual EOAI was slightly lower with 0.845 cm2/m2, representing a moderate mismatch. Fig. 1 shows the distribution of EOAI values per valve size.

Distribution of EOAI values per valve size (median and range). Based on EOA values by Dalmau et al. 2006. Total does not add up to 270 due to occasional missing values and no EOA values for 27 and 29 mm valves.
The echocardiographic follow-up (Table 4 ) was completed in 140 patients (52%) after a mean duration of 150 (±91) days. Nine patients died during follow-up. Echocardiographic examination showed very satisfactory hemodynamic performance with no paravalvular leakage in any patient. The mean transvalvular gradient was 11.8±4.8 mmHg, combined for all valve sizes. The distribution of the mean transvalvular gradient per valve size compared to the Perimount Magna standard valve is shown in Fig. 2 .
| Follow-up data | |
| Echocardiographic follow-up retrieved | 140 (52%) |
| Mean duration (days) | 150 (90.9) |
| Mean transvalvular gradient (mmHg) | 11.8 (4.8) |
| Follow-up data | |
| Echocardiographic follow-up retrieved | 140 (52%) |
| Mean duration (days) | 150 (90.9) |
| Mean transvalvular gradient (mmHg) | 11.8 (4.8) |
All continuous variables are given as mean (standard deviation). All categorical variables given as numbers (%).
| Follow-up data | |
| Echocardiographic follow-up retrieved | 140 (52%) |
| Mean duration (days) | 150 (90.9) |
| Mean transvalvular gradient (mmHg) | 11.8 (4.8) |
| Follow-up data | |
| Echocardiographic follow-up retrieved | 140 (52%) |
| Mean duration (days) | 150 (90.9) |
| Mean transvalvular gradient (mmHg) | 11.8 (4.8) |
All continuous variables are given as mean (standard deviation). All categorical variables given as numbers (%).
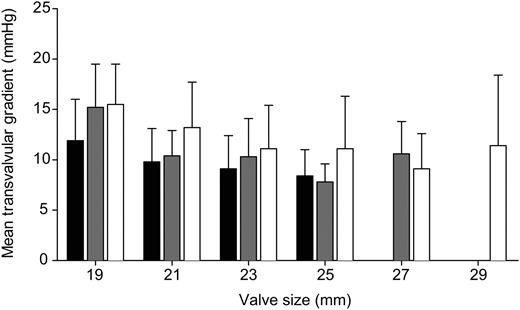
Distribution of mean transvalvular gradient per valve size (mean and standard deviation). MAGNA Dalmau et al. 2006 vs. MAGNA Dalmau et al. 2007 vs. EASE University Hospital Bern.
4. Discussion
The sewing ring as well as the stent itself may contribute to reducing the existing orifice area and therefore lead to a PPM following AVR with a stented aortic valve bioprosthesis. The clinical implication of PPM is still controversial. Clearly a severe mismatch contributes to increased mortality and the number of cardiac events after AVR [2, 3]. Nowadays there is a trend towards implantation of biological valves in younger patients, because transcatheter valve technology is already able to implant a new stented valve within a degenerated tissue valve.
Notably this group of patients, age <60 years [11] and age <70 years [2], shows impaired long-term survival when severe PPM is present. Hence, long-term durability and avoidance of PPM is of utmost importance. There is agreement concerning the effect of severe PPM after AVR, particularly in the presence of preoperative left ventricular dysfunction [2] but large prospective randomized trials are still lacking. Projected EOA values have been commonly used. This enables the surgeon to be certain of avoiding PPM during the planning of the procedure and raises the awareness of a possible mismatch [10].
In this study, we assessed the incidence of PPM and the early clinical results. Furthermore, we assessed the hemodynamic performance at a mean follow-up of ∼5 months. In this series of 270 consecutive PMEAV implantations not a single severe mismatch could be observed using different projected in vivo EOA values. Additionally, we had a low rate of moderate PPM of 0.5–4.1% in our series. Valve sizes of 29 mm were not included due to the absence of representative EOA values for this size.
In-hospital mortality was low at 2.2%, considering the fact that a variety of additional procedures were performed in a large number of patients and that the additive EuroSCORE was as high as 7.6%. This is comparable to other large volume centers with similar patient cohorts: additive and logistic EuroSCORE models substantially overestimate mortality [12]. Results of percutaneous and transapical aortic valve implantations in a skilled center show 30-day mortality of 11.1% and 3.6%, respectively, with a predicted risk by median logistic EuroSCOREs of 35.0 (Q1–Q3; 20.0–50.3) and 25.0 (16.0–37.0) [13]. Following percutaneous implantation the incidence of severe PPM is reported to be as high as 6% [14]. This should not be forgotten when transcatheter aortic valve implantation is recommended.
The previous series of Edwards Lifesciences, the Perimount Magna valve, has demonstrated good long-term durability [15]. The PMEAV offers a lower profile and cusp height, as well as a scalloped and compliant sewing ring. These changes are not major ones, but have led to some advantages for implantation, particularly in patients with a small aortic annulus and/or with a narrowed sino-tubular junction. We did not observe the need for down-sizing in our series and furthermore performed annulus enlargement in only 3.3% of the patients. Supra-annular valve implantation seems to keep the blood stream free of stent and sewing material and allows for a physiological flow. The narrowing by the aortic annulus is therefore not worsened by prosthetic valve material itself thus supporting good hemodynamic performance.
Patients after AVR are advised to attend a first echocardiographic follow-up ∼3 months after surgery. We were able to collect data on 140 patients. The echocardiographic follow-up confirms our clinical impression with very satisfying hemodynamic performance: the mean transvalvular gradients are similar to that of previous series with the Perimount Magna valve.
4.1. Study limitations
The echocardiographic follow-up was not performed in a single center due to various reasons (e.g. distance, age) but at the patients' local cardiologist's practice in ∼60% of the cases. These measurements were therefore performed by different cardiologists and they were not standardized. EOA measurements were not performed in the echocardiographic follow-up. Therefore, we used the available reference values previously published. To reduce this limitation, we present indexed EOA values on the basis of three different studies. Still, the rate of PPM remains low. EOA values used in this study are similar to those reported for St. Jude Medical Regent or On-X mechanical bi-leaflet prostheses. To our knowledge, there has not been any publication on the EOA of the PMEAV. Due to the unchanged internal diameter and external sewing ring diameter compared to the Perimount Magna bioprosthesis the two valves have presumably a very similar EOA. Follow-up data include echocardiographic results of 52% of the patients. Further follow-up supported by more detailed echocardiographic data including actual EOA values will be carried out to strengthen our results.
5. Conclusions
The Carpentier–Edwards PMEAV shows good clinical results and hemodynamic performance. It can be implanted without producing severe PPM. Overall, our experience with this new xenograft is promising.
Presented at the 23rd Annual Meeting of the European Association for Cardio-thoracic Surgery, Vienna, Austria, October 18–21, 2009.
References
Conference discussion
Dr. R. Fuster (Valencia, Spain): Your presentation deals with an issue which is, of course, an old concept and an old controversy. It was first defined by Rahimtoola in the 60s as being present when the effective orifice area of the prosthesis being implanted is less than that of the normal human valve. According to this general definition, most patients undergoing aortic valve replacement would have at least mild mismatch, or, in other words, mismatch is inevitable in aortic valve replacement. So, as a fact of terminology, absence of mismatch as is written in your presentation, should not be completely true and perhaps we must say absence of moderate or severe mismatch. But this is only a terminology issue. The first question should be, what is your opinion about this terminology?
In the second place, the main controversy about mismatch is not if mismatch is frequent or not; it is if moderate or severe mismatch, when present, is deleterious in all patients or in a subset of patients. It is quite logical that with the development of new high-performance prostheses, the effective orifice area will increase, moderate–severe mismatch will decrease and practically disappear in most patients. Thus, we can make the statement that with the end of mismatch will come the end of its controversy.
Nowadays, the general agreement is that severe mismatch must be ideally avoided in every patient and that moderate mismatch should only be avoided in a specific subset of patients, high-risk patients, those with depressed left ventricular function and/or severe left ventricular hypertrophy. In this sense, your paper does not clarify this controversy because moderate or severe mismatch is extremely infrequent in your experience with this valve. So the second question is, what is your opinion about this issue?
And finally, I would like to point out an important limitation, that is, the lack of calculated effective orifice area by echocardiography, because this Carpentier–Edwards Magna Ease is quite similar to Carpentier–Edwards Magna with respect to diameters, external sewing ring or internal orifice diameters, but Carpentier–Edwards Magna Ease enhances implantability with a lower profile. Perhaps, and this is a hypothesis, these elements might also be translated into a real hemodynamic benefit that only studies with echocardiographic effective orifice area measurements can clarify. What is your opinion about this? This is the third question.
And, finally, in obese patients have you used a correction to calculate the indexed effective orifice area?
Dr. Wyss: To address the first question about the title, we could change it to absence of severe patient–prosthesis mismatch. The second, we showed very good transvalvular gradients, and we think that reflects an adequate effective orifice area as well. The effective orifice area is certainly a very good parameter, but the transvalvular gradient is one as well. So we think it is a mixture of both.
We did avoid any severe mismatch, as you stated, and I think that is the most important fact about it. The new valve has the same diameters as the predecessor valve. Therefore, we hypothesise that it has the same effective orifice area as well. We will look at that in a further study to really check that.
Dr. Fuster: In obese patients have you used a correction for the calculation of indexed orifice area? Because in obese patients the body surface area is magnified by an increase in weight, and the incidence of mismatch is overestimated. Some authors report that we can correct the calculation by means of height or body mass index.
Dr. Wyss: In obese patients we didn't correct the value of the body surface area, no.




