-
PDF
- Split View
-
Views
-
Cite
Cite
Ana Veríssimo, Leonel Gordo, Ivone Figueiredo, Reproductive biology and embryonic development of Centroscymnus coelolepis in Portuguese mainland waters, ICES Journal of Marine Science, Volume 60, Issue 6, 2003, Pages 1335–1341, https://doi.org/10.1016/S1054-3139(03)00146-2
Close - Share Icon Share
Abstract
A total of 871 females (76–122 cm) and 86 males (68–100 cm) of Centroscymnus coelolepis caught in Portuguese mainland waters were examined for reproductive characteristics. One hermaphroditic individual of 95 cm was found. Males were few in numbers and dominated by immature specimens. Female length at first sexual maturity was 98.5 cm. Mean ovarian and uterine fecundity were 13.2 oocytes per female and 9.9 embryos per pregnant female, respectively. Total length of embryos with completely absorbed external yolk sacs ranged between 233 and 300 mm. Sex ratio (F/M) of embryos with completely absorbed external yolk sac was 0.9. All stages of reproduction were found during the 12-month sampling period. Results were compared with those from other regions. A hypothesis is formulated on the existence of several regional populations based on morphometric and reproductive parameters observed in different areas.
Introduction
Centroscymnus coelolepis Bocage & Capello, 1864 is a deep-water squaloid shark caught regularly on the Portuguese mainland slope as an important bycatch in the longline fishery for black scabbard fish. For many years, Portugal has been the only European country exploiting this resource commercially. Official landings statistics are available only for the last decade, ranging between 503 (in 2000) and 927 tonnes (in 1997). Around 1990, French bottom trawlers, operating west of the British Isles, began landing deep-water sharks (Girard and Du Buit, 1999; Girard et al., 2000). Landings are usually reported as “siki” representing a mixture of C. coelolepis and Centrophorus squamosus (Bonnaterre, 1788). This fishery produced 302 tonnes in 1991, reached a peak of 3284 tonnes in 1996, and then declined to 1939 tonnes in 1999 (Clarke et al., 2001). The level of exploitation has raised serious concerns about the sustainability of this resource. Biological characteristics, such as slow growth, long life spans and low fecundity and natural mortality, makes sharks particularly vulnerable to intense fishing pressure (Stevens et al., 2000). Our main purpose is to determine reproductive parameters of C. coelolepis in Portuguese mainland waters and to provide further information on the biology of the species as a basis for future population dynamics studies and management plans.
Materials and methods
Specimens were sampled from landings of commercial longliners operating at 700–1500 m depth in slope waters about 30 miles off Sesimbra (central Portugal). Sesimbra was selected for sampling deep-water sharks because this fishing port accounts for 80–95% of the total landings in Portugal. Samples were taken twice a month from August 2000 to July 2001, except for January, February and June when only one sample was collected.
Total weight (g), total length (cm) and sex were registered for each individual. For males, clasper length (distance from anterior margin of the cloaca to distal clasper tip) was also measured. Reproductive organs were removed and frozen. After thawing, ovaries, testes, mature oocytes, embryos and yolk sacs were weighed (g) in the laboratory. For females, uterine and nidamental glands width, mature oocytes diameter, embryo total length and villosities (internal extensions from the inner uterine wall) were measured to the nearest millimetre. Embryo sex was determined based on presence/absence of claspers.
We adapted the maturation scale developed by Stehmann (2002) for this species (Table 1). Mean ovarian fecundity of Stage-3 females (ripe oocyte stage) is expressed as the mean number of mature oocytes (>40 mm in diameter) present in the two ovaries and mean uterine fecundity per pregnant female as the mean number of embryos in the uteri.
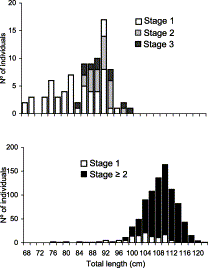
Length frequency distribution of C. coelolepis by maturity stage of (top) males and (bottom) females.
Maturity scale (T: testes; C: claspers; O: ovaries; U: uteri; E: embryos) for C. coelolepis adapted from Stehmann (2002).
| Reproductive stage . | Stage description . |
|---|---|
| Male | |
| 1. Immature | T: threadlike; C: small and flexible |
| 2. Maturing | T: enlarged; C: formed but still flexible |
| 3. Mature | T: large and convoluted; C: fully formed and calcified |
| Female | |
| 1. Immature | O: small; undifferentiated ova; U: threadlike |
| 2. Maturing | O: enlarged; differentiated ova; U: wider |
| 3. Ripe | O: large; large yolky oocytes of similar size |
| 4. Developing | U: filled with large yellow segmented ova |
| 5. Differentiating | E: undeveloped and unpigmented, some with external gill filaments |
| 6. Expecting | E: fully formed, yolk sacs reduced |
| 7. Post-natal | O: similar to Stage 2; U: empty but still widened |
| Reproductive stage . | Stage description . |
|---|---|
| Male | |
| 1. Immature | T: threadlike; C: small and flexible |
| 2. Maturing | T: enlarged; C: formed but still flexible |
| 3. Mature | T: large and convoluted; C: fully formed and calcified |
| Female | |
| 1. Immature | O: small; undifferentiated ova; U: threadlike |
| 2. Maturing | O: enlarged; differentiated ova; U: wider |
| 3. Ripe | O: large; large yolky oocytes of similar size |
| 4. Developing | U: filled with large yellow segmented ova |
| 5. Differentiating | E: undeveloped and unpigmented, some with external gill filaments |
| 6. Expecting | E: fully formed, yolk sacs reduced |
| 7. Post-natal | O: similar to Stage 2; U: empty but still widened |
Maturity scale (T: testes; C: claspers; O: ovaries; U: uteri; E: embryos) for C. coelolepis adapted from Stehmann (2002).
| Reproductive stage . | Stage description . |
|---|---|
| Male | |
| 1. Immature | T: threadlike; C: small and flexible |
| 2. Maturing | T: enlarged; C: formed but still flexible |
| 3. Mature | T: large and convoluted; C: fully formed and calcified |
| Female | |
| 1. Immature | O: small; undifferentiated ova; U: threadlike |
| 2. Maturing | O: enlarged; differentiated ova; U: wider |
| 3. Ripe | O: large; large yolky oocytes of similar size |
| 4. Developing | U: filled with large yellow segmented ova |
| 5. Differentiating | E: undeveloped and unpigmented, some with external gill filaments |
| 6. Expecting | E: fully formed, yolk sacs reduced |
| 7. Post-natal | O: similar to Stage 2; U: empty but still widened |
| Reproductive stage . | Stage description . |
|---|---|
| Male | |
| 1. Immature | T: threadlike; C: small and flexible |
| 2. Maturing | T: enlarged; C: formed but still flexible |
| 3. Mature | T: large and convoluted; C: fully formed and calcified |
| Female | |
| 1. Immature | O: small; undifferentiated ova; U: threadlike |
| 2. Maturing | O: enlarged; differentiated ova; U: wider |
| 3. Ripe | O: large; large yolky oocytes of similar size |
| 4. Developing | U: filled with large yellow segmented ova |
| 5. Differentiating | E: undeveloped and unpigmented, some with external gill filaments |
| 6. Expecting | E: fully formed, yolk sacs reduced |
| 7. Post-natal | O: similar to Stage 2; U: empty but still widened |
Based on the fraction of mature specimens (M) by length class (L), length at 50% sexual maturity was calculated by adjusting the simple logistic model (Zar, 1996) expressed by M=1/(exp(−(a+b×L))+1), where a and b are model parameters.
Ordinary least-squares regression was used for determining the length–weight relationship according to W=aLb, where W represents total weight (g) and L total length (cm).
Results
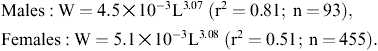
Monthly frequency distribution by reproductive stage (cf. Table 1) of males and females sampled (in italics: fraction females fF).
| Reproductive stage . | Aug. . | Sept. . | Oct. . | Nov. . | Dec. . | Jan. . | Feb. . | Mar. . | Apr. . | May . | Jun. . | Jul. . | Total . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Females | |||||||||||||
| 1 | 8 | 8 | 6 | 11 | 17 | 6 | 14 | 20 | 22 | 4 | 10 | 126 | |
| 2 | 1 | 22 | 27 | 13 | 9 | 20 | 13 | 17 | 25 | 15 | 8 | 15 | 185 |
| 3 | 11 | 16 | 15 | 11 | 3 | 13 | 10 | 19 | 16 | 8 | 14 | 136 | |
| 4 | 7 | 15 | 11 | 22 | 18 | 1 | 33 | 10 | 32 | 40 | 12 | 6 | 207 |
| 5 | 7 | 2 | 1 | 4 | 8 | 3 | 4 | 11 | 7 | 7 | 54 | ||
| 6 | 3 | 11 | 4 | 18 | 9 | 2 | 17 | 4 | 11 | 32 | 1 | 6 | 118 |
| 7 | 5 | 6 | 2 | 3 | 3 | 2 | 6 | 10 | 1 | 6 | 44 | ||
| Males | |||||||||||||
| 1 | 1 | 11 | 12 | 28 | 52 | ||||||||
| 2 | 8 | 2 | 9 | 3 | 22 | ||||||||
| 3 | 1 | 6 | 1 | 5 | 13 | ||||||||
| Total | 18 | 74 | 74 | 80 | 78 | 62 | 88 | 61 | 125 | 168 | 41 | 88 | 957 |
| fF | 1.0 | 1.0 | 1.0 | 1.00 | 0.9 | 0.7 | 1.0 | 1.0 | 1.0 | 0.9 | 1.0 | 0.7 | 0.9 |
| Reproductive stage . | Aug. . | Sept. . | Oct. . | Nov. . | Dec. . | Jan. . | Feb. . | Mar. . | Apr. . | May . | Jun. . | Jul. . | Total . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Females | |||||||||||||
| 1 | 8 | 8 | 6 | 11 | 17 | 6 | 14 | 20 | 22 | 4 | 10 | 126 | |
| 2 | 1 | 22 | 27 | 13 | 9 | 20 | 13 | 17 | 25 | 15 | 8 | 15 | 185 |
| 3 | 11 | 16 | 15 | 11 | 3 | 13 | 10 | 19 | 16 | 8 | 14 | 136 | |
| 4 | 7 | 15 | 11 | 22 | 18 | 1 | 33 | 10 | 32 | 40 | 12 | 6 | 207 |
| 5 | 7 | 2 | 1 | 4 | 8 | 3 | 4 | 11 | 7 | 7 | 54 | ||
| 6 | 3 | 11 | 4 | 18 | 9 | 2 | 17 | 4 | 11 | 32 | 1 | 6 | 118 |
| 7 | 5 | 6 | 2 | 3 | 3 | 2 | 6 | 10 | 1 | 6 | 44 | ||
| Males | |||||||||||||
| 1 | 1 | 11 | 12 | 28 | 52 | ||||||||
| 2 | 8 | 2 | 9 | 3 | 22 | ||||||||
| 3 | 1 | 6 | 1 | 5 | 13 | ||||||||
| Total | 18 | 74 | 74 | 80 | 78 | 62 | 88 | 61 | 125 | 168 | 41 | 88 | 957 |
| fF | 1.0 | 1.0 | 1.0 | 1.00 | 0.9 | 0.7 | 1.0 | 1.0 | 1.0 | 0.9 | 1.0 | 0.7 | 0.9 |
Monthly frequency distribution by reproductive stage (cf. Table 1) of males and females sampled (in italics: fraction females fF).
| Reproductive stage . | Aug. . | Sept. . | Oct. . | Nov. . | Dec. . | Jan. . | Feb. . | Mar. . | Apr. . | May . | Jun. . | Jul. . | Total . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Females | |||||||||||||
| 1 | 8 | 8 | 6 | 11 | 17 | 6 | 14 | 20 | 22 | 4 | 10 | 126 | |
| 2 | 1 | 22 | 27 | 13 | 9 | 20 | 13 | 17 | 25 | 15 | 8 | 15 | 185 |
| 3 | 11 | 16 | 15 | 11 | 3 | 13 | 10 | 19 | 16 | 8 | 14 | 136 | |
| 4 | 7 | 15 | 11 | 22 | 18 | 1 | 33 | 10 | 32 | 40 | 12 | 6 | 207 |
| 5 | 7 | 2 | 1 | 4 | 8 | 3 | 4 | 11 | 7 | 7 | 54 | ||
| 6 | 3 | 11 | 4 | 18 | 9 | 2 | 17 | 4 | 11 | 32 | 1 | 6 | 118 |
| 7 | 5 | 6 | 2 | 3 | 3 | 2 | 6 | 10 | 1 | 6 | 44 | ||
| Males | |||||||||||||
| 1 | 1 | 11 | 12 | 28 | 52 | ||||||||
| 2 | 8 | 2 | 9 | 3 | 22 | ||||||||
| 3 | 1 | 6 | 1 | 5 | 13 | ||||||||
| Total | 18 | 74 | 74 | 80 | 78 | 62 | 88 | 61 | 125 | 168 | 41 | 88 | 957 |
| fF | 1.0 | 1.0 | 1.0 | 1.00 | 0.9 | 0.7 | 1.0 | 1.0 | 1.0 | 0.9 | 1.0 | 0.7 | 0.9 |
| Reproductive stage . | Aug. . | Sept. . | Oct. . | Nov. . | Dec. . | Jan. . | Feb. . | Mar. . | Apr. . | May . | Jun. . | Jul. . | Total . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Females | |||||||||||||
| 1 | 8 | 8 | 6 | 11 | 17 | 6 | 14 | 20 | 22 | 4 | 10 | 126 | |
| 2 | 1 | 22 | 27 | 13 | 9 | 20 | 13 | 17 | 25 | 15 | 8 | 15 | 185 |
| 3 | 11 | 16 | 15 | 11 | 3 | 13 | 10 | 19 | 16 | 8 | 14 | 136 | |
| 4 | 7 | 15 | 11 | 22 | 18 | 1 | 33 | 10 | 32 | 40 | 12 | 6 | 207 |
| 5 | 7 | 2 | 1 | 4 | 8 | 3 | 4 | 11 | 7 | 7 | 54 | ||
| 6 | 3 | 11 | 4 | 18 | 9 | 2 | 17 | 4 | 11 | 32 | 1 | 6 | 118 |
| 7 | 5 | 6 | 2 | 3 | 3 | 2 | 6 | 10 | 1 | 6 | 44 | ||
| Males | |||||||||||||
| 1 | 1 | 11 | 12 | 28 | 52 | ||||||||
| 2 | 8 | 2 | 9 | 3 | 22 | ||||||||
| 3 | 1 | 6 | 1 | 5 | 13 | ||||||||
| Total | 18 | 74 | 74 | 80 | 78 | 62 | 88 | 61 | 125 | 168 | 41 | 88 | 957 |
| fF | 1.0 | 1.0 | 1.0 | 1.00 | 0.9 | 0.7 | 1.0 | 1.0 | 1.0 | 0.9 | 1.0 | 0.7 | 0.9 |
In males, immature fish (Stage 1; length range 68–97 cm) dominated (Figure 1). Maturing (Stage 2) fish were observed in the range of 87–98 cm and very few mature (Stage 3) males were observed (range 85–100 cm). Clasper length and testes weight increased with size (range 0.5–65.4 g and 22–94 mm, respectively; Figure 2).
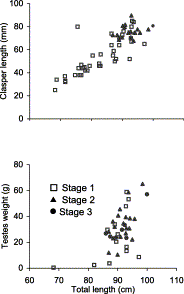
Relationship between (top) clasper length (mm) and (bottom) testis weight (g) and total length (cm) of male C. coelolepis by maturity stage.
Testis weight appears to be a bad indicator of maturity stage, because the values for the three stages largely overlap. While clasper length of maturing and mature fish was always in the upper range of the total distribution (>61 mm), claspers of this size were also recorded among immature fish. The length at 50% sexual maturity could not be accurately determined owing to the preponderance of immature males in most length classes, but the data suggest a value around 90 cm (Figure 1(top)).
In females, ovary weight and uterine width showed clear variations with reproductive stage (Table 3). Gonad weight increased with ovary development and oocyte maturation. Ripe females (Stage 3) showed the highest values of gonad weight and oocyte diameter (65.1±6.0; range 45–88 mm).
Mean gonad weight and uterine width of C. coelolepis by maturity stage (±s.d.).
| Stage . | Length range (cm) . | Gonad weight (g) . | Uterine width (mm) . |
|---|---|---|---|
| 1 | 76–119 | 26.3±17.8 | <26 |
| 2 | 100–120 | 62.1±54.2 | 34.6±7.9 |
| 3 | 98–120 | 1515.7±512.1 | 32.8±7.2 |
| 4 | 80–122 | 175.7±146.7 | 69.8±10.6 |
| 5 | 100–117 | 77.0±55.7 | 8.2±1.6 |
| 6 | 84–120 | 56.2±24.6 | 9.9±1.7 |
| 7 | 99–116 | 83.4±90.3 | 6.9±8.8 |
| Stage . | Length range (cm) . | Gonad weight (g) . | Uterine width (mm) . |
|---|---|---|---|
| 1 | 76–119 | 26.3±17.8 | <26 |
| 2 | 100–120 | 62.1±54.2 | 34.6±7.9 |
| 3 | 98–120 | 1515.7±512.1 | 32.8±7.2 |
| 4 | 80–122 | 175.7±146.7 | 69.8±10.6 |
| 5 | 100–117 | 77.0±55.7 | 8.2±1.6 |
| 6 | 84–120 | 56.2±24.6 | 9.9±1.7 |
| 7 | 99–116 | 83.4±90.3 | 6.9±8.8 |
Mean gonad weight and uterine width of C. coelolepis by maturity stage (±s.d.).
| Stage . | Length range (cm) . | Gonad weight (g) . | Uterine width (mm) . |
|---|---|---|---|
| 1 | 76–119 | 26.3±17.8 | <26 |
| 2 | 100–120 | 62.1±54.2 | 34.6±7.9 |
| 3 | 98–120 | 1515.7±512.1 | 32.8±7.2 |
| 4 | 80–122 | 175.7±146.7 | 69.8±10.6 |
| 5 | 100–117 | 77.0±55.7 | 8.2±1.6 |
| 6 | 84–120 | 56.2±24.6 | 9.9±1.7 |
| 7 | 99–116 | 83.4±90.3 | 6.9±8.8 |
| Stage . | Length range (cm) . | Gonad weight (g) . | Uterine width (mm) . |
|---|---|---|---|
| 1 | 76–119 | 26.3±17.8 | <26 |
| 2 | 100–120 | 62.1±54.2 | 34.6±7.9 |
| 3 | 98–120 | 1515.7±512.1 | 32.8±7.2 |
| 4 | 80–122 | 175.7±146.7 | 69.8±10.6 |
| 5 | 100–117 | 77.0±55.7 | 8.2±1.6 |
| 6 | 84–120 | 56.2±24.6 | 9.9±1.7 |
| 7 | 99–116 | 83.4±90.3 | 6.9±8.8 |
After ovulation and during gestation (Stages 4–7), no development of oocytes within the ovary was observed and ovary weight tended to decrease to the value observed in the beginning of the reproductive cycle (Stage 2). Uterine width increased with length in immature females and remained fairly constant once sexual maturity was achieved during ovary development (Stages 2 and 3). During ovulation, the transfer of large ova further increased uterine width (Stage 4). The increase continued throughout gestation (Stages 5 and 6). After birth, the uteri were still wide (Stage 7). Estimated female length at 50% sexual maturity was 98.5 cm (Figure 3), with the smallest mature female measuring 80 cm and the largest immature one 119 cm. Mature females dominated almost all length classes (Figure 1(bottom)). Gravid females (Stages 4–6) represented nearly 50% of those mature, followed by Stage 2 with 26% and by Stage 3 with 17%. Stage 7 females represented only ≈6%.
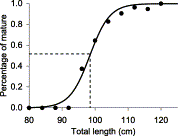
Maturity ogive for female C. coelolepis (M = 1/(exp(−32.6 + 0.331 × L) + 1); variance explained: 98.6%; length at 50% maturity: 98.5 cm).
One specimen (95 cm) had both types of gonads (Figure 4), an ovary with developing and atretic oocytes (2–15 and 40 mm in diameter, respectively) on the right side and a testis (weight 34.6 g) on the left side. Only the right oviduct, nidamental gland and uterus were present and claspers were absent. This observation constitutes the first record of hermaphroditism in C. coelolepis.

Photo of the gonads of a 95 cm C. coelolepis hermaphrodite. (OV, ovary; UT, uterus; TE, testis).
Among 175 pregnant females, a total of 1752 embryos were found in different stages of development (length range: 28–300 mm). For all embryos allowing for distinction of gender (n=1553), sex ratio (F/M) was 1, and for only those with completely absorbed yolk sacs (n=30) it was 0.9. All embryos present in a female were of similar size and in the same developmental stage. Egg capsules, embryonic membranes or uterine compartments were never found.
In the early stage of gestation, uteri contained several large ova, well separated and covered by a thin hyaline membrane. The smallest embryo observed (28 mm) had long filamentous and heavily irrigated external gills, undifferentiated sex and lacked pigmentation. Claspers were observed at a minimum embryo size of 92 mm and for larger embryos gender distinction was always possible. Pigmentation occurred at a length of 100–150 mm and coincided with regression of the external gills (largest embryo with visible external gills: 133 mm). All embryos >150 mm had completed differentiation and attained the juvenile body appearance.
The external yolk sac (EYS) weighed 120–130 g in the smallest embryos. By the end of the gestation period, the EYS was completely absorbed and had regressed to a scar. The presence of an internal yolk sac (IYS) was only observed in embryos >140 mm. Embryos with completely absorbed EYS measured 233–300 mm (mean: 256 ± 14 mm). Females bearing embryos >240 mm (i.e. in the final gestation stage) were infrequent (n=8).
Embryo weight increased exponentially with length (Figure 5(top)) while embryo weight plus EYS showed a more moderate increase. Compared to the mean total weight (embryo+EYS) of the smallest embryos observed (<60 mm: 124.5 ± 3.3 g; n=79), the mean weight of embryos with completely absorbed EYS (159.0 ± 23.2 g; n=164) suggests an increase of 22% from the initial value. EYS weight showed a pronounced decline with embryo length while IYS weight increased up to a size of 250 mm (Figure 5(bottom)). Beyond 250 mm, IYS decreased until birth coinciding with EYS depletion.
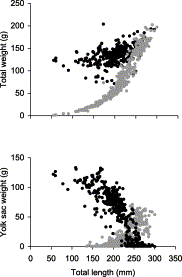
Relationship between (top) embryo weight (g) with (black dots) and without external yolk sac (grey dots) and (bottom) weight of the external (black dots) and of the internal yolk sac (grey dots) and embryo length (mm) of C. coelolepis.
Mean ovarian fecundity (Stage 3) was 13.2±4.5 oocytes (range: 5–30; n=82) and uterine fecundity (per pregnant female) 9.9±4.9 embryos (range 1–25; n=1738). The number of oocytes was not significantly correlated with length of fish or oocyte diameter and also embryo number was not significantly correlated with length of the mother or of the embryos.
Females were present in most reproductive stages during the entire year although relative frequencies by month and stage varied considerably (Table 2).
Discussion
The reproductive cycle of C. coelolepis on the Portuguese mainland slope is similar to the one described for Japanese waters (Yano and Tanaka, 1988) and for the area west of the British Isles (Girard and Du Buit, 1999). A female dominance has also been noticed in other studies (Yano and Tanaka, 1988; Clarke et al., 2001), which considered this as indicative of sexual segregation with depth. These authors found that immature fish occur at larger depths. Monthly differences in the depth interval exploited by the fleet may be partly responsible for variations in sex ratio and in the frequency distribution of the different maturity stages.
In males, testis weight showed a considerable overlap among the three reproductive stages, immature males having sometimes even higher testis weights than maturing or mature males. Overlap among stages was not nearly so large with respect of clasper length, suggesting that maturation and testes enlargement are independent of clasper growth and may even occur before clasper development.
So far, sperm has never been found in female reproductive tracts, suggesting that ova are fertilised immediately after copulation. Considering that all oocytes are fertilised when they reach the uteri, fertilisation should occur during their transfer. Consequently, ovulation must coincide with, and may be induced by, copulation. Observations related to reproductive depth segregation support this hypothesis: females with mature oocytes are most abundant in the same depth interval as active mature males (Yano and Tanaka, 1988; Girard and Du Buit, 1999; Clarke et al., 2001).
Our data on embryonic development are in agreement with earlier descriptions by Girard and Du Buit (1999). Embryo growth concurs with EYS depletion but appears not to be limited by these reserves: the embryo continues to grow by consuming the IYS after the EYS has been completely absorbed. However, yolk may not be the unique source of energy. Although no placentas, uterine compartments or embryonic membranes are formed during gestation, the presence of villosities in the inner uterine layer and their increase in length and irrigation during gestation suggests an additional source of nutrition for the embryos. Support for this hypothesis is provided by the 22% (35 g) increase in total embryo weight at the end of gestation. An even larger increase of 60 g was found by Girard and Du Buit (1999). Biochemical characterisation of the uterine fluid in successive developmental stages is required to certify this hypothesis.
Most stages of reproduction and embryonic development were found in all months, indicating that reproduction and parturition occur throughout the year. Nonetheless, the differential monthly frequency distributions observed in each gestation stage suggest that high fractions of mature females were reproductively synchronous, especially Stages 5 and 6. Embryo length frequency distribution by month could not confirm this apparent synchronism because of the low number of observations. Future studies should consider a larger time interval covering the whole reproductive cycle that is thought to be over 2 years (Yano and Tanaka, 1988) and pay special attention to the depth strata where the fish were actually caught.
The available data on reproduction of the species in different geographical areas are summarised in Table 4. Mediterranean samples presented the most obvious differences: the length range is displaced to smaller sizes with a maximum (65 cm; Clò et al., 2002) only reaching the lower end of the range observed in other areas. In Japanese and Atlantic waters, the length ranges caught are more similar, but the former had higher mean ovarian and uterine fecundity and smaller oocytes before ovulation. While fecundity and size at birth appear to be higher in the area west of the British Isles than off Portugal, oocyte diameter was lower. Thus, the reproductive strategy on the Portuguese slope seems to favour the accumulation of yolk reserves for embryo development at the expense of the number of embryos and length at birth. Another difference between the two Atlantic regions refers to clasper length in relation to maturity stage. Applying the same morphological criteria for distinguishing maturity stages, mature males off the British Isles had claspers measuring >30 mm (Girard and Du Buit, 1999), while those in Portuguese waters were >70 mm. In addition, clasper length ranges were quite different (6–37 and 22–94 mm), despite a similar length range sampled.
Comparison of reproductive studies in C. coelolepis from different geographical areas (M: males; F: females; n.d.: no data; range in brackets).
| Characteristics . | Sex . | Yano and Tanaka (1988) . | Girard and Du Buit (1999) . | Clò et al. (2002) . | This study . |
|---|---|---|---|---|---|
| Geographic area | Suruga Bay, Japan | West of the British Isles | Mediterranean | Off Portugal mainland | |
| Length range (cm) | M | 60–95 | 62–108 | 35–56 | 68–102 |
| F | 50–115 | 70–122 | 32–65 | 77–119 | |
| Length at 50% maturity (cm) | M | >70.0a | 86.0 | >53.0b | – |
| F | 95.0 | 102.0 | 98.5 | ||
| Mean ovarian fecundityc | 25.7 (20–28) | 17 (8–22) | n.d. | 13.2 (2–30) | |
| Oocyte diameter before ovulation (mm) | 50–60 | 60 | n.d. | 60–80 | |
| Mean uterine fecundity | 22.0 (15–29) | 14.0 (8–19) | n.d. | 9.9 (1–25) | |
| Sex ratio at birth (F/M) | 0.9 | 0.9 | n.d. | 0.9 | |
| Mean TL at birth (mm) | – | 300 | n.d. | 268 (233–300) |
| Characteristics . | Sex . | Yano and Tanaka (1988) . | Girard and Du Buit (1999) . | Clò et al. (2002) . | This study . |
|---|---|---|---|---|---|
| Geographic area | Suruga Bay, Japan | West of the British Isles | Mediterranean | Off Portugal mainland | |
| Length range (cm) | M | 60–95 | 62–108 | 35–56 | 68–102 |
| F | 50–115 | 70–122 | 32–65 | 77–119 | |
| Length at 50% maturity (cm) | M | >70.0a | 86.0 | >53.0b | – |
| F | 95.0 | 102.0 | 98.5 | ||
| Mean ovarian fecundityc | 25.7 (20–28) | 17 (8–22) | n.d. | 13.2 (2–30) | |
| Oocyte diameter before ovulation (mm) | 50–60 | 60 | n.d. | 60–80 | |
| Mean uterine fecundity | 22.0 (15–29) | 14.0 (8–19) | n.d. | 9.9 (1–25) | |
| Sex ratio at birth (F/M) | 0.9 | 0.9 | n.d. | 0.9 | |
| Mean TL at birth (mm) | – | 300 | n.d. | 268 (233–300) |
100% Maturation.
Minimum.
Ø > 40 mm.
Comparison of reproductive studies in C. coelolepis from different geographical areas (M: males; F: females; n.d.: no data; range in brackets).
| Characteristics . | Sex . | Yano and Tanaka (1988) . | Girard and Du Buit (1999) . | Clò et al. (2002) . | This study . |
|---|---|---|---|---|---|
| Geographic area | Suruga Bay, Japan | West of the British Isles | Mediterranean | Off Portugal mainland | |
| Length range (cm) | M | 60–95 | 62–108 | 35–56 | 68–102 |
| F | 50–115 | 70–122 | 32–65 | 77–119 | |
| Length at 50% maturity (cm) | M | >70.0a | 86.0 | >53.0b | – |
| F | 95.0 | 102.0 | 98.5 | ||
| Mean ovarian fecundityc | 25.7 (20–28) | 17 (8–22) | n.d. | 13.2 (2–30) | |
| Oocyte diameter before ovulation (mm) | 50–60 | 60 | n.d. | 60–80 | |
| Mean uterine fecundity | 22.0 (15–29) | 14.0 (8–19) | n.d. | 9.9 (1–25) | |
| Sex ratio at birth (F/M) | 0.9 | 0.9 | n.d. | 0.9 | |
| Mean TL at birth (mm) | – | 300 | n.d. | 268 (233–300) |
| Characteristics . | Sex . | Yano and Tanaka (1988) . | Girard and Du Buit (1999) . | Clò et al. (2002) . | This study . |
|---|---|---|---|---|---|
| Geographic area | Suruga Bay, Japan | West of the British Isles | Mediterranean | Off Portugal mainland | |
| Length range (cm) | M | 60–95 | 62–108 | 35–56 | 68–102 |
| F | 50–115 | 70–122 | 32–65 | 77–119 | |
| Length at 50% maturity (cm) | M | >70.0a | 86.0 | >53.0b | – |
| F | 95.0 | 102.0 | 98.5 | ||
| Mean ovarian fecundityc | 25.7 (20–28) | 17 (8–22) | n.d. | 13.2 (2–30) | |
| Oocyte diameter before ovulation (mm) | 50–60 | 60 | n.d. | 60–80 | |
| Mean uterine fecundity | 22.0 (15–29) | 14.0 (8–19) | n.d. | 9.9 (1–25) | |
| Sex ratio at birth (F/M) | 0.9 | 0.9 | n.d. | 0.9 | |
| Mean TL at birth (mm) | – | 300 | n.d. | 268 (233–300) |
100% Maturation.
Minimum.
Ø > 40 mm.
Based on these regional differences in reproduction and the existence of major topographic barriers within the wide geographic distribution area, it may be hypothesised that there is limited gene exchange among C. coelolepis populations in different regions. The existence of a segregated population has been suggested for the Mediterranean based on morphometric data (Clò et al., 2002). Likewise, no exchange can be expected between populations in Japanese and Atlantic waters. However, the observed differences in reproductive characteristics and strategies suggest that distinct local populations may also exist within the Atlantic. To develop an appropriate management plan for this species, it is important to further clarify the potential existence of regional populations.
A common feature on Japanese and Atlantic fishing grounds is the absence of small fish and the low abundance of females in the latest stage of gestation. Absence of small fish does not seem to be caused by gear selectivity. Although small fish trying to take bait from longlines may be scared off by larger fish (Clarke et al., 2002), the use of smaller hooks did not lead to the catch of small specimens (Yano and Tanaka, 1988). The explanation put forward was that somewhere a nursery area exists where females give birth and where juveniles grow up during their first years of life (Yano and Tanaka, 1988; Clarke et al., 2001). According to our data, new cohorts recruit to the exploited stock approximately when they reach sexual maturity. However, the depth range selected by the fishery implicates a predominance of mature females in the catch, a large proportion of which is pregnant. Because the number of young produced by deep-sea sharks is closely related to the breeding biomass (Stevens et al., 2000), a high fishing pressure on females and uncontrolled fisheries may disrupt the reproduction capacity.
On the Portuguese slope (ICES Sub-area IXa), number of boats targeting deep-water sharks and total landings of C. coelolepis has remained stable during the last decade. This may indicate that at present fishing mortality is sustained by its reproductive capacity. In Sub-area VI, the stock status of this species appears to be quite different: CPUE values as well as mean length in the catch have declined markedly (ICES, 2002). The difference between the two areas may be explained by the artisanal character of the Portuguese fishery compared to the French fishery.



