-
PDF
- Split View
-
Views
-
Cite
Cite
Lynn Kobeissi, Kristi Briggs, Caya McFalls, Mark Lazarev, Joanna Melia, CARE OF PATIENTS WITH INFLAMMATORY BOWEL DISEASE WITH INADEQUATE RESPONSE OR INTOLERANCE TO UPADACITINIB: WHAT TO DO NEXT?, Inflammatory Bowel Diseases, Volume 31, Issue Supplement_1, February 2025, Pages S68–S69, https://doi.org/10.1093/ibd/izae282.160
Close - Share Icon Share
Abstract
Upadacitinib, a selective Janus kinase 1 inhibitor, is approved for TNF-alpha-experienced patients with inflammatory bowel disease. Phase III trials showed remission rates near 50%, while real-world studies report up to 80%.These advancements aim to overcome the therapeutic ceiling seen in patients with prior anti-TNF exposure. However, the challenge remains in managing those intolerant or unresponsive to upadacitinib.
To investigate management strategies for patients who fail or are intolerant to upadacitinib.
A retrospective cohort study at Johns Hopkins on patients aged 18+ with Crohn’s disease (CD), Ulcerative Colitis (UC), or Indeterminate Colitis (ID) treated with upadacitinib between January 2020 and September 2024. Patients were excluded if they achieved remission, had an endoscopic response, or were new starts (≤60 days). Clinical remission was defined by a colitis activity index ≤2, Harvey-Bradshaw index ≤3, or symptom resolution off steroids. Non-responders were patients with persistent symptoms, steroid dependence, or worsened after initial improvement.
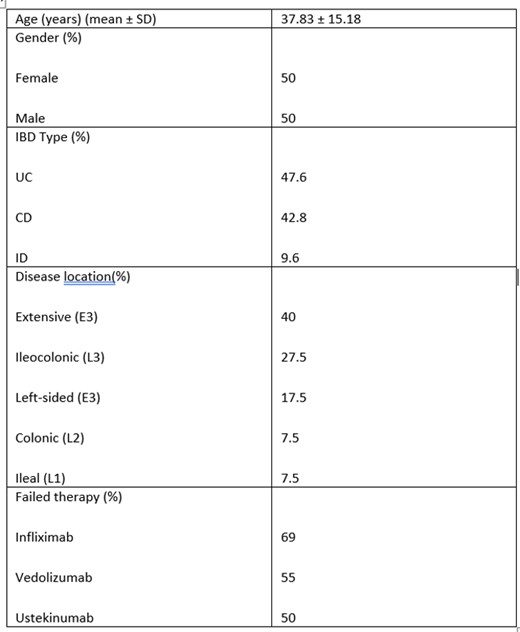
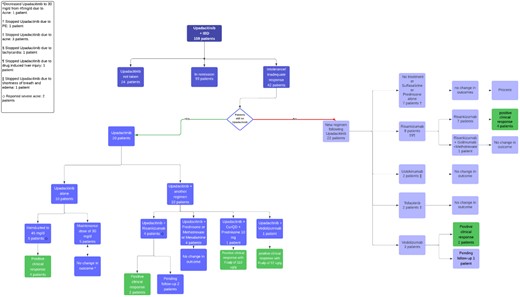
Of 135 patients receiving upadacitinib, 42 did not achieve a clinical response, patient factors can be found in table 1. All had prior anti-TNF exposure, with an average upadacitinib duration of 9.5 months. All received induction dosing of 45 mg/day; 37 remained on 30 mg/day, and 5 on 15 mg/day. The flowchart in figure 1 outlines the treatment pathways taken. Twenty patients remained on upadacitinib, 5 were increased back to 45 mg/day after 12 months, with 4 showing improvement and 40% normalizing fecal calprotectin. One patient achieved full remission after two re-inductions. Re-induction significantly improved response rates (p-value of 0.00253).
Four patients remaining on upadacitinib had risankizumab added, all showing improved clinical response. One UC patient received vedolizumab and upadacitinib, improving calprotectin levels. Twenty-two patients switched to other agents; 8 (mostly CD) transitioned to risankizumab, with 50% improving after 3 months. Three UC patients switched to vedolizumab, all improving clinically.
Upadacitinib re-induction (45 mg/day) shows promise for patients with persistent disease despite maintenance therapy. Risankizumab may also be an effective option after upadacitinib failure or in combination. Further studies are needed to confirm the benefits of re-induction and combination therapy in avoiding surgical interventions and improving disease management.
Table 1: Patient factors
- tumor necrosis factors
- crohn's disease
- inflammatory bowel disease
- ulcerative colitis
- endoscopy
- phase 3 clinical trials
- colitis
- combined modality therapy
- feces
- steroids
- surgical procedures, operative
- disease management
- persistence
- janus kinases
- colitis, indeterminate
- steroid dependency
- frequency of responses
- complete remission
- leukocyte l1 antigen complex
- disease remission
- vedolizumab
- risankizumab
- upadacitinib



