-
PDF
- Split View
-
Views
-
Cite
Cite
Viveca Söderström-Anttila, Ulla-Britt Wennerholm, Anne Loft, Anja Pinborg, Kristiina Aittomäki, Liv Bente Romundstad, Christina Bergh, Surrogacy: outcomes for surrogate mothers, children and the resulting families—a systematic review, Human Reproduction Update, Volume 22, Issue 2, March/April 2016, Pages 260–276, https://doi.org/10.1093/humupd/dmv046
Close - Share Icon Share
Abstract
Surrogacy is a highly debated method mainly used for treating women with infertility caused by uterine factors. This systematic review summarizes current levels of knowledge of the obstetric, medical and psychological outcomes for the surrogate mothers, the intended parents and children born as a result of surrogacy.
PubMed, Cochrane and Embase databases up to February 2015 were searched. Cohort studies and case series were included. Original studies published in English and the Scandinavian languages were included. In case of double publications, the latest study was included. Abstracts only and case reports were excluded. Studies with a control group and case series (more than three cases) were included. Cohort studies, but not case series, were assessed for methodological quality, in terms of risk of bias. We examined a variety of main outcomes for the surrogate mothers, children and intended mothers, including obstetric outcome, relationship between surrogate mother and intended couple, surrogate's experiences after relinquishing the child, preterm birth, low birthweight, birth defects, perinatal mortality, child psychological development, parent–child relationship, and disclosure to the child.
The search returned 1795 articles of which 55 met the inclusion criteria. The medical outcome for the children was satisfactory and comparable to previous results for children conceived after fresh IVF and oocyte donation. The rate of multiple pregnancies was 2.6–75.0%. Preterm birth rate in singletons varied between 0 and 11.5% and low birthweight occurred in between 0 and 11.1% of cases. At the age of 10 years there were no major psychological differences between children born after surrogacy and children born after other types of assisted reproductive technology (ART) or after natural conception. The obstetric outcomes for the surrogate mothers were mainly reported from case series. Hypertensive disorders in pregnancy were reported in between 3.2 and 10% of cases and placenta praevia/placental abruption in 4.9%. Cases with hysterectomies have also been reported. Most surrogate mothers scored within the normal range on personality tests. Most psychosocial variables were satisfactory, although difficulties related to handing over the child did occur. The psychological well-being of children whose mother had been a surrogate mother between 5 and 15 years earlier was found to be good. No major differences in psychological state were found between intended mothers, mothers who conceived after other types of ART and mothers whose pregnancies were the result of natural conception.
Most studies reporting on surrogacy have serious methodological limitations. According to these studies, most surrogacy arrangements are successfully implemented and most surrogate mothers are well-motivated and have little difficulty separating from the children born as a result of the arrangement. The perinatal outcome of the children is comparable to standard IVF and oocyte donation and there is no evidence of harm to the children born as a result of surrogacy. However, these conclusions should be interpreted with caution. To date, there are no studies on children born after cross-border surrogacy or growing up with gay fathers.
Introduction
Definitions
Surrogacy implies that a woman becomes pregnant and gives birth to a child with the intention of giving away this child to another person or couple, commonly referred to as the ‘intended’ or ‘commissioning’ parents (Shenfield et al., 2005). A surrogate mother is the woman who carries and gives birth to the child and the intended parent is the person who intends to raise the child. The definition from the European Society for Human Reproduction and Embryology (ESHRE) (Shenfield et al., 2005) does not state the sexuality of the intended parents. There are two main types of surrogacy, traditional and gestational. The first traditional surrogacy arrangement is believed to have happened about 2000 years before the birth of Christ and was mentioned in the Old Testament of the Bible. Sarah and Abraham were unable to conceive and Sarah hired her maiden Hagar to carry a child for her husband. Subsequently Hagar gave birth to a son, Ishmael, for Sarah and Abraham. Nowadays traditional (also called genetic or partial) surrogacy is the result of artificial insemination of the surrogate mother with the intended father's sperm. This means that the surrogate mother's eggs are used, making her a genetic parent along with the intended father. Gestational or IVF surrogacy (also called host or full surrogacy) is defined as an arrangement in which an embryo from the intended parents, or from a donated oocyte or sperm, is transferred to the surrogate's uterus. In gestational surrogacy, the woman who carries the child (the gestational carrier) has no genetic connection to the child (Zegers-Hochschild et al., 2009). The first successful IVF surrogate pregnancy was reported by Utian et al. in 1985 (Utian et al., 1985). In this review, we have decided to use the terms ‘traditional surrogacy’ and ‘gestational surrogacy’ for the two different types of surrogacy treatment.
Surrogacy may be commercial or altruistic, depending upon whether the surrogate receives financial reward for her pregnancy. In commercial surrogacy the surrogate is usually recruited through an agency, reimbursed for medical costs and paid for her gestational services. With altruistic surrogacy, the surrogate is found through friends, acquaintances or advertisement. She may be reimbursed for medical costs directly related to the pregnancy and for loss of income due to the pregnancy (FIGO, Committee for Ethical Aspects of Human Reproduction and Women's Health, 2008; Dempsey, 2013).
Indications for surrogacy treatment
The main indication for surrogacy treatment is congenital or acquired absence of a functioning uterus. Müllerian aplasia, including congenital absence of the uterus such as Mayer-Rokitansky-Kuster-Hauser (MRKH) syndrome, is relatively rare with an incidence of one per 4000–5000 newborn girls (Lindenman et al., 1997; Aittomaki et al., 2001). Young fertile women with normally functioning ovaries might lose their uterus in connection with serious obstetric complications, such as intra- or post-partum heavy bleeding or rupture of the uterus. Such obstetric complications will often lead to the death of the baby as well. Medical diseases of the uterus, for example cervical cancer, will also lead to hysterectomy and uterine infertility. Significant structural abnormalities, an inoperably scarred uterus or repeated miscarriages are other indications for considering using a surrogate mother. Severe medical conditions (e.g. heart and renal diseases), which might be life-threatening for a woman during pregnancy, are also indications that a surrogacy may be considered, provided that the intended mother is healthy enough to take care of a child after birth and that her life expectancy is reasonable (Brinsden, 2003). A further indication is the biological inability to conceive or bear a child, which applies to same-sex male couples or single men (Dempsey, 2013). In some countries gestational carriers may be considered when an unidentified endometrial factor exists, such as for couples with repeated unexplained IVF failures despite retrieval of good-quality embryos (Practice Committee of American Society for Reproductive Medicine; ASRM, 2015).
Choice of a surrogate mother
The choice of a surrogate mother is of the highest importance for the successful outcome of the treatment. She might be a member of the family, such as a sister or a mother, or an anonymous or known unrelated person. According to recommendations from ESHRE and the American Society for Reproductive Medicine (ASRM), a gestational carrier should preferably be between the ages of 21 and 45 years and she should have at least one child. Her previous pregnancies should have been full-term and uncomplicated (Shenfield et al., 2005; ASRM, 2015). Ideally, the carrier should not have had more than a total of five previous deliveries and three deliveries via Caesarean section (ASRM, 2015). General requirements as to the screening and testing of gestational carriers and the latest recommendations related to psychosocial consultations have been summarized by the expert groups from ESHRE and ASRM (Shenfield et al., 2005; ASRM, 2015). According to an International Federation of Gynecology & Obstetrics (FIGO) committee report only gestational surrogacy is nowadays acceptable. It was also decided that the autonomy of the surrogate mother should be respected at all stages, including any decision about her pregnancy, which may conflict with the commissioning couple's interest. Surrogacy arrangements should not be commercial (FIGO Committee for Ethical Aspects of Human Reproduction and Women's Health, 2008).
Pregnancy and delivery rates after surrogacy treatment
In gestational surrogacy programmes, the clinical pregnancy rate per embryo transfer has been reported as being between 19 and 33%, with between 30 and 70% of the couples succeeding in becoming parents as a result of the arrangement (Meniru and Craft, 1997; Corson et al., 1998; Parkinson et al., 1998; Wood et al., 1999; Beski et al., 2000; Brinsden et al., 2000; Goldfarb et al., 2000; Soderstrom-Anttila et al., 2002; Raziel et al., 2005; Dar et al., 2015).
In the recent, and thus far largest, report including 333 consecutive surrogacy treatments in Canada, the pregnancy, miscarriage and delivery rates did not differ between patient groups with different indications for surrogacy treatment (Dar et al., 2015).
Legislation in different countries
In Europe, surrogacy is not officially allowed in Austria, Bulgaria, Denmark, Finland, France, Germany, Italy, Malta, Norway, Portugal, Spain and Sweden. Altruistic, but not commercial, surrogacy is allowed in Belgium, Greece, the Netherlands and the UK. Some European countries, such as Poland and the Czech Republic, currently have no laws regulating surrogacy (Brunet et al., 2013; Deomampo, 2015). Commercial surrogacy is legal in Georgia, Israel, Ukraine, Russia, India and California, USA, while in many states of the USA only altruistic surrogacy is allowed. Altruistic surrogacy is also allowed in Australia, Canada and New Zealand.
Cross-border surrogacy
As surrogacy treatment is illegal in the majority of Western countries, infertile couples are seeking commercial surrogacy arrangements elsewhere, for example in Russia, Ukraine and India, where the treatment is available. It has been estimated that more than 25 000 children have been born or are to be born to surrogates in India, of which 50% are from the West (Shetty, 2012). Cross-border gestational surrogacy is an activity that challenges legal and ethical norms in many countries. It puts both intended parents and gestational surrogates at risk and can leave the offspring of these arrangements vulnerable in a variety of ways (Pande, 2011; Crockin, 2013). There is uncertainty about the status of the parent and child, as well as legal issues regarding immigration and citizenship (Crockin, 2013; Deomampo, 2015; Schover, 2014). By legalizing surrogacy, potential harm to the health and well-being of all parties involved in unregulated cross-border surrogacy arrangements can be avoided (Ekberg, 2014). Another way of reducing the legal uncertainties is to regulate the legal implications of surrogacy (i.e. legal parenthood) without making surrogacy itself legal. This has been suggested by the Hague Convention on Private International Law. In Austria and Germany, the best interest of the child has been decided to outweigh the reservations of the national legislation concerning surrogacy (http://www.hcch.net/index).
Concern about surrogacy arrangements
In many Western countries surrogacy practice has been made illegal because of concern for the surrogate mother, the welfare of the child and the family created by the birth of the new baby. There have been worries about the possibility of exploitation or coercion of women to act as gestational carriers (Tieu, 2009; Pande, 2011; Deonandan et al., 2012). A surrogate undergoes risks during pregnancy similar to any other pregnant woman. She is exposed to the possibility of miscarriage, ectopic pregnancy and common obstetric complications, which are increased by the risk of multiple pregnancies. There has also been concern that psychological reactions may occur post-partum in relation to surrendering the child, as the carrier may develop emotional attachments to the child she has carried (FIGO Committee for Ethical Aspects of Human Reproduction and Women's Health, 2008). Furthermore, there have been fears that the baby might be abandoned by the intended parents and/or the surrogate mother in the case of unexpected complications or birth defects. Potential harm to the health of the offspring includes the negative effects of multiple pregnancies, as well as the possible effects of gamete donation on the well-being of the child (FIGO Committee for Ethical Aspects of Human Reproduction and Women's Health, 2008).
During recent years there have been discussions in many European countries, and all Nordic countries, about whether surrogacy should be allowed in the future. This has led to an urgent need to summarize what we currently know about surrogacy in a systematic way. This systematic review summarizes current levels of knowledge of the obstetric, medical and psychological outcomes for surrogate mothers, the intended parents and the children born as a result of surrogacy.
Methods
We searched the PubMed, Cochrane and Embase databases up to February 2015. The main outcomes we examined were as follows.
For the surrogate mothers: obstetric outcome, psychological characteristics, personality, motivation, relationship with intended couple, contact with the couple and child, experiences after relinquishing child, openness, psychological well-being, satisfaction with surrogacy.
For the children: preterm birth, low birthweight, birth defects, perinatal mortality, child psychological development, child psychological adjustment.
For the intended mothers: quality of life, parent psychological status, parent–child relationship, quality of parenting, marital quality and stability, relationship with surrogate mother, motivation, experience of surrogacy, disclosure to the child.
Systematic search for evidence
The terms used in the searches were (‘Surrogate Mothers’[Mesh]) OR (ivf-surroga*[tiab] OR surrogate mother*[tiab] OR surrogate parent*[tiab] OR surrogacy*[tiab] OR gestational carrier*[tiab] OR surrogate pregnanc*[tiab])) NOT (Editorial[ptyp] OR Letter[ptyp] OR Comment[ptyp]).
OR ((animals[mh]) NOT (animals[mh] AND humans[mh])) NOT (‘News’[Publication Type] OR ‘Newspaper Article’[Publication Type]).
We also manually searched reference lists of identified articles for additional references. Guidelines for meta-analysis and systematic reviews of observational studies were followed (Stroup et al., 2000).
Literature searches and abstract screening were performed by three researchers (CB, UBW and VSA) and one librarian. Differences of opinion in the team were solved by discussion and consensus.
Inclusion and exclusion of studies
Original studies published in English and the Scandinavian languages were included. In the case of double publication the latest study was included. Studies published only as abstracts and case reports were excluded. Studies with a control group and case series (more than three cases) were included.
Appraisal of quality of evidence
The methodological quality of the studies, in terms of risk bias, was assessed by two reviewers (CB and UBW). They used the tools developed by Swedish Agency for Health Technology Assessment and Assessment of Social Services (SBU) for assessing original articles, which grade articles as of low, moderate and high quality. Only cohort studies, not case series, were assessed for methodological quality. For quality of evidence we used the GRADE system (Guyatt et al., 2008).
The GRADE system evaluates the following variables for all studies, both combined and per outcome: Design, study limitations, consistency, directness, precision, publication bias, magnitude of effect, relative effect and absolute effect. Quality levels are divided into high, moderate, low and very low quality. Quality levels are based on our confidence in the effect estimate, which in turn is based on the number of studies, design of studies, consistency of associations between studies, study limitations, directness, precision, publication bias, effect size, and relative and absolute effect.
The quality levels are; very confident = high quality, moderately confident = moderate quality, limited confidence = low quality and very little confidence = very low quality. If conclusions are based on RCTs, GRADE starts at high quality level (level 4) but can be downgraded, while if conclusions are based on observational studies GRADE starts at low quality level (level 2) but might be upgraded (or downgraded). If conclusions were based on case series, no assessment of GRADE was performed.
Results
The search strategy identified a total of 1795 abstracts, of which 55 were selected for inclusion in the systematic review (Fig. 1).
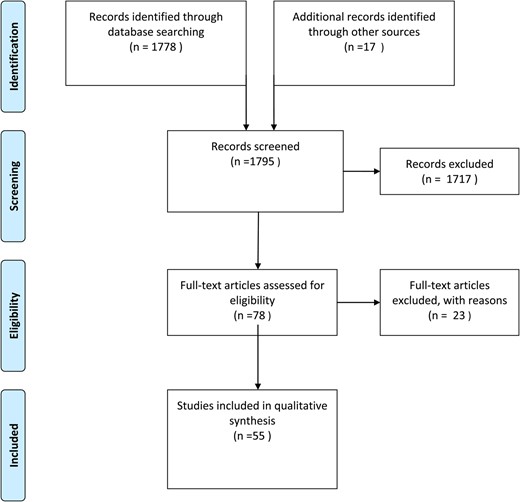
Selection process of publications for a systematic review of surrogacy.
Thirty studies were cohort studies, 22 were case series and three were qualitative studies (Supplementary Table SI). Excluded studies are presented in Supplementary Table SII.
Quality assessment of included cohort studies is presented in Supplementary Table SIII. Of the cohort studies one article was of high quality, 16 were of moderate quality and 13 of low quality.
Obstetric outcome in surrogate mothers
Five studies were identified that reported on pregnancy complications, one cohort study with historical controls and four case series. Three were from the USA, one was from Canada and one from Finland (Table I). The studies included between 8 and 133 deliveries after surrogacy, out of a total of 284 deliveries. Hypertensive disorders in pregnancy (HDP) were reported in between 3.2 and 10% of subjects and placenta praevia/placental abruption in between 1.1 and 7.9% of singleton surrogate pregnancies. The cohort study and one of the case series also reported on pregnancy complications in twin pregnancies. HDP occurred in between 2.9 and 7.4%, and placenta previa/placental abruption in 1.1 and 3.7% in twin pregnancies (Parkinson et al., 1998; Dar et al., 2015). Three cases of hysterectomies were reported. The reasons for hysterectomy were uterine atony, placenta accreta and uterine rupture. Two of these three complications occurred in multiple pregnancies.
| Author, year, country . | Study design . | Number of deliveries and children . | Result . | |
|---|---|---|---|---|
| Intervention . | Control . | |||
| Dar et al. (2015), Canada | Case series | 133 GC deliveries 175 GC children | Hypertensive disorders in pregnancy:
| |
| Duffy et al. (2005), USA | Case series | 8 GC deliveries 11 GC children | Caesarean section:
| |
| Parkinson et al. (1998), USA* | Cohort study | 95 GC deliveries 128 GC children Numbers of IVF children NA | GC deliveries:Hypertensive disorders in pregnancy:
| IVF deliveries:Hypertensive disorders in pregnancy:
|
| Reame and Parker (1990), USA | Case series | 38 deliveries, 39 surrogate (traditional) children | Hypertensive disorders in pregnancy:
| |
| Soderstrom-Anttila et al. (2002), Finland | Case series | 10 GC deliveries 11 GC children | Hypertensive disorders in pregnancy:
| |
| Author, year, country . | Study design . | Number of deliveries and children . | Result . | |
|---|---|---|---|---|
| Intervention . | Control . | |||
| Dar et al. (2015), Canada | Case series | 133 GC deliveries 175 GC children | Hypertensive disorders in pregnancy:
| |
| Duffy et al. (2005), USA | Case series | 8 GC deliveries 11 GC children | Caesarean section:
| |
| Parkinson et al. (1998), USA* | Cohort study | 95 GC deliveries 128 GC children Numbers of IVF children NA | GC deliveries:Hypertensive disorders in pregnancy:
| IVF deliveries:Hypertensive disorders in pregnancy:
|
| Reame and Parker (1990), USA | Case series | 38 deliveries, 39 surrogate (traditional) children | Hypertensive disorders in pregnancy:
| |
| Soderstrom-Anttila et al. (2002), Finland | Case series | 10 GC deliveries 11 GC children | Hypertensive disorders in pregnancy:
| |
GC, gestational carrier; NA, not available.
*IVF pregnancies from Brinsden and Rizk (1992).
| Author, year, country . | Study design . | Number of deliveries and children . | Result . | |
|---|---|---|---|---|
| Intervention . | Control . | |||
| Dar et al. (2015), Canada | Case series | 133 GC deliveries 175 GC children | Hypertensive disorders in pregnancy:
| |
| Duffy et al. (2005), USA | Case series | 8 GC deliveries 11 GC children | Caesarean section:
| |
| Parkinson et al. (1998), USA* | Cohort study | 95 GC deliveries 128 GC children Numbers of IVF children NA | GC deliveries:Hypertensive disorders in pregnancy:
| IVF deliveries:Hypertensive disorders in pregnancy:
|
| Reame and Parker (1990), USA | Case series | 38 deliveries, 39 surrogate (traditional) children | Hypertensive disorders in pregnancy:
| |
| Soderstrom-Anttila et al. (2002), Finland | Case series | 10 GC deliveries 11 GC children | Hypertensive disorders in pregnancy:
| |
| Author, year, country . | Study design . | Number of deliveries and children . | Result . | |
|---|---|---|---|---|
| Intervention . | Control . | |||
| Dar et al. (2015), Canada | Case series | 133 GC deliveries 175 GC children | Hypertensive disorders in pregnancy:
| |
| Duffy et al. (2005), USA | Case series | 8 GC deliveries 11 GC children | Caesarean section:
| |
| Parkinson et al. (1998), USA* | Cohort study | 95 GC deliveries 128 GC children Numbers of IVF children NA | GC deliveries:Hypertensive disorders in pregnancy:
| IVF deliveries:Hypertensive disorders in pregnancy:
|
| Reame and Parker (1990), USA | Case series | 38 deliveries, 39 surrogate (traditional) children | Hypertensive disorders in pregnancy:
| |
| Soderstrom-Anttila et al. (2002), Finland | Case series | 10 GC deliveries 11 GC children | Hypertensive disorders in pregnancy:
| |
GC, gestational carrier; NA, not available.
*IVF pregnancies from Brinsden and Rizk (1992).
Conclusion: Rates of HDP and placental complications in surrogate pregnancies were similar to those for IVF. Rates of HDP were lower than reported in OD pregnancies. Peripartum hysterectomies were reported as severe complications. Since most data was derived from case series, no GRADE assessment was performed.
The outcome for children
Gestational age
Two cohort studies and five case series reported on the gestational age of children born after surrogate pregnancies (Table II). Most studies come from the USA. The cohort studies included in total 1308 children, while the case series included a total of 271 children born after surrogacy. The preterm birth rate (PTB) in surrogate singletons varied between 0 and 11.5% as compared with 14% for IVF singletons. In the largest cohort study (Gibbons et al., 2011) including 1180 surrogacy singletons the mean gestational age was 37.2 weeks as compared with 37.7 weeks for IVF singletons and 37.4 weeks for singletons from OD. The rate of multiple pregnancies was 2.6–75.0% (Table II). The cohort study with historical controls and the case series also reported on gestational age in twin pregnancies (n = 1–38 twin pregnancies). PTB occurred in 20.4–100% of surrogate twin pregnancies. In the cohort study, mean gestational age was 36.2 (SD 0.4) weeks in surrogate twins and 36.0 (SD 2) weeks in IVF twins (Parkinson et al., 1998).
| Author, year, country . | Study design . | Number of deliveries and children . | Result . | ||
|---|---|---|---|---|---|
| Intervention . | Control . | ||||
| Corson et al. (1998), USA | Case series | 27 GC deliveries 33 GC children | PTB:
| Plurality:
| |
| Dar et al. (2015), Canada | Case series | 133 GC deliveries 175 GC children | PTB (<37 weeks):
| Plurality:
| |
| Dermout et al. (2010), The Netherlands | Case series | 13 GC deliveries 16 GC children | PTB (<37 weeks):
| Plurality:
| |
| Duffy et al. (2005), USA | Case series | 8 GC deliveries 11 GC children | PTB (<37 weeks):
| Plurality:
| |
| Gibbons et al. (2011), USA | Cohort study | 1180 GC singletons 49 252 fresh IVF singletons 10 785 frozen IVF singletons 10 176 fresh OD singletons | GA, mean (SD), weeks:
| Not reported | GA, mean (SD), weeks:
|
| Goldfarb et al. (2000) | Case series | 18 GC deliveries | Plurality 38.9% (7/18) | ||
| Meniru and Craft (1997), UK | Case series | 4 GC deliveries | Plurality 75% (1/4) | ||
| Parkinson et al. (1998)*, USA | Cohort study | 95 GC deliveries 128 GC children Numbers on IVF children NA | GA, mean (SEM), weeks:
| Not reported | GA, mean (SEM), weeks:
|
| Reame and Parker (1990), USA | Case series | 38 surrogate (traditional) deliveries, 39 surrogate (traditional) children | PTB:
| Plurality:
| |
| SART (1993), USA | Cohort study | 35 GC deliveries, 3215 fresh IVF deliveries, 431 frozen IVF deliveries, 268 donor deliveries | Plurality:
| Plurality:
| |
| SART (1994), USA | Cohort study | 51 GC deliveries, 4206 fresh IVF deliveries, 619 frozen IVF deliveries, 534 donor deliveries | Plurality:
| Plurality:
| |
| SART (1995), USA | Cohort study | 78 GC deliveries, 5101 fresh IVF deliveries, 791 frozen IVF deliveries, 716 donor deliveries | Plurality:
| Plurality:
| |
| SART (1996), USA | Cohort study | 56 GC deliveries, 4912 fresh IVF deliveries, 1076 frozen IVF deliveries, 929 donor deliveries | Plurality:
| Plurality:
| |
| SART (1998), USA | Cohort study | 45 GC deliveries, 6754 fresh IVF deliveries, 1185 fresh ICSI deliveries, 1136 frozen IVF/ICSI deliveries, 1206 fresh donor deliveries, 146 frozen donor deliveries | Plurality:
| Plurality:
| |
| SART (1999), USA | Cohort study | 187 GC deliveries, 6379 fresh IVF deliveries, 3632 fresh ICSI deliveries, 1457 frozen IVF/ICSI deliveries, 1309 fresh donor deliveries, 214 frozen donor deliveries | Plurality:
| Plurality:
| |
| SART (2000), USA | Cohort study | 187 GC deliveries, 7353 fresh IVF deliveries, 4949 fresh ICSI deliveries, 1719 frozen IVF/ICSI, 1650 fresh donor deliveries, 325 frozen donor deliveries | Plurality:
| Plurality:
| |
| SART (2002a), USA | Cohort study | 235 GC deliveries, 14 789 fresh IVF deliveries, 7712 fresh ICSI deliveries, 1941 frozen IVF/ICSI, 1972 fresh donor deliveries, 410 frozen donor deliveries | Plurality: 38.2% | Plurality:
| |
| SART (2002b), USA | Cohort study | 245 GC deliveries, 16 175 fresh IVF deliveries, 8982 fresh ICSI deliveries, 1956 frozen IVF/ICSI, 2340 fresh donor deliveries, 536 frozen donor deliveries | Plurality: 36.7% | Plurality:
| |
| SART (2004), USA | Cohort study | 382 GC deliveries, 18 793 fresh IVF/ICSI deliveries, 2324 frozen IVF/ICSI deliveries, 2920 fresh donor deliveries, 563 frozen donor deliveries | Plurality: 37.4% | Plurality:
| |
| SART (2007), USA | Cohort study | 464 GC deliveries, 21 475 fresh IVF/ICSI deliveries, 3046 frozen IVF/ICSI deliveries, 3461 fresh donor deliveries, 770 frozen donor deliveries | Plurality: 40.9% | Plurality:
| |
| Utian et al. (1989), USA | Case series | 5 GC deliveries | Plurality: 40% (2/5) | ||
| Author, year, country . | Study design . | Number of deliveries and children . | Result . | ||
|---|---|---|---|---|---|
| Intervention . | Control . | ||||
| Corson et al. (1998), USA | Case series | 27 GC deliveries 33 GC children | PTB:
| Plurality:
| |
| Dar et al. (2015), Canada | Case series | 133 GC deliveries 175 GC children | PTB (<37 weeks):
| Plurality:
| |
| Dermout et al. (2010), The Netherlands | Case series | 13 GC deliveries 16 GC children | PTB (<37 weeks):
| Plurality:
| |
| Duffy et al. (2005), USA | Case series | 8 GC deliveries 11 GC children | PTB (<37 weeks):
| Plurality:
| |
| Gibbons et al. (2011), USA | Cohort study | 1180 GC singletons 49 252 fresh IVF singletons 10 785 frozen IVF singletons 10 176 fresh OD singletons | GA, mean (SD), weeks:
| Not reported | GA, mean (SD), weeks:
|
| Goldfarb et al. (2000) | Case series | 18 GC deliveries | Plurality 38.9% (7/18) | ||
| Meniru and Craft (1997), UK | Case series | 4 GC deliveries | Plurality 75% (1/4) | ||
| Parkinson et al. (1998)*, USA | Cohort study | 95 GC deliveries 128 GC children Numbers on IVF children NA | GA, mean (SEM), weeks:
| Not reported | GA, mean (SEM), weeks:
|
| Reame and Parker (1990), USA | Case series | 38 surrogate (traditional) deliveries, 39 surrogate (traditional) children | PTB:
| Plurality:
| |
| SART (1993), USA | Cohort study | 35 GC deliveries, 3215 fresh IVF deliveries, 431 frozen IVF deliveries, 268 donor deliveries | Plurality:
| Plurality:
| |
| SART (1994), USA | Cohort study | 51 GC deliveries, 4206 fresh IVF deliveries, 619 frozen IVF deliveries, 534 donor deliveries | Plurality:
| Plurality:
| |
| SART (1995), USA | Cohort study | 78 GC deliveries, 5101 fresh IVF deliveries, 791 frozen IVF deliveries, 716 donor deliveries | Plurality:
| Plurality:
| |
| SART (1996), USA | Cohort study | 56 GC deliveries, 4912 fresh IVF deliveries, 1076 frozen IVF deliveries, 929 donor deliveries | Plurality:
| Plurality:
| |
| SART (1998), USA | Cohort study | 45 GC deliveries, 6754 fresh IVF deliveries, 1185 fresh ICSI deliveries, 1136 frozen IVF/ICSI deliveries, 1206 fresh donor deliveries, 146 frozen donor deliveries | Plurality:
| Plurality:
| |
| SART (1999), USA | Cohort study | 187 GC deliveries, 6379 fresh IVF deliveries, 3632 fresh ICSI deliveries, 1457 frozen IVF/ICSI deliveries, 1309 fresh donor deliveries, 214 frozen donor deliveries | Plurality:
| Plurality:
| |
| SART (2000), USA | Cohort study | 187 GC deliveries, 7353 fresh IVF deliveries, 4949 fresh ICSI deliveries, 1719 frozen IVF/ICSI, 1650 fresh donor deliveries, 325 frozen donor deliveries | Plurality:
| Plurality:
| |
| SART (2002a), USA | Cohort study | 235 GC deliveries, 14 789 fresh IVF deliveries, 7712 fresh ICSI deliveries, 1941 frozen IVF/ICSI, 1972 fresh donor deliveries, 410 frozen donor deliveries | Plurality: 38.2% | Plurality:
| |
| SART (2002b), USA | Cohort study | 245 GC deliveries, 16 175 fresh IVF deliveries, 8982 fresh ICSI deliveries, 1956 frozen IVF/ICSI, 2340 fresh donor deliveries, 536 frozen donor deliveries | Plurality: 36.7% | Plurality:
| |
| SART (2004), USA | Cohort study | 382 GC deliveries, 18 793 fresh IVF/ICSI deliveries, 2324 frozen IVF/ICSI deliveries, 2920 fresh donor deliveries, 563 frozen donor deliveries | Plurality: 37.4% | Plurality:
| |
| SART (2007), USA | Cohort study | 464 GC deliveries, 21 475 fresh IVF/ICSI deliveries, 3046 frozen IVF/ICSI deliveries, 3461 fresh donor deliveries, 770 frozen donor deliveries | Plurality: 40.9% | Plurality:
| |
| Utian et al. (1989), USA | Case series | 5 GC deliveries | Plurality: 40% (2/5) | ||
GA gestational age; OD, oocyte donation; PTB, preterm birth.
*IVF children from Brinsden and Rizk (1992).
| Author, year, country . | Study design . | Number of deliveries and children . | Result . | ||
|---|---|---|---|---|---|
| Intervention . | Control . | ||||
| Corson et al. (1998), USA | Case series | 27 GC deliveries 33 GC children | PTB:
| Plurality:
| |
| Dar et al. (2015), Canada | Case series | 133 GC deliveries 175 GC children | PTB (<37 weeks):
| Plurality:
| |
| Dermout et al. (2010), The Netherlands | Case series | 13 GC deliveries 16 GC children | PTB (<37 weeks):
| Plurality:
| |
| Duffy et al. (2005), USA | Case series | 8 GC deliveries 11 GC children | PTB (<37 weeks):
| Plurality:
| |
| Gibbons et al. (2011), USA | Cohort study | 1180 GC singletons 49 252 fresh IVF singletons 10 785 frozen IVF singletons 10 176 fresh OD singletons | GA, mean (SD), weeks:
| Not reported | GA, mean (SD), weeks:
|
| Goldfarb et al. (2000) | Case series | 18 GC deliveries | Plurality 38.9% (7/18) | ||
| Meniru and Craft (1997), UK | Case series | 4 GC deliveries | Plurality 75% (1/4) | ||
| Parkinson et al. (1998)*, USA | Cohort study | 95 GC deliveries 128 GC children Numbers on IVF children NA | GA, mean (SEM), weeks:
| Not reported | GA, mean (SEM), weeks:
|
| Reame and Parker (1990), USA | Case series | 38 surrogate (traditional) deliveries, 39 surrogate (traditional) children | PTB:
| Plurality:
| |
| SART (1993), USA | Cohort study | 35 GC deliveries, 3215 fresh IVF deliveries, 431 frozen IVF deliveries, 268 donor deliveries | Plurality:
| Plurality:
| |
| SART (1994), USA | Cohort study | 51 GC deliveries, 4206 fresh IVF deliveries, 619 frozen IVF deliveries, 534 donor deliveries | Plurality:
| Plurality:
| |
| SART (1995), USA | Cohort study | 78 GC deliveries, 5101 fresh IVF deliveries, 791 frozen IVF deliveries, 716 donor deliveries | Plurality:
| Plurality:
| |
| SART (1996), USA | Cohort study | 56 GC deliveries, 4912 fresh IVF deliveries, 1076 frozen IVF deliveries, 929 donor deliveries | Plurality:
| Plurality:
| |
| SART (1998), USA | Cohort study | 45 GC deliveries, 6754 fresh IVF deliveries, 1185 fresh ICSI deliveries, 1136 frozen IVF/ICSI deliveries, 1206 fresh donor deliveries, 146 frozen donor deliveries | Plurality:
| Plurality:
| |
| SART (1999), USA | Cohort study | 187 GC deliveries, 6379 fresh IVF deliveries, 3632 fresh ICSI deliveries, 1457 frozen IVF/ICSI deliveries, 1309 fresh donor deliveries, 214 frozen donor deliveries | Plurality:
| Plurality:
| |
| SART (2000), USA | Cohort study | 187 GC deliveries, 7353 fresh IVF deliveries, 4949 fresh ICSI deliveries, 1719 frozen IVF/ICSI, 1650 fresh donor deliveries, 325 frozen donor deliveries | Plurality:
| Plurality:
| |
| SART (2002a), USA | Cohort study | 235 GC deliveries, 14 789 fresh IVF deliveries, 7712 fresh ICSI deliveries, 1941 frozen IVF/ICSI, 1972 fresh donor deliveries, 410 frozen donor deliveries | Plurality: 38.2% | Plurality:
| |
| SART (2002b), USA | Cohort study | 245 GC deliveries, 16 175 fresh IVF deliveries, 8982 fresh ICSI deliveries, 1956 frozen IVF/ICSI, 2340 fresh donor deliveries, 536 frozen donor deliveries | Plurality: 36.7% | Plurality:
| |
| SART (2004), USA | Cohort study | 382 GC deliveries, 18 793 fresh IVF/ICSI deliveries, 2324 frozen IVF/ICSI deliveries, 2920 fresh donor deliveries, 563 frozen donor deliveries | Plurality: 37.4% | Plurality:
| |
| SART (2007), USA | Cohort study | 464 GC deliveries, 21 475 fresh IVF/ICSI deliveries, 3046 frozen IVF/ICSI deliveries, 3461 fresh donor deliveries, 770 frozen donor deliveries | Plurality: 40.9% | Plurality:
| |
| Utian et al. (1989), USA | Case series | 5 GC deliveries | Plurality: 40% (2/5) | ||
| Author, year, country . | Study design . | Number of deliveries and children . | Result . | ||
|---|---|---|---|---|---|
| Intervention . | Control . | ||||
| Corson et al. (1998), USA | Case series | 27 GC deliveries 33 GC children | PTB:
| Plurality:
| |
| Dar et al. (2015), Canada | Case series | 133 GC deliveries 175 GC children | PTB (<37 weeks):
| Plurality:
| |
| Dermout et al. (2010), The Netherlands | Case series | 13 GC deliveries 16 GC children | PTB (<37 weeks):
| Plurality:
| |
| Duffy et al. (2005), USA | Case series | 8 GC deliveries 11 GC children | PTB (<37 weeks):
| Plurality:
| |
| Gibbons et al. (2011), USA | Cohort study | 1180 GC singletons 49 252 fresh IVF singletons 10 785 frozen IVF singletons 10 176 fresh OD singletons | GA, mean (SD), weeks:
| Not reported | GA, mean (SD), weeks:
|
| Goldfarb et al. (2000) | Case series | 18 GC deliveries | Plurality 38.9% (7/18) | ||
| Meniru and Craft (1997), UK | Case series | 4 GC deliveries | Plurality 75% (1/4) | ||
| Parkinson et al. (1998)*, USA | Cohort study | 95 GC deliveries 128 GC children Numbers on IVF children NA | GA, mean (SEM), weeks:
| Not reported | GA, mean (SEM), weeks:
|
| Reame and Parker (1990), USA | Case series | 38 surrogate (traditional) deliveries, 39 surrogate (traditional) children | PTB:
| Plurality:
| |
| SART (1993), USA | Cohort study | 35 GC deliveries, 3215 fresh IVF deliveries, 431 frozen IVF deliveries, 268 donor deliveries | Plurality:
| Plurality:
| |
| SART (1994), USA | Cohort study | 51 GC deliveries, 4206 fresh IVF deliveries, 619 frozen IVF deliveries, 534 donor deliveries | Plurality:
| Plurality:
| |
| SART (1995), USA | Cohort study | 78 GC deliveries, 5101 fresh IVF deliveries, 791 frozen IVF deliveries, 716 donor deliveries | Plurality:
| Plurality:
| |
| SART (1996), USA | Cohort study | 56 GC deliveries, 4912 fresh IVF deliveries, 1076 frozen IVF deliveries, 929 donor deliveries | Plurality:
| Plurality:
| |
| SART (1998), USA | Cohort study | 45 GC deliveries, 6754 fresh IVF deliveries, 1185 fresh ICSI deliveries, 1136 frozen IVF/ICSI deliveries, 1206 fresh donor deliveries, 146 frozen donor deliveries | Plurality:
| Plurality:
| |
| SART (1999), USA | Cohort study | 187 GC deliveries, 6379 fresh IVF deliveries, 3632 fresh ICSI deliveries, 1457 frozen IVF/ICSI deliveries, 1309 fresh donor deliveries, 214 frozen donor deliveries | Plurality:
| Plurality:
| |
| SART (2000), USA | Cohort study | 187 GC deliveries, 7353 fresh IVF deliveries, 4949 fresh ICSI deliveries, 1719 frozen IVF/ICSI, 1650 fresh donor deliveries, 325 frozen donor deliveries | Plurality:
| Plurality:
| |
| SART (2002a), USA | Cohort study | 235 GC deliveries, 14 789 fresh IVF deliveries, 7712 fresh ICSI deliveries, 1941 frozen IVF/ICSI, 1972 fresh donor deliveries, 410 frozen donor deliveries | Plurality: 38.2% | Plurality:
| |
| SART (2002b), USA | Cohort study | 245 GC deliveries, 16 175 fresh IVF deliveries, 8982 fresh ICSI deliveries, 1956 frozen IVF/ICSI, 2340 fresh donor deliveries, 536 frozen donor deliveries | Plurality: 36.7% | Plurality:
| |
| SART (2004), USA | Cohort study | 382 GC deliveries, 18 793 fresh IVF/ICSI deliveries, 2324 frozen IVF/ICSI deliveries, 2920 fresh donor deliveries, 563 frozen donor deliveries | Plurality: 37.4% | Plurality:
| |
| SART (2007), USA | Cohort study | 464 GC deliveries, 21 475 fresh IVF/ICSI deliveries, 3046 frozen IVF/ICSI deliveries, 3461 fresh donor deliveries, 770 frozen donor deliveries | Plurality: 40.9% | Plurality:
| |
| Utian et al. (1989), USA | Case series | 5 GC deliveries | Plurality: 40% (2/5) | ||
GA gestational age; OD, oocyte donation; PTB, preterm birth.
*IVF children from Brinsden and Rizk (1992).
Conclusion: Similar rates of PTB (<37 weeks) were reported after surrogacy and in pregnancies which were the result of fresh IVF. Low quality of evidence (GRADE⊕○○○).
Birthweight
Birthweights were recorded in three cohort studies, including a total of 1775 children, and in five case series, including a total of 252 children (Table III). Studies came mainly from the USA, Canada and Brazil. Mean birthweight for surrogate singletons varied between 3309 and 3536 g, compared with 3100–3240 g for IVF singletons and 3226 g for OD singletons (OD comparison in only one study). In two small case series from Europe (Soderstrom-Anttila et al., 2002; Dermout et al., 2010) the mean birthweights of surrogate singletons were 3536 and 3498 g, respectively. Low birthweight (LBW; <2500 g) occurred in between 0 and 11.1% for surrogate singletons, in 13.6–14.0% for IVF singletons and in 14.0% for OD singletons.
| Author, year, country . | Study design . | Number of deliveries and children . | Result . | |
|---|---|---|---|---|
| Intervention . | Control . | |||
| Dar et al. (2015), Canada | Case series | 133 GC deliveries 175 GC children | LBW (<2500 g):
| |
| Dermout et al. (2010), The Netherlands | Case series | 13 GC deliveries 16 GC children | Mean birthweight in singletons: 3536 g
| |
| Duffy et al. (2005), USA | Case series | 8 GC deliveries 11 GC children | LBW (<2500 g):
| |
| Gibbons et al. (2011), USA | Cohort study | 1180 GC singletons 49 252 fresh IVF singletons 10 785 frozen IVF singletons 10 176 fresh OD singletons | Mean (SD) birthweight:
| Mean (SD) birthweight (SD):
|
| Parkinson et al. (1998), USA* | Cohort study | 95 GC deliveries 128 GC children numbers of IVF children NA | Mean (SEM) birthweight:
| Mean (SEM) birthweight in IVF:
|
| Reame and Parker (1990), USA | Case series | 38 surrogate (traditional) deliveries, 39 surrogate (traditional) children | Mean (SEM) birthweight:
| |
| Schieve et al. (2002), USA | Cohort study | 467 GC 33 121 fresh IVF 3779 frozen IVF 4458 fresh OD 679 frozen OD 3 389 098 SC | LBW (<2500 g):
| LBW in fresh IVF:
|
| Soderstrom-Anttila et al. (2002), Finland | Case series | 11 GC children | Mean birthweight:
| |
| Author, year, country . | Study design . | Number of deliveries and children . | Result . | |
|---|---|---|---|---|
| Intervention . | Control . | |||
| Dar et al. (2015), Canada | Case series | 133 GC deliveries 175 GC children | LBW (<2500 g):
| |
| Dermout et al. (2010), The Netherlands | Case series | 13 GC deliveries 16 GC children | Mean birthweight in singletons: 3536 g
| |
| Duffy et al. (2005), USA | Case series | 8 GC deliveries 11 GC children | LBW (<2500 g):
| |
| Gibbons et al. (2011), USA | Cohort study | 1180 GC singletons 49 252 fresh IVF singletons 10 785 frozen IVF singletons 10 176 fresh OD singletons | Mean (SD) birthweight:
| Mean (SD) birthweight (SD):
|
| Parkinson et al. (1998), USA* | Cohort study | 95 GC deliveries 128 GC children numbers of IVF children NA | Mean (SEM) birthweight:
| Mean (SEM) birthweight in IVF:
|
| Reame and Parker (1990), USA | Case series | 38 surrogate (traditional) deliveries, 39 surrogate (traditional) children | Mean (SEM) birthweight:
| |
| Schieve et al. (2002), USA | Cohort study | 467 GC 33 121 fresh IVF 3779 frozen IVF 4458 fresh OD 679 frozen OD 3 389 098 SC | LBW (<2500 g):
| LBW in fresh IVF:
|
| Soderstrom-Anttila et al. (2002), Finland | Case series | 11 GC children | Mean birthweight:
| |
LBW, low birthweight; SC, spontaneous conception; SGA, small for gestational age; VLBW, very low birthweight.
*IVF children from Brinsden and Rizk (1992).
| Author, year, country . | Study design . | Number of deliveries and children . | Result . | |
|---|---|---|---|---|
| Intervention . | Control . | |||
| Dar et al. (2015), Canada | Case series | 133 GC deliveries 175 GC children | LBW (<2500 g):
| |
| Dermout et al. (2010), The Netherlands | Case series | 13 GC deliveries 16 GC children | Mean birthweight in singletons: 3536 g
| |
| Duffy et al. (2005), USA | Case series | 8 GC deliveries 11 GC children | LBW (<2500 g):
| |
| Gibbons et al. (2011), USA | Cohort study | 1180 GC singletons 49 252 fresh IVF singletons 10 785 frozen IVF singletons 10 176 fresh OD singletons | Mean (SD) birthweight:
| Mean (SD) birthweight (SD):
|
| Parkinson et al. (1998), USA* | Cohort study | 95 GC deliveries 128 GC children numbers of IVF children NA | Mean (SEM) birthweight:
| Mean (SEM) birthweight in IVF:
|
| Reame and Parker (1990), USA | Case series | 38 surrogate (traditional) deliveries, 39 surrogate (traditional) children | Mean (SEM) birthweight:
| |
| Schieve et al. (2002), USA | Cohort study | 467 GC 33 121 fresh IVF 3779 frozen IVF 4458 fresh OD 679 frozen OD 3 389 098 SC | LBW (<2500 g):
| LBW in fresh IVF:
|
| Soderstrom-Anttila et al. (2002), Finland | Case series | 11 GC children | Mean birthweight:
| |
| Author, year, country . | Study design . | Number of deliveries and children . | Result . | |
|---|---|---|---|---|
| Intervention . | Control . | |||
| Dar et al. (2015), Canada | Case series | 133 GC deliveries 175 GC children | LBW (<2500 g):
| |
| Dermout et al. (2010), The Netherlands | Case series | 13 GC deliveries 16 GC children | Mean birthweight in singletons: 3536 g
| |
| Duffy et al. (2005), USA | Case series | 8 GC deliveries 11 GC children | LBW (<2500 g):
| |
| Gibbons et al. (2011), USA | Cohort study | 1180 GC singletons 49 252 fresh IVF singletons 10 785 frozen IVF singletons 10 176 fresh OD singletons | Mean (SD) birthweight:
| Mean (SD) birthweight (SD):
|
| Parkinson et al. (1998), USA* | Cohort study | 95 GC deliveries 128 GC children numbers of IVF children NA | Mean (SEM) birthweight:
| Mean (SEM) birthweight in IVF:
|
| Reame and Parker (1990), USA | Case series | 38 surrogate (traditional) deliveries, 39 surrogate (traditional) children | Mean (SEM) birthweight:
| |
| Schieve et al. (2002), USA | Cohort study | 467 GC 33 121 fresh IVF 3779 frozen IVF 4458 fresh OD 679 frozen OD 3 389 098 SC | LBW (<2500 g):
| LBW in fresh IVF:
|
| Soderstrom-Anttila et al. (2002), Finland | Case series | 11 GC children | Mean birthweight:
| |
LBW, low birthweight; SC, spontaneous conception; SGA, small for gestational age; VLBW, very low birthweight.
*IVF children from Brinsden and Rizk (1992).
Two cohort studies (exact numbers of twins not available) and five case series (n = 2–76 twins) reported on birthweight in surrogate twins. In surrogate twins, LBW occurred in 29.6–50%. In the largest cohort study, LBW occurred in 50 versus 56% in fresh IVF twins and 53.6% in fresh OD twins (Schieve et al., 2002). The other cohort study also reported on mean birthweight in surrogate twins, which was 2.7 (SD 0.06) kg as compared with 2.4 (SD 0.04) kg in IVF twins (Parkinson et al., 1998).
Conclusion: Numerically similar or lower rates of LBW were reported after surrogacy and in pregnancies resulting from fresh IVF. Low quality of evidence (GRADE⊕⊕○○).
Birth defects
Birth defects were reported in eight cohort studies, of which seven were annual reports from Society for Assisted Reproductive Technologies (SART), and in three case series (Table IV). In total, data from 1238 children born after surrogacy were identified. Birth defects were reported in 0 to 6.5% of the surrogacy children, as compared to 1.1 to 2.9% for IVF children and 0.6 to 2.1% for children born after OD.
| Author, year, country . | Study design . | Number of deliveries and children . | Result . | |
|---|---|---|---|---|
| Intervention . | Control . | |||
| Corson et al. (1998), USA | Case series | 27 GC deliveries 30 GC children +7 ongoing GC pregnancies | One chromosomal aberration (XO/XX) in an ongoing pregnancy | |
| Dar et al. (2015), Canada | Case series | 133 GC deliveries 175 GC children | 1.8% (3/175) birth defects (one renal, two cardiac) | |
| Dermout et al. (2010), The Netherlands | Case series | 13 GC deliveries 16 GC children | 6.3% (1/16) birth defects (spina bifida and hydrocephalus in a twin) | |
| Parkinson et al. (1998), USA** | Cohort study | 95 GC deliveries 128 GC children Number of IVF children NA | Singletons: 0 major, 4.9% minor Twins: 7.4% major, 0 minor Triplets: 0 minor and 0 major | IVF singletons: 2.9% major |
| SART (1993), USA | Cohort study (1991) | 50 GC children | 2.6% (1/39) with structural or functional defects, 11 not reported | Fresh IVF: 1.5% Frozen IVF: 0.8% OD: 2.1% |
| SART (1994), USA | Cohort study (1992) | 72 GC children | 6.5% (4/62) with structural or functional defects, 10 not reported | Fresh IVF: 1.9% Frozen IVF: 1.3% OD: 1.7% |
| SART (1995), USA | Cohort study (1993) | 104 GC children | 2.0% (2/102) with structural or functional defects, 2 not reported | Fresh IVF: 2.3% Frozen IVF: 1.8% OD: 1.8% |
| SART (1996), USA | Cohort study (1994) | 70 GC children | 2.9% (2/69) with structural or functional defects, 1 not reported | Fresh IVF: 2.7% Frozen IVF: 2.6% OD: 2.1% |
| SART (1998), USA | Cohort study (1995) | 65 GC children | 0% (0/65) with structural or functional defects | Fresh IVF/ICSI: 1.1% Frozen IVF/ICSI: 1.0% Fresh OD: 0.6% |
| SART (1999), USA | Cohort study (1996) | 258 GC children | 1.6% (4/258) with structural or functional defects | Fresh IVF/ICSI: 1.8% Frozen IVF/ICSI: 1.9% Fresh OD: 1.3% |
| SART (2000), USA | Cohort study (1997) | 270 GC children | 1.9% (5/270) with structural or functional defects | Fresh IVF/ICSI: 1.7% Frozen IVF/ICSI: 1.8% Fresh OD: 1.9% |
| Author, year, country . | Study design . | Number of deliveries and children . | Result . | |
|---|---|---|---|---|
| Intervention . | Control . | |||
| Corson et al. (1998), USA | Case series | 27 GC deliveries 30 GC children +7 ongoing GC pregnancies | One chromosomal aberration (XO/XX) in an ongoing pregnancy | |
| Dar et al. (2015), Canada | Case series | 133 GC deliveries 175 GC children | 1.8% (3/175) birth defects (one renal, two cardiac) | |
| Dermout et al. (2010), The Netherlands | Case series | 13 GC deliveries 16 GC children | 6.3% (1/16) birth defects (spina bifida and hydrocephalus in a twin) | |
| Parkinson et al. (1998), USA** | Cohort study | 95 GC deliveries 128 GC children Number of IVF children NA | Singletons: 0 major, 4.9% minor Twins: 7.4% major, 0 minor Triplets: 0 minor and 0 major | IVF singletons: 2.9% major |
| SART (1993), USA | Cohort study (1991) | 50 GC children | 2.6% (1/39) with structural or functional defects, 11 not reported | Fresh IVF: 1.5% Frozen IVF: 0.8% OD: 2.1% |
| SART (1994), USA | Cohort study (1992) | 72 GC children | 6.5% (4/62) with structural or functional defects, 10 not reported | Fresh IVF: 1.9% Frozen IVF: 1.3% OD: 1.7% |
| SART (1995), USA | Cohort study (1993) | 104 GC children | 2.0% (2/102) with structural or functional defects, 2 not reported | Fresh IVF: 2.3% Frozen IVF: 1.8% OD: 1.8% |
| SART (1996), USA | Cohort study (1994) | 70 GC children | 2.9% (2/69) with structural or functional defects, 1 not reported | Fresh IVF: 2.7% Frozen IVF: 2.6% OD: 2.1% |
| SART (1998), USA | Cohort study (1995) | 65 GC children | 0% (0/65) with structural or functional defects | Fresh IVF/ICSI: 1.1% Frozen IVF/ICSI: 1.0% Fresh OD: 0.6% |
| SART (1999), USA | Cohort study (1996) | 258 GC children | 1.6% (4/258) with structural or functional defects | Fresh IVF/ICSI: 1.8% Frozen IVF/ICSI: 1.9% Fresh OD: 1.3% |
| SART (2000), USA | Cohort study (1997) | 270 GC children | 1.9% (5/270) with structural or functional defects | Fresh IVF/ICSI: 1.7% Frozen IVF/ICSI: 1.8% Fresh OD: 1.9% |
SART, Society for assisted reproductive technology.
*Birth defects as defined by authors.
**IVF children from Brinsden and Rizk (1992).
| Author, year, country . | Study design . | Number of deliveries and children . | Result . | |
|---|---|---|---|---|
| Intervention . | Control . | |||
| Corson et al. (1998), USA | Case series | 27 GC deliveries 30 GC children +7 ongoing GC pregnancies | One chromosomal aberration (XO/XX) in an ongoing pregnancy | |
| Dar et al. (2015), Canada | Case series | 133 GC deliveries 175 GC children | 1.8% (3/175) birth defects (one renal, two cardiac) | |
| Dermout et al. (2010), The Netherlands | Case series | 13 GC deliveries 16 GC children | 6.3% (1/16) birth defects (spina bifida and hydrocephalus in a twin) | |
| Parkinson et al. (1998), USA** | Cohort study | 95 GC deliveries 128 GC children Number of IVF children NA | Singletons: 0 major, 4.9% minor Twins: 7.4% major, 0 minor Triplets: 0 minor and 0 major | IVF singletons: 2.9% major |
| SART (1993), USA | Cohort study (1991) | 50 GC children | 2.6% (1/39) with structural or functional defects, 11 not reported | Fresh IVF: 1.5% Frozen IVF: 0.8% OD: 2.1% |
| SART (1994), USA | Cohort study (1992) | 72 GC children | 6.5% (4/62) with structural or functional defects, 10 not reported | Fresh IVF: 1.9% Frozen IVF: 1.3% OD: 1.7% |
| SART (1995), USA | Cohort study (1993) | 104 GC children | 2.0% (2/102) with structural or functional defects, 2 not reported | Fresh IVF: 2.3% Frozen IVF: 1.8% OD: 1.8% |
| SART (1996), USA | Cohort study (1994) | 70 GC children | 2.9% (2/69) with structural or functional defects, 1 not reported | Fresh IVF: 2.7% Frozen IVF: 2.6% OD: 2.1% |
| SART (1998), USA | Cohort study (1995) | 65 GC children | 0% (0/65) with structural or functional defects | Fresh IVF/ICSI: 1.1% Frozen IVF/ICSI: 1.0% Fresh OD: 0.6% |
| SART (1999), USA | Cohort study (1996) | 258 GC children | 1.6% (4/258) with structural or functional defects | Fresh IVF/ICSI: 1.8% Frozen IVF/ICSI: 1.9% Fresh OD: 1.3% |
| SART (2000), USA | Cohort study (1997) | 270 GC children | 1.9% (5/270) with structural or functional defects | Fresh IVF/ICSI: 1.7% Frozen IVF/ICSI: 1.8% Fresh OD: 1.9% |
| Author, year, country . | Study design . | Number of deliveries and children . | Result . | |
|---|---|---|---|---|
| Intervention . | Control . | |||
| Corson et al. (1998), USA | Case series | 27 GC deliveries 30 GC children +7 ongoing GC pregnancies | One chromosomal aberration (XO/XX) in an ongoing pregnancy | |
| Dar et al. (2015), Canada | Case series | 133 GC deliveries 175 GC children | 1.8% (3/175) birth defects (one renal, two cardiac) | |
| Dermout et al. (2010), The Netherlands | Case series | 13 GC deliveries 16 GC children | 6.3% (1/16) birth defects (spina bifida and hydrocephalus in a twin) | |
| Parkinson et al. (1998), USA** | Cohort study | 95 GC deliveries 128 GC children Number of IVF children NA | Singletons: 0 major, 4.9% minor Twins: 7.4% major, 0 minor Triplets: 0 minor and 0 major | IVF singletons: 2.9% major |
| SART (1993), USA | Cohort study (1991) | 50 GC children | 2.6% (1/39) with structural or functional defects, 11 not reported | Fresh IVF: 1.5% Frozen IVF: 0.8% OD: 2.1% |
| SART (1994), USA | Cohort study (1992) | 72 GC children | 6.5% (4/62) with structural or functional defects, 10 not reported | Fresh IVF: 1.9% Frozen IVF: 1.3% OD: 1.7% |
| SART (1995), USA | Cohort study (1993) | 104 GC children | 2.0% (2/102) with structural or functional defects, 2 not reported | Fresh IVF: 2.3% Frozen IVF: 1.8% OD: 1.8% |
| SART (1996), USA | Cohort study (1994) | 70 GC children | 2.9% (2/69) with structural or functional defects, 1 not reported | Fresh IVF: 2.7% Frozen IVF: 2.6% OD: 2.1% |
| SART (1998), USA | Cohort study (1995) | 65 GC children | 0% (0/65) with structural or functional defects | Fresh IVF/ICSI: 1.1% Frozen IVF/ICSI: 1.0% Fresh OD: 0.6% |
| SART (1999), USA | Cohort study (1996) | 258 GC children | 1.6% (4/258) with structural or functional defects | Fresh IVF/ICSI: 1.8% Frozen IVF/ICSI: 1.9% Fresh OD: 1.3% |
| SART (2000), USA | Cohort study (1997) | 270 GC children | 1.9% (5/270) with structural or functional defects | Fresh IVF/ICSI: 1.7% Frozen IVF/ICSI: 1.8% Fresh OD: 1.9% |
SART, Society for assisted reproductive technology.
*Birth defects as defined by authors.
**IVF children from Brinsden and Rizk (1992).
Conclusion: Similar rates of birth defects in singletons were reported after surrogacy and after fresh IVF and oocyte donation. Low quality of evidence (GRADE⊕⊕○○).
Psychological follow-up
Eight studies, made up of six cohort studies and two case series, were identified as dealing with the psychological outcome for children born after surrogacy (Table V). Six of these papers were published by Golombok and co-workers (Golombok et al., 2004, 2006a, b, 2011, 2013; Jadva et al., 2012). The authors followed 42 children from 1 to 10 years of age. No major differences in psychological development were found between children born after surrogacy, children born after OD and children born after natural conception. However, at the age of 7 years children born after surrogacy showed higher levels of adjustment problems than children born after gamete donation. At the age of 10 years this difference had disappeared. In another study from the UK (Shelton et al., 2009) 21 children born after surrogacy were compared with children born after different kinds of assisted reproduction (IVF, OD, insemination and embryo donation) and followed up for between 4 and 10 years. No differences in psychological adjustment between the groups were detected. Jadva et al. (2012) investigated children's views of surrogacy at the ages of 7 and 10 years. A majority of the children had some knowledge of modes of conception. Fourteen out of 42 children had met their surrogate mothers in the past year and all were either positive or indifferent to surrogacy births. Lastly, a large case series of 110 surrogate children was reported from Brazil (Serafini, 2001). Outcomes included speech delay, as well as growth and motor development. A low rate of slow physical growth was also reported. Speech delay declined with age and was 3.8% at 2 years of age. No motor delays were reported.
| Author, year, country . | Study design . | Number of children . | Methods . | Results . | Comments . |
|---|---|---|---|---|---|
| Golombok et al. (2004), UK | Cohort study | 42 surrogacy children* 51 OD children 80 SC children | Follow-up at 1 year: Infant temperament: Infant Characteristics Questionnaire | No significant difference between groups | Around 69 invited families, response rate ∼61% (42/69) |
| Golombok et al. (2006a), UK | Cohort study | 37 surrogacy children* 48 OD children 68 SC children | Follow-up at 2 years: Children's psychological development: Brief Infant Toddler Social and Emotional Assessment (BITSEA) and Mental Scale of the Bailey Scales of Infant Development (BSID II) | No significant difference between groups for BITSEA, BSID II, Developmental delay (Mental Developmental Index): Surrogacy 6%, OD 9% and SC 10%, respectively (NS) | Same cohort as Golombok et al. (2004), Response rate 54% (37/69), representing 88% (37/42) from Golombok et al. (2004) |
| Golombok et al. (2006b), UK | Cohort study | 34 surrogacy children* 41 OD children 41 DI children 67 SC children | Follow-up at 3 years: Children's psychological adjustment Strengths and Difficulties Questionnaire (SDQ) | No significant difference between groups for SDQ | Same cohort as Golombok et al. (2004), Response rate 49% (34/69), representing 81% (34/42) from Golombok et al. (2004) |
| Golombok et al. (2011), UK | Cohort study | 32 surrogacy children* 32 OD children 54 SC children | Follow-up at 7 years: Children's psychological adjustment SDQ | No significant difference between groups for SDQ | Same cohort as Golombok et al. (2004), Response rate 46% (32/69), representing 76% (32/42) from Golombok et al. (2004) |
| Golombok et al. (2013), UK | Cohort study | 30 surrogacy children* 31 OD children 35 DI children 53 SC children | Follow-up at 7 and 10 years: Children's psychological adjustment SDQ | Surrogacy children showed higher levels of adjustment problems than children conceived by gamete donation (OD + DI) at age 7. No significant difference between groups for SDQ at age 10. | Same cohort as Golombok et al. (2004), Response rate 43% (30/69), representing 71% (30/42) from Golombok et al. (2004) |
| Jadva et al. (2012), UK | Case series | 42 families created by surrogacy*, 1 year after delivery | Surrogate children's view on surrogacy at ages 7 and 10 | Majority showed some knowledge about mode of conception. 14 had seen their surrogate mother in the past year. All were positive or neutral/indifferent regarding surrogacy birth. | Age 7: 21 traditional, 12 GC, 67% (22/33) answered, representing 52% (22/42) of 1 year cohort Age 10: 21 traditional, 12 GC, 63% (21/33) answered, representing 50% (21/42) of 1 year cohort |
| Serafini (2001), Brazil | Case series | 110 GC children (63 singletons, 47 multiples) | Follow-up at 1 and 2 years: Speech, motor development, physical growth | Slow physical growth: Singletons: 1.7% (in SC up to 10%) Speech delay: Singletons: 1 year 9.4%, 2 year 3.8%. Multiples: 1 year 21.3%, 2 year 10.5%. Motor delay: Singletons and multiples 0% | Birthweight and gestational age reported in Parkinson (1998) |
| Shelton et al. (2009), UK | Cohort study | 21 GC children 386 IVF children 182 DI children 153 OD children 27 embryo donation children | Follow-up at 4–10 years: Children's psychological adjustment SDQ (Conduct problems, peer problems, prosocial behaviour), DuPaul ADHD rating Scale, DSM (diagnostic and statistical manual of mental disorders) IV (Oppositional disorders, depression and anxiety), Child Behaviour Disorders (Somatic problems), Mood and Feeling Questionnaire (MFQ) (depressive symptoms) | No significant difference between GC children and other groups for any outcomes | Unclear response rate |
| Author, year, country . | Study design . | Number of children . | Methods . | Results . | Comments . |
|---|---|---|---|---|---|
| Golombok et al. (2004), UK | Cohort study | 42 surrogacy children* 51 OD children 80 SC children | Follow-up at 1 year: Infant temperament: Infant Characteristics Questionnaire | No significant difference between groups | Around 69 invited families, response rate ∼61% (42/69) |
| Golombok et al. (2006a), UK | Cohort study | 37 surrogacy children* 48 OD children 68 SC children | Follow-up at 2 years: Children's psychological development: Brief Infant Toddler Social and Emotional Assessment (BITSEA) and Mental Scale of the Bailey Scales of Infant Development (BSID II) | No significant difference between groups for BITSEA, BSID II, Developmental delay (Mental Developmental Index): Surrogacy 6%, OD 9% and SC 10%, respectively (NS) | Same cohort as Golombok et al. (2004), Response rate 54% (37/69), representing 88% (37/42) from Golombok et al. (2004) |
| Golombok et al. (2006b), UK | Cohort study | 34 surrogacy children* 41 OD children 41 DI children 67 SC children | Follow-up at 3 years: Children's psychological adjustment Strengths and Difficulties Questionnaire (SDQ) | No significant difference between groups for SDQ | Same cohort as Golombok et al. (2004), Response rate 49% (34/69), representing 81% (34/42) from Golombok et al. (2004) |
| Golombok et al. (2011), UK | Cohort study | 32 surrogacy children* 32 OD children 54 SC children | Follow-up at 7 years: Children's psychological adjustment SDQ | No significant difference between groups for SDQ | Same cohort as Golombok et al. (2004), Response rate 46% (32/69), representing 76% (32/42) from Golombok et al. (2004) |
| Golombok et al. (2013), UK | Cohort study | 30 surrogacy children* 31 OD children 35 DI children 53 SC children | Follow-up at 7 and 10 years: Children's psychological adjustment SDQ | Surrogacy children showed higher levels of adjustment problems than children conceived by gamete donation (OD + DI) at age 7. No significant difference between groups for SDQ at age 10. | Same cohort as Golombok et al. (2004), Response rate 43% (30/69), representing 71% (30/42) from Golombok et al. (2004) |
| Jadva et al. (2012), UK | Case series | 42 families created by surrogacy*, 1 year after delivery | Surrogate children's view on surrogacy at ages 7 and 10 | Majority showed some knowledge about mode of conception. 14 had seen their surrogate mother in the past year. All were positive or neutral/indifferent regarding surrogacy birth. | Age 7: 21 traditional, 12 GC, 67% (22/33) answered, representing 52% (22/42) of 1 year cohort Age 10: 21 traditional, 12 GC, 63% (21/33) answered, representing 50% (21/42) of 1 year cohort |
| Serafini (2001), Brazil | Case series | 110 GC children (63 singletons, 47 multiples) | Follow-up at 1 and 2 years: Speech, motor development, physical growth | Slow physical growth: Singletons: 1.7% (in SC up to 10%) Speech delay: Singletons: 1 year 9.4%, 2 year 3.8%. Multiples: 1 year 21.3%, 2 year 10.5%. Motor delay: Singletons and multiples 0% | Birthweight and gestational age reported in Parkinson (1998) |
| Shelton et al. (2009), UK | Cohort study | 21 GC children 386 IVF children 182 DI children 153 OD children 27 embryo donation children | Follow-up at 4–10 years: Children's psychological adjustment SDQ (Conduct problems, peer problems, prosocial behaviour), DuPaul ADHD rating Scale, DSM (diagnostic and statistical manual of mental disorders) IV (Oppositional disorders, depression and anxiety), Child Behaviour Disorders (Somatic problems), Mood and Feeling Questionnaire (MFQ) (depressive symptoms) | No significant difference between GC children and other groups for any outcomes | Unclear response rate |
ADHD, attention deficit hyperactivity disorder; DI, donor insemination.
*Mix of traditional and gestational carrier surrogacy.
| Author, year, country . | Study design . | Number of children . | Methods . | Results . | Comments . |
|---|---|---|---|---|---|
| Golombok et al. (2004), UK | Cohort study | 42 surrogacy children* 51 OD children 80 SC children | Follow-up at 1 year: Infant temperament: Infant Characteristics Questionnaire | No significant difference between groups | Around 69 invited families, response rate ∼61% (42/69) |
| Golombok et al. (2006a), UK | Cohort study | 37 surrogacy children* 48 OD children 68 SC children | Follow-up at 2 years: Children's psychological development: Brief Infant Toddler Social and Emotional Assessment (BITSEA) and Mental Scale of the Bailey Scales of Infant Development (BSID II) | No significant difference between groups for BITSEA, BSID II, Developmental delay (Mental Developmental Index): Surrogacy 6%, OD 9% and SC 10%, respectively (NS) | Same cohort as Golombok et al. (2004), Response rate 54% (37/69), representing 88% (37/42) from Golombok et al. (2004) |
| Golombok et al. (2006b), UK | Cohort study | 34 surrogacy children* 41 OD children 41 DI children 67 SC children | Follow-up at 3 years: Children's psychological adjustment Strengths and Difficulties Questionnaire (SDQ) | No significant difference between groups for SDQ | Same cohort as Golombok et al. (2004), Response rate 49% (34/69), representing 81% (34/42) from Golombok et al. (2004) |
| Golombok et al. (2011), UK | Cohort study | 32 surrogacy children* 32 OD children 54 SC children | Follow-up at 7 years: Children's psychological adjustment SDQ | No significant difference between groups for SDQ | Same cohort as Golombok et al. (2004), Response rate 46% (32/69), representing 76% (32/42) from Golombok et al. (2004) |
| Golombok et al. (2013), UK | Cohort study | 30 surrogacy children* 31 OD children 35 DI children 53 SC children | Follow-up at 7 and 10 years: Children's psychological adjustment SDQ | Surrogacy children showed higher levels of adjustment problems than children conceived by gamete donation (OD + DI) at age 7. No significant difference between groups for SDQ at age 10. | Same cohort as Golombok et al. (2004), Response rate 43% (30/69), representing 71% (30/42) from Golombok et al. (2004) |
| Jadva et al. (2012), UK | Case series | 42 families created by surrogacy*, 1 year after delivery | Surrogate children's view on surrogacy at ages 7 and 10 | Majority showed some knowledge about mode of conception. 14 had seen their surrogate mother in the past year. All were positive or neutral/indifferent regarding surrogacy birth. | Age 7: 21 traditional, 12 GC, 67% (22/33) answered, representing 52% (22/42) of 1 year cohort Age 10: 21 traditional, 12 GC, 63% (21/33) answered, representing 50% (21/42) of 1 year cohort |
| Serafini (2001), Brazil | Case series | 110 GC children (63 singletons, 47 multiples) | Follow-up at 1 and 2 years: Speech, motor development, physical growth | Slow physical growth: Singletons: 1.7% (in SC up to 10%) Speech delay: Singletons: 1 year 9.4%, 2 year 3.8%. Multiples: 1 year 21.3%, 2 year 10.5%. Motor delay: Singletons and multiples 0% | Birthweight and gestational age reported in Parkinson (1998) |
| Shelton et al. (2009), UK | Cohort study | 21 GC children 386 IVF children 182 DI children 153 OD children 27 embryo donation children | Follow-up at 4–10 years: Children's psychological adjustment SDQ (Conduct problems, peer problems, prosocial behaviour), DuPaul ADHD rating Scale, DSM (diagnostic and statistical manual of mental disorders) IV (Oppositional disorders, depression and anxiety), Child Behaviour Disorders (Somatic problems), Mood and Feeling Questionnaire (MFQ) (depressive symptoms) | No significant difference between GC children and other groups for any outcomes | Unclear response rate |
| Author, year, country . | Study design . | Number of children . | Methods . | Results . | Comments . |
|---|---|---|---|---|---|
| Golombok et al. (2004), UK | Cohort study | 42 surrogacy children* 51 OD children 80 SC children | Follow-up at 1 year: Infant temperament: Infant Characteristics Questionnaire | No significant difference between groups | Around 69 invited families, response rate ∼61% (42/69) |
| Golombok et al. (2006a), UK | Cohort study | 37 surrogacy children* 48 OD children 68 SC children | Follow-up at 2 years: Children's psychological development: Brief Infant Toddler Social and Emotional Assessment (BITSEA) and Mental Scale of the Bailey Scales of Infant Development (BSID II) | No significant difference between groups for BITSEA, BSID II, Developmental delay (Mental Developmental Index): Surrogacy 6%, OD 9% and SC 10%, respectively (NS) | Same cohort as Golombok et al. (2004), Response rate 54% (37/69), representing 88% (37/42) from Golombok et al. (2004) |
| Golombok et al. (2006b), UK | Cohort study | 34 surrogacy children* 41 OD children 41 DI children 67 SC children | Follow-up at 3 years: Children's psychological adjustment Strengths and Difficulties Questionnaire (SDQ) | No significant difference between groups for SDQ | Same cohort as Golombok et al. (2004), Response rate 49% (34/69), representing 81% (34/42) from Golombok et al. (2004) |
| Golombok et al. (2011), UK | Cohort study | 32 surrogacy children* 32 OD children 54 SC children | Follow-up at 7 years: Children's psychological adjustment SDQ | No significant difference between groups for SDQ | Same cohort as Golombok et al. (2004), Response rate 46% (32/69), representing 76% (32/42) from Golombok et al. (2004) |
| Golombok et al. (2013), UK | Cohort study | 30 surrogacy children* 31 OD children 35 DI children 53 SC children | Follow-up at 7 and 10 years: Children's psychological adjustment SDQ | Surrogacy children showed higher levels of adjustment problems than children conceived by gamete donation (OD + DI) at age 7. No significant difference between groups for SDQ at age 10. | Same cohort as Golombok et al. (2004), Response rate 43% (30/69), representing 71% (30/42) from Golombok et al. (2004) |
| Jadva et al. (2012), UK | Case series | 42 families created by surrogacy*, 1 year after delivery | Surrogate children's view on surrogacy at ages 7 and 10 | Majority showed some knowledge about mode of conception. 14 had seen their surrogate mother in the past year. All were positive or neutral/indifferent regarding surrogacy birth. | Age 7: 21 traditional, 12 GC, 67% (22/33) answered, representing 52% (22/42) of 1 year cohort Age 10: 21 traditional, 12 GC, 63% (21/33) answered, representing 50% (21/42) of 1 year cohort |
| Serafini (2001), Brazil | Case series | 110 GC children (63 singletons, 47 multiples) | Follow-up at 1 and 2 years: Speech, motor development, physical growth | Slow physical growth: Singletons: 1.7% (in SC up to 10%) Speech delay: Singletons: 1 year 9.4%, 2 year 3.8%. Multiples: 1 year 21.3%, 2 year 10.5%. Motor delay: Singletons and multiples 0% | Birthweight and gestational age reported in Parkinson (1998) |
| Shelton et al. (2009), UK | Cohort study | 21 GC children 386 IVF children 182 DI children 153 OD children 27 embryo donation children | Follow-up at 4–10 years: Children's psychological adjustment SDQ (Conduct problems, peer problems, prosocial behaviour), DuPaul ADHD rating Scale, DSM (diagnostic and statistical manual of mental disorders) IV (Oppositional disorders, depression and anxiety), Child Behaviour Disorders (Somatic problems), Mood and Feeling Questionnaire (MFQ) (depressive symptoms) | No significant difference between GC children and other groups for any outcomes | Unclear response rate |
ADHD, attention deficit hyperactivity disorder; DI, donor insemination.
*Mix of traditional and gestational carrier surrogacy.
Conclusion: Up to the age of 10 years there were no major psychological differences between children born after surrogacy and children born after other types of ART, or after natural conception. Low quality of evidence (GRADE⊕⊕○○).
Psychological outcome for surrogate mothers
Sixteen studies, eight cohort studies, six case series and two qualitative studies including between 8 and 61 surrogate mothers, examined psychological outcome (Supplementary Table SIV). No serious psychopathology among the surrogate mothers was noted. The motives for surrogacy were mostly altruistic but financial reasons were also noted. The rate of immediate post-partum depression was between 0 and 20% (Parkinson et al., 1998; Soderstrom-Anttila et al., 2002; Jadva et al., 2003; van den Akker, 2007, Imrie and Jadva, 2014). Six studies assessed relinquishing issues. In one study from the UK (Jadva et al., 2003) including 34 surrogate mothers, 35% initially had some/moderate difficulties handing over the child. One year on, 6% still reported some negative feelings related to relinquishment. The majority of the surrogates in this study were traditional surrogates. In two other studies relinquishing the child was a problem in 1/33 and 1/15, respectively (Blyth, 1994; Pashmi et al., 2010).
In studies which assessed contact between the surrogate mother and the intended mother/family, in the vast majority of cases contact was harmonious and regular, both during pregnancy and after birth (Jadva et al., 2003; Imrie and Jadva, 2014). The frequency of contacts decreased over time while the quality of the relationship seemed to continue to a similar degree, also after 10 years (Jadva et al., 2012, 2015). One study assessed the psychological well-being, family relationships and experiences of the surrogates’ own children, born prior to the surrogacy arrangements (Jadva and Imrie, 2014). The children whose mother had been a surrogate between 5 and 15 years earlier did not experience any negative consequences as a result of their mother's decision to be a surrogate, irrespective of whether the surrogate mother had used her own oocytes or not (Jadva and Imrie, 2014).
Conclusion: Most surrogate mothers are within the normal range on personality tests. Most psychosocial variables were satisfactory, although relinquishing problems sometimes occurred. Very low quality of evidence (GRADE⊕○○○).
Psychological outcome for intended parents
We identified 16 studies, 11 cohort studies, four case series and one qualitative study that reported on outcomes for the intended mothers and their families (Supplementary Table SV). Most studies were from the UK, seven from Golombok and co-workers (Golombok et al., 2004, 2006a, b, 2011, 2013; Blake et al., 2012; Jadva et al., 2012). No major differences in the parents’ psychological states or mother-child interactions were observed in groups made up of commissioning mothers, mothers who had received OD and mothers who had conceived naturally. The mothers’ and fathers’ marital quality was compared at five time points between different family types when the children were between 1 and 10 years of age (Blake et al., 2012). The mothers and fathers of children born through surrogacy had similar marital satisfaction as parents in gamete donation families. When the children were 2 years old the mothers in natural conception families had higher levels of marital satisfaction than their counterparts in ART families. This difference had disappeared when the children were 3, 7 and 10 years (Blake et al., 2012). In families with a 2-year-old child born through surrogacy fathers reported lower levels of parenting stress than their natural conception counterparts (Golombok et al., 2006a).
Four studies were reported by another UK group (van den Akker, 2000, 2005a, b, 2007). Genetic links were found to be more important to mothers whose children were the result of gestational surrogacy than those who had children after traditional surrogacy. These differences were observed both during pregnancy and after birth. In a study from the Netherlands (Dermout et al., 2010) more than 500 couples enquired about surrogacy by telephone or e-mail, but only 35 couples actually entered the IVF programme. After this extensive screening, no negative or harmful consequences were detected among the parties involved. This meant that there were no problems in accepting even a disabled child. Disclosure of surrogacy to the children was recorded in one study. In 29 out of 33 families, disclosure to the child had been made by the age of 7 years (Readings et al., 2011). In three other studies, 97–100% of parents aimed to tell the child about the surrogacy (Blyth, 1995; van den Akker, 2000; MacCallum et al., 2003).
Conclusion: There were no major differences in the psychological states of mothers of children who were the product of surrogacies, mothers of children conceived after other types of ART and mothers of children who were conceived naturally. Low quality of evidence (GRADE⊕⊕○○).
Discussion
This systematic review summarizes the published literature on surrogacy, including both medical and psychological outcomes for surrogate mothers, intended parents and the children born after surrogate arrangements. Although these arrangements, including gestational carrier programmes, have been carried out since the late 1980s, research on outcomes for the parties involved is very limited. Most studies have significant methodological limitations, such as small sample size. Also, for the studies on psychological follow-up in particular, a low response rate is noted, introducing a risk of selection bias. The research literature on surrogacy is sparse for many reasons. It is believed that financial support for such controversial research is difficult to secure and that it is difficult to track the number of children born, especially those born as a result of traditional surrogacy. Furthermore, given the social stigma associated with surrogacy arrangements, it is thought that intended parents are perhaps unwilling to sacrifice their privacy to participate in research in the field (Ciccarelli and Beckman, 2005).
One objection to allowing surrogacy is that the woman carrying the child will be exposed to unexpected health risks related to the pregnancy and delivery. Only five studies have looked into the risks of pregnancy complications; four of these involved gestational and one traditional surrogacy. The incidence of HDP was between 4.3 and 10% in singleton gestational carrier pregnancies compared to between 16 and 40% usually reported in OD pregnancies (Parkinson et al., 1998; Dar et al., 2015; van der Hoorn et al., 2010). The high frequency of HDP noted in OD pregnancies has been associated with the fact that the oocyte recipient is immunologically unrelated to the donor (van der Hoorn et al., 2010). In theory, the same situation occurs in gestational surrogacy, as in such cases the entire fetal genome is allogeneic to the carrier. Based on the few reports on GC outcome available, there has been speculation that a healthy carrier with a normal reproductive background might somehow compensate for atypical immunological reactions related to a foreign embryo, and that the surrogates might have a more hospitable uterine environment than infertile oocyte recipients (Gibbons et al., 2011). Although the number of cases studied is small, the lower HDP rate in surrogate mothers, most of whom have already experienced normal pregnancies and deliveries, supports the view of a connection between nulliparity and HDP (Parkinson et al., 1998).
The duration of singleton surrogacy pregnancies was similar to or shorter than that of singleton standard IVF pregnancies, and the incidence of preterm birth (<37 weeks) was, in general, numerically lower than in standard IVF singletons. The shorter mean gestational age seen in one study is interesting as the rate of obstetric complications in general was low and, according to selection criteria for surrogate mothers, they should previously have experienced uncomplicated pregnancies (Gibbons et al., 2011). The articles give no information on the rate of induction of labour or the rate of Caesarean section performed for non-medical reasons. The surrogate mother might also have had one of a number of risk factors, as reported in the early paper by Reame and Parker (1990) in which the profiles of 66% of the traditional surrogate mothers had risk factors, including smoking or having had no previous deliveries. These factors may have had implications for pregnancy length.
There were three reports of hysterectomies related to delivery, two of which occurred in multiple pregnancies, in a twin and a triplet pregnancy. The third hysterectomy occurred in a singleton pregnancy after uterine rupture in a gestational carrier with three previous, full-term, normal vaginal deliveries, indicating that there are always potential maternal risks, even if the carrier has no obstetric risk factors in her case history.
One of the cornerstones in surrogacy arrangements is the importance of choosing the surrogate mother with extreme caution. To minimize the medical risks to the surrogate mother recommendations drawn up by expert groups in ESHRE, ASRM and FIGO should be followed. Gestational carrier candidates, who have had previous adverse obstetric outcomes should not be accepted. The pregnancy history of the surrogate candidate might be more predictive of obstetric complications than her age (Duffy et al., 2005). Furthermore, the risk of almost all maternal complications is increased by multiple pregnancies (HDP, haemorrhage during pregnancy and delivery, preterm labour and delivery, operative delivery). To avoid unnecessary endangerment of the health of the surrogate and the future child it is strongly recommended that only one embryo at a time is transferred to the surrogate (Shenfield et al., 2005).
The most important concern related to surrogacy treatment is anxiety about possible harmful medical and psychological consequences for the child. For infants born after surrogacy, perinatal outcome has been satisfactory. The mean birthweight of gestational carrier singleton children was higher than average and the incidence of LBW was low (Table III). This positive outcome is probably because in surrogacy pregnancies the pregnant women have better reproductive health than infertile women with a history of reproductive illness. As in standard IVF and OD treatments, the rate of PTB and LBW in multiple births in gestational carriers was high, at between 30 and 100%. This underlines the importance of avoiding multiple pregnancies in surrogacy arrangements.
Two papers give the incidence of birth defects as 6% (SART 1994; Dermout et al., 2010). However, all SART reports from 1995 onwards, and including much higher numbers of children, showed a lower rate of birth defects, at between 0 and 2.9%, which was comparable to SART data for standard IVF and OD treatments. Another Dutch study by Dermout included only 13 deliveries out of which one twin baby had severe malformations (Dermout et al., 2010). Published studies show that the risk of birth defects in infants born after surrogate pregnancies is similar to risks after IVF and OD treatment.
Follow-ups of the psychological development of older children belonging to the surrogate group are very limited and almost all studies come from the UK. An initial group of 42 surrogacy children was followed up for 10 years from the age of 1 year. Of the children, 71% were still participating in the study at the age of 10 years. Through the years the children's psychological adjustment was normal and comparable to that of OD and naturally conceived children. The higher levels of adjustment problems noted in surrogacy children at 7 years of age proved to be temporary and had disappeared 3 years later. Good psychological adjustment in surrogacy children was also reported by Shelton and associates (Shelton et al., 2009). It is possible that families and children taking part in these studies are not representative of the outcome in general, but the studies from the UK following these families for up to 10 years are the largest and most representative samples so far collected.
The research literature on psychological implications for surrogate mothers and intended parents is sparse and the heterogeneity of the groups of surrogate mothers included in published studies makes it impossible to drawn any firm conclusions about this group. Some of the studies examine commercial and others altruistic non-paid surrogates. Other studies include both gestational and traditional arrangements where the surrogate might be unknown, or known, to the intended couple.
An interesting question relates to the characteristics which make women willing to act as surrogates. Do these women have some special traits which set them apart? Again, non-representative samples, a lack of control groups and ambiguous comparisons with test norms make it difficult to reach firm conclusions (Ciccarelli and Beckman, 2005). A cautious summary of published research indicates that the psychological profiles and characteristics of most surrogate mothers are in the normal range of personality tests (Pizitz et al., 2013). However, it has been suggested that some characteristics of surrogate mothers make them more flexible regarding moral and ethical principles to do with traditional family values and the meaning of motherhood (Kleinpeter and Hohman, 2000; Ciccarelli and Beckman, 2005).
The primary motivation reported by surrogate mothers is altruistic concern for infertile couples. Money was named as a prime motive by only a small number of the women. However, financial interests are probably also present in many cases where the main motivation is reported to be empathy for childless couples (Braverman and Corson, 1992; Blyth, 1994; Kleinpeter and Hohman, 2000; Hohman and Hagan, 2001; Baslington, 2002; Jadva et al., 2003; van den Akker, 2003; Pashmi et al., 2010).
Surrogate mothers generally report being satisfied with their experiences (Jadva et al., 2003). The frequency of post-partum depression seems to be low. Important factors that determine the surrogate mother's satisfaction after the birth of the baby have been related to the quality of the relationship with the intended couple, especially the intended mother, and circumstances to do with the relinquishment of the child (Blyth, 1994; Hohman and Hagan, 2001; MacCallum et al., 2003). One reason for regarding surrogacy as problematic and controversial is the risk of dispute between the surrogacy mother and the intended parents as to custody of the child. What if the surrogate mother decides to keep the child or the intended parents are not willing to welcome a disabled child? There have been reports highlighted in the media about situations when problems arise, such as the Baby M case and more recently the case of Baby Gammy (Peterson, 1987; BBC News Asia, 2014; Schover, 2014; Topping and Foster, 2014). Baby M was born in 1986 as a result of traditional surrogacy and after the birth the surrogate mother decided to keep the child. In court, the genetic father was awarded legal custody, with the surrogate mother having visiting rights.
Follow-up studies show that generally there were no significant difficulties for the surrogate mothers to hand over the children to the intended parents (Fischer and Gillman, 1991; Blyth, 1994; Baslington, 2002; Jadva et al., 2003; van den Akker, 2003; Pashmi et al., 2010). It has been suggested that surrogate mothers may not view the child they are carrying as theirs, thereby facilitating relinquishment (Jadva et al., 2003; van den Akker, 2003; Ahmari Tehran et al., 2014;,Lorenceau et al., 2015). However, in a minority of cases the woman experienced some difficulties in giving up the child. The reasons for these problems have not been reported, but it might have to do with insufficient screening of the surrogate mothers or, for example, lack of emotional support. Furthermore, it was not always clear whether the child was the result of traditional or gestational surrogacy. There is evidence to suggest that where the surrogate mother has a genetic link to the child the potential for disputes between the parties is increased (Trowse, 2011).
Recently, the case of Baby Gammy has made international news. The intended Australian parents of twins born to a Thai surrogate mother did not accept baby Gammy, who had Down syndrome. They took baby Gammy's healthy sister back to Australia leaving the critically ill baby Gammy with the surrogate mother. Fortunately, such cases are rare events. In Western countries, the risk that the intended parents involved in gestational surrogacy would not want to welcome their own child must be estimated as small (Brinsden, 2003). Those couples, who after screening and counselling are committed to going further with this time-consuming and complicated treatment, are in general highly motivated to become parents. However, pretreatment counselling is extremely important and requires high attention. It should include descriptions of all the risks and benefits of gestational surrogacy as well as information on the rights and responsibilities of all parties.
In general, at least in countries where surrogacy is legal, a large majority of the intended parents plan to inform their child about the method of conception (van den Akker, 2000; MacCallum et al., 2003; Readings et al., 2011). In this respect the surrogacy families are more open with their children than families created through other forms of ART, for example OD and donor insemination (Söderström-Anttila et al., 2010; Readings et al., 2011; Sälevaara et al., 2013). Genetically related surrogate mothers have been shown to be more likely than genetically unrelated surrogate mothers to wish the child to be told about the surrogacy arrangement (Jadva et al., 2003). Continuation of contact between the family and the surrogate mother will depend on whether the child has been informed or not. The type of surrogacy has been shown to be associated with the frequency of contact with the child's parents, with the parties involved in traditional surrogacy arrangements maintaining less frequent contact than those involved in gestational surrogacy (Jadva et al., 2012; Imrie and Jadva, 2014). Most families created by surrogacy have reported a harmonious relationship with the surrogate mother (Kleinpeter, 2002; Imrie and Jadva, 2014). Again, this refers to countries that have regulated surrogacy. In cross-country surrogacy, there are no studies on the relationship between the intended parents and the surrogate mother, who might even be unknown to the commissioning couple.
Surrogacy arrangements have been used by same-sex men to become fathers, as it allows one of the couple's spermatozoa to provide a genetic link to the child and an opportunity to raise the child from birth (Norton et al., 2013). However, there is very limited research on gay men and surrogacy. A few studies have addressed questions of counselling, decision-making and the significance of biogenetic paternity to gay male couples becoming parents through surrogacy (Greenfeld and Seli, 2011; Dempsey, 2013; Norton et al., 2013). Outcomes for the children and the families have not been reported.
It is obvious that surrogacy involves deep ethical considerations. Normally the person who wants the child takes the medical risks of the pregnancy. In surrogacy arrangements the carrier takes the risks. Relationships between the parties may change during the procedure and disagreements between the intended parents and the carrier may occur over issues related to the pregnancy and the relinquishment of the child. However, in Western countries, many of these risks can be minimized by careful counselling and psychological support. This should be offered to all parties, not only before and during the treatment, but also after the child has been born. Emotional and legal support can best be offered in the home country of the partners involved. The risks of conflicts between the intended parents and the surrogate mother will probably rise in line with increasing cross-border ‘reproductive tourism’ and the lack of regulations in countries with commercial surrogacy. Ethical issues specific to the engaging of surrogate mothers from low and middle income countries include questions about custody rights, limits of surrogacy care, remuneration, multiple embryo transfer, medical advocacy and the exploitation of poor women (Deonandan et al., 2012). In commercial cross-border surrogacy, it might be argued that children are transformed into something one can buy. If wealthy couples go to poor countries to find a surrogate mother, that woman might be put under pressure to live under certain conditions while she is being paid. The woman might also agree to enter into a surrogacy arrangement simply because of her poor financial situation. These are strong arguments against commercial surrogacy and these are situations which do not arise in altruistic surrogacy.
It seems clear there is a growing practice of surrogacy treatments worldwide and that many of these arrangements cross national borders. During the last years, prohibition of surrogacy treatment has led to critical discussion in several European countries. It is an ethical dilemma that couples have to turn to the commercial surrogacy industry abroad to receive help for their infertility problem. In Iceland the Government has, at time of writing, prepared a law proposing legalization of altruistic gestational surrogacy. Recently, the National Advisory Board on Social Welfare and Health Care Ethics in Finland (ETENE, www.etene.fi) and Swedish Medical Advisory Board in Ethics (SMER; www.smer.se) have suggested that surrogacy treatments should be allowed in restricted medical situations. Details of the current legal regime of surrogacy in European Union (EU) member states, as well as reports on solutions of legal implications of cross-border surrogacy, can be found in papers published by the EU and Hague Convention on Private International Law (Brunet et al., 2013; http://www.hcch.net/index).
Strength and limitations
The major strength of this review is the comprehensive appraisal of the literature regarding outcome for the children as well as outcomes for the surrogate and intended mothers, and including both medical and psychological variables. Limitations are the lack of high quality studies. Most studies have significant methodological limitations such as small sample size, lack of controls and a low response rate. Gestational and traditional surrogacy was not always separated in the studies. To date there are no studies on children growing up with gay fathers. There are no follow-up studies on families created via commercial cross-border surrogacy in countries such as India, Russia and Ukraine, where surrogacy seems to be increasing.
Conclusions
Most studies reporting on surrogacy have serious methodological limitations. According to these studies most surrogacy arrangements are successfully implemented, and most surrogate mothers are well motivated and have little difficulty separating from children born as a result of the arrangement. The perinatal outcome of the children is comparable to standard IVF and OD and there is no evidence of harm to the children born as a result of surrogacy. However, these conclusions should be interpreted with caution. We have not found any publications on outcome for families and surrogate mothers involved in commercial cross-country surrogacy in less well developed countries where surrogacy is a growing industry. Furthermore, there are no studies on children growing up with gay fathers. Long-term follow-up studies on surrogacy children and families will be needed in the future.
Supplementary data
Supplementary data are available at http://humupd.oxfordjournals.org/.
Authors' roles
All authors contributed substantially to conception and design of the study. C.B., U.-B.W. and V.S.-A. selected and appraised the articles. V.S.-A., C.B. and U.-B.W. wrote the article. All authors revised the article critically and approved the final version.
Funding
The Nordic Expert group's research work was unconditionally supported by MSD in Finland, Norway and Denmark and by an agreement concerning research and the education of doctors (ALFGBG-70 940).
Conflict of interest
The authors confirm that they have no conflict of interest in relation to this work.
Acknowledgements
The authors wish to thank librarian Therese Svanberg for searching of literature and Gwyneth Olofsson for correction of the English language.
References



