-
PDF
- Split View
-
Views
-
Cite
Cite
Stella Iacovides, Ingrid Avidon, Fiona C. Baker, What we know about primary dysmenorrhea today: a critical review, Human Reproduction Update, Volume 21, Issue 6, November/December 2015, Pages 762–778, https://doi.org/10.1093/humupd/dmv039
Close - Share Icon Share
Abstract
Primary dysmenorrhea, or painful menstruation in the absence of pelvic pathology, is a common, and often debilitating, gynecological condition that affects between 45 and 95% of menstruating women. Despite the high prevalence, dysmenorrhea is often poorly treated, and even disregarded, by health professionals, pain researchers, and the women themselves, who may accept it as a normal part of the menstrual cycle. This review reports on current knowledge, particularly with regards to the impact and consequences of recurrent menstrual pain on pain sensitivity, mood, quality of life and sleep in women with primary dysmenorrhea.
Comprehensive literature searches on primary dysmenorrhea were performed using the electronic databases PubMed, Google Scholar and the Cochrane Library. Full-text manuscripts published between the years 1944 and 2015 were reviewed for relevancy and reference lists were cross-checked for additional relevant studies. In combination with the word ‘dysmenorrhea’ one or more of the following search terms were used to obtain articles published in peer-reviewed journals only: pain, risk factors, etiology, experimental pain, clinical pain, adenomyosis, chronic pain, women, menstrual cycle, hyperalgesia, pain threshold, pain tolerance, pain sensitivity, pain reactivity, pain perception, central sensitization, quality of life, sleep, treatment, non-steroidal anti-inflammatory drugs.
Women with dysmenorrhea, compared with women without dysmenorrhea, have greater sensitivity to experimental pain both within and outside areas of referred menstrual pain. Importantly, the enhanced pain sensitivity is evident even in phases of the menstrual cycle when women are not experiencing menstrual pain, illustrating that long-term differences in pain perception extend outside of the painful menstruation phase. This enhanced pain sensitivity may increase susceptibility to other chronic pain conditions in later life; dysmenorrhea is a risk factor for fibromyalgia. Further, dysmenorrheic pain has an immediate negative impact on quality of life, for up to a few days every month. Women with primary dysmenorrhea have a significantly reduced quality of life, poorer mood and poorer sleep quality during menstruation compared with their pain-free follicular phase, and compared with the menstruation phase of pain-free control women. The prescribed first-line therapy for menstrual pain remains non-steroidal anti-inflammatory drugs, which are effective in relieving daytime and night-time pain.
Further study is needed to determine whether effectively blocking dysmenorrheic pain ameliorates risk for the development of chronic pain disorders and to explore whether it is possible to prevent the development—and not just treat—severe dysmenorrheic pain in adolescent girls. In conclusion, we demonstrate the extensive multi-factorial impact of dysmenorrhea and we encourage and direct researchers to necessary future studies.
Introduction
Dysmenorrhea, defined as painful menstrual cramps of uterine origin, is the most common gynecological condition among women of reproductive age (Coco, 1999). Despite its common occurrence, however, it is under-diagnosed and under-treated (Campbell and McGrath, 1997; Coco, 1999; Proctor and Farquhar, 2006). This review describes our current knowledge about the pathophysiology of primary dysmenorrhea, particularly the role of prostaglandins (PG) in the uterus. It also highlights pain sensitivity in women with dysmenorrhea and makes the case for it being classified as a central sensitization syndrome. In the second part of the review, we describe the impact of primary dysmenorrhea on quality of life (QoL), including mood and sleep (see Fig. 1), and discuss current treatment strategies. Despite primary dysmenorrhea being so common, there are still major gaps in our knowledge of this disorder. We highlight some of these gaps and suggest future directions for research in the conclusion.
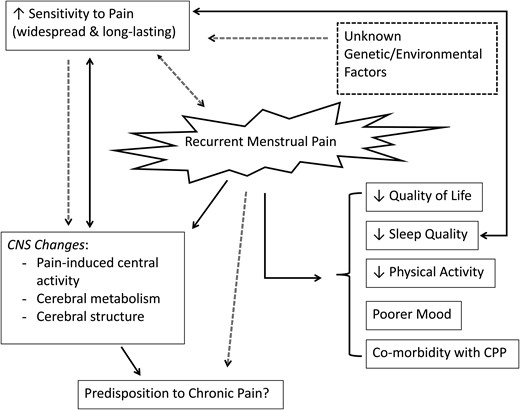
A schematic representation of the proposed (dotted lines) and known (solid lines) effects of recurrent dysmenorrheic pain, as well as the inter-relationships of these effects. Women with dysmenorrhea have reduced sleep quality, quality of life, physical activity, and poorer mood when in pain, as well as increased co-morbidity with chronic pelvic pain (CPP) conditions. Throughout the menstrual cycle, women with dysmenorrhea have an increased sensitivity to painful stimuli, however, it is unknown whether the increased sensitivity to pain is the cause or effect of recurrent menstrual pain. Unknown genetic or environmental factors and underlying differences in the pain processing of the central nervous system (CNS) may also play a role in predisposing these women to greater pain sensitivity and consequently recurrent menstrual pain.
Methods
Using the electronic databases, PubMed, Google Scholar and the Cochrane Library, comprehensive literature searches were conducted on epidemiological studies, as well as clinical and experimental pain studies in women with primary dysmenorrhea. For this narrative review, in combination with the word ‘dysmenorrhea’, one or more of the following search terms were used to obtain articles published in peer-reviewed journals only: pain, risk factors, etiology, experimental pain, clinical pain, chronic pain, adenomyosis, women, menstrual cycle, hyperalgesia, pain threshold, pain tolerance, pain sensitivity, pain reactivity, pain perception, central sensitization, QoL, sleep, treatment, non-steroidal anti-inflammatory drugs. Full-text manuscripts published between the years 1944 and 2015 were reviewed for relevancy and importantly, reference lists were cross-checked for additional relevant studies.
Definition, prevalence and etiology of primary dysmenorrhea
Definition of dysmenorrhea
Based on pathophysiology, as illustrated in Fig. 2, dysmenorrhea can be subclassified as either primary or secondary dysmenorrhea (Proctor and Farquhar, 2006). Primary dysmenorrhea is defined as painful, spasmodic cramping in the lower abdomen, just before and/or during menstruation, in the absence of any discernable macroscopic pelvic pathology (Dawood, 1987). The onset of primary dysmenorrhea usually occurs in adolescence, at or shortly after (6–24 months) menarche (Hofmeyr, 1996; Dawood, 2006). The onset of primary dysmenorrheic pain usually has a clear and predictable temporal pattern, beginning just before or at the start of menstruation (Dawood, 1987; Harel, 2008). The pain typically lasts for 8–72 h, is most severe during the first or second day of menstruation, and may radiate to the back and thighs (Hofmeyr, 1996; Proctor et al., 2002; Ruoff and Lema, 2003). In addition, systemic symptoms such as nausea, vomiting, diarrhea, fatigue and insomnia frequently accompany the pain (Hofmeyr, 1996; Ruoff and Lema, 2003).
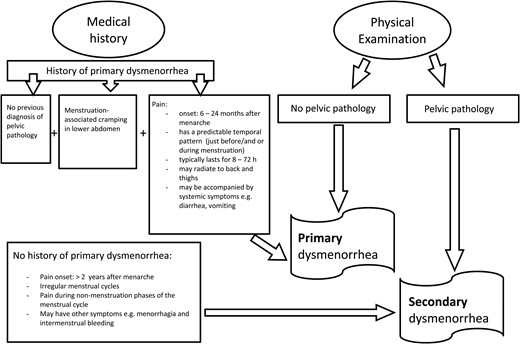
A diagnostic flow diagram for the differential diagnosis of primary and secondary dysmenorrhea.
Secondary dysmenorrheic pain, in contrast, may originate from a number of identifiable pathological conditions, including endometriosis, adenomyosis, fibroids (myomas) and pelvic inflammatory disease. The onset of secondary dysmenorrhea can occur any time, usually >2 years, after menarche, and depending on the underlying condition, may be accompanied by other gynecological symptoms such as intermenstrual bleeding and menorrhagia. In addition, the timing and intensity of secondary dysmenorrheic pain during the menstrual cycle may be constant or diffuse, and is not necessarily associated with menses (Hofmeyr, 1996; Proctor and Farquhar, 2006). The most common cause of secondary dysmenorrhea is endometriosis, described as the presence of endometrial tissue on extra-uterine locations, with an overall prevalence of 62% in adolescents (Janssen et al., 2013). Adenomyosis is another cause of secondary dysmenorrhea, defined as the benign invasion of endometrial tissue into the myometrium (Bird et al., 1972), and historically was only identifiable from histological examination of the uterus following a hysterectomy. With such a selection bias, adenomyosis was commonly thought to be a condition confined to adulthood, with limited clinical cases in adolescents (Ryan et al., 2006). Recent advancements in imaging have led to improvements in the non-invasive identification of adenomyosis, and demonstrate that the prevalence is likely underestimated and that it may not be confined to older women (Kunz et al., 2007; Benagiano et al., 2015). However, further research is needed in adolescent girls, particularly those whose pain is refractory to treatment with non-steroidal anti-inflammatories and/or oral contraceptives, to determine whether some cases of primary dysmenorrhea may actually be early stages of adenomyosis (Dietrich, 2010). While women with secondary dysmenorrhea share some of the same characteristics and pathways to pain as women with primary dysmenorrhea, for example increased uterine PG (Koike et al., 1992), the focus of this review is on primary dysmenorrhea. The reader is referred to excellent recent reviews on adenomyosis (Benagiano et al., 2012, 2015) and endometriosis (Burney and Giudice, 2012; Janssen et al., 2013).
Prevalence and risk factors for primary dysmenorrhea
The prevalence of primary dysmenorrhea is highly underestimated, yet difficult to determine, because few affected women seek medical treatment, despite the substantial distress experienced, as many consider the pain to be a normal part of the menstrual cycle rather than a disorder (Wong, 2010). Many cases thus remain undocumented (Gould, 1998; Jones, 2004; Chen et al., 2006; Daley, 2008). Due to the different definitions of the condition, and the lack of standard methods for assessing severity of dysmenorrhea, prevalence estimates vary between 45 and 95% of menstruating women (Jamieson and Steege, 1996; Proctor and Farquhar, 2006; Unsal et al., 2010), with very severe primary dysmenorrhea estimated to affect ∼10–25% of women of reproductive age (Andersch and Milsom, 1982; Dawood, 1987; Sundell et al., 1990; Hofmeyr, 1996). As such, dysmenorrhea appears to be the most common gynecological disorder in women irrespective of nationality and age (Harlow and Park, 1996; Proctor and Farquhar, 2002; Patel et al., 2006).
Risk factors for dysmenorrhea, but not specifically primary dysmenorrhea, include smoking (Messing et al., 1993; Parazzini et al., 1994), earlier age at menarche (Sundell et al., 1990; Harlow and Park, 1996), longer and heavier menstrual flow (Sundell et al., 1990; Harlow and Park, 1996), higher BMI (Harlow and Park, 1996), alcohol consumption (Parazzini et al., 1994; Harlow and Park, 1996), family history of dysmenorrhea (Ju et al., 2014), age (Pullon et al., 1988; Sundell et al., 1990; Messing et al., 1993) and nulliparity (Sundell et al., 1990; Juang et al., 2006). Dysmenorrheic pain severity may decrease after childbirth and with increasing age (Sundell et al., 1990; Juang et al., 2006), although not always (Pullon et al., 1988; Messing et al., 1993; Weissman et al., 2004). Despite the identification of a range of risk factors for developing dysmenorrhea, researchers have not always been able to agree (Ju et al., 2014), and given the conflicting results, more studies are required to further our understanding of environmental and genetic risk factors for primary dysmenorrhea.
Etiology of primary dysmenorrhea
The most widely accepted explanation for the pathogenesis of primary dysmenorrhea is the overproduction of uterine PGs (Dawood, 1987). Enhanced release of PGs, allegedly from disintegrating cells during endometrial sloughing, is believed to cause myometrial hypercontractility, resulting in ischemia and hypoxia of the uterine muscle, and, ultimately, pain (Fig. 3) (Dawood, 1987). PGs are ubiquitously distributed intracellular substances which are derived from long-chain polyunsaturated fatty acids, such as arachidonic acid, a common component of cell membrane phospholipids (Hayaishi and Matsumura, 1995). PGs have been shown to have a range of biological effects on a wide variety of physiological as well as pathological activities including pain, inflammation, body temperature, and sleep regulation (Hayaishi and Matsumura, 1995).
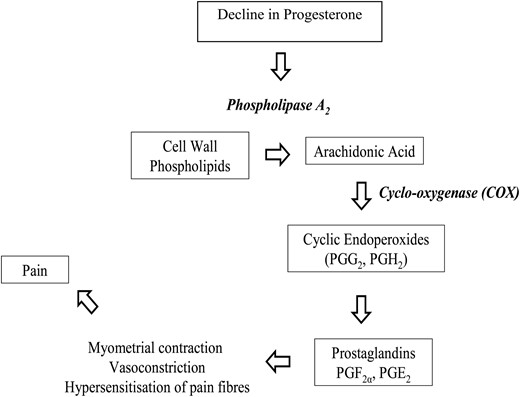
The arachidonic acid cascade displaying the cyclo-oxygenase (COX) pathway, the biosynthesis of cyclic endoperoxides (PGG2 and PGH2) and finally the synthesis of prostaglandins (PGF2α and PGE2). Prostaglandins F2α and E2 mediate myometrial contractions, vasoconstriction, hypersensitization of pain nerve fibers and, ultimately, pain. Enzymes are shown in bold italics.
PG synthesis is limited by the availability of the free fatty acid precursors for arachidonic acid, which is regulated by cyclic adenosine phosphate. Via cyclic adenosine phosphate, PG production can be stimulated by substances such as adrenalin, peptide hormones and steroid hormones, but also by mechanical stimuli and tissue trauma (Vander et al., 1998; Funk, 2001). Arachidonic acid is derived from phospholipids by the lysosomal enzyme phospholipase A2. The stability of lysosomal activity is regulated by several factors, one of which is progesterone levels; high progesterone levels tend to stabilize the activity of lysosomes, while falling levels tend to labilize lysosome activity (Dawood, 1995; Hofmeyr, 1996). Therefore, the decrease in progesterone that accompanies the regression of the corpus luteum in the late luteal phase of the menstrual cycle results in the removal of this stabilizing effect on endometrial lysosomes, the release of phospholipase A2, menstrual flow and hydrolysis of phospholipids from the cell membrane to generate additional arachidonic acid (see Fig. 3). Consequently, the enduring availability of arachidonic acid together with the intracellular destruction and tissue trauma during menstruation, favor the production of PGs (Dawood, 1995).
All women have increased levels of PGs during the luteal phase compared with the follicular phase of ovulatory cycles. However, compared with eumenorrheic women, dysmenorrheic women have higher levels of PGs, as measured in luteal phase endometrial biopsies, endometrial jet washings and menstrual fluids (Chanand Hill, 1978; Dawood, 2006). Higher circulating levels of PGs (PGF2α and PGE2) have been reported in women with dysmenorrhea compared with asymptomatic women during menstruation, and these PG levels are highest during the first 48 h of menses, when symptoms peak (Lundstrom and Green, 1978; Dawood, 1987; Hofmeyr, 1996; Coco, 1999). Further, the severity of menstrual pain and associated symptoms of dysmenorrhea are directly proportional to the amount of PGs released (Chan et al., 1981; Dawood, 2006). In addition, clinical administration of exogenous PGs results in uterine contraction and often also produces the same systemic symptoms that frequently accompany dysmenorrhea, including nausea, vomiting and diarrhea (Dawood, 1995; Coco, 1999). Taken together, these findings, in addition to the many clinical trials that demonstrate the effective relief of dysmenorrheic pain through PG suppression (further discussed under ‘Treatment of primary dysmenorrhea’, below), support the hypothesis that PGs are responsible for the painful uterine contractions and the associated systemic symptoms that accompany dysmenorrheic pain.
On the basis that exposure of the endometrium to luteal phase progesterone is crucial for the increased production of uterine PGs, dysmenorrhea is believed to occur only in ovulatory menstrual cycles (Dawood, 1987); this notion has, however, more recently been challenged in a study in which basal body temperature was used to distinguish between ovulatory and spontaneous anovulatory menstrual cycles. There was no difference in the severity of menstrual symptoms, including pain, between ovulatory and anovulatory menstrual cycles in women with dysmenorrhea (Espin Lopez et al., 2010). These data are preliminary and need to be replicated before making the conclusion that exposure of the uterus to progesterone and its subsequent withdrawal are not critical for the development of dysmenorrhea.
There are nine classes of PGs: PGA through PGI; within which individual PG are denoted by numerical subscripts. The two types of PGs that are implicated in the pathogenesis of primary dysmenorrheic pain are PGF2α and PGE2; however, PGF2α appears to be of particular importance (Ruoff and Lema, 2003). While PGE2 may result in either myometrial contraction or relaxation, PGF2α always causes potent vasoconstriction of uterine blood vessels, and myometrial contractions (Hofmeyr, 1996; Ruoff and Lema, 2003; Harel, 2004). There also is evidence that PGF2α lowers the threshold for pain perception by sensitizing nerve endings to pain (Hofmeyr, 1996; Ruoff and Lema, 2003; Harel, 2004).
Coupled with their elevated PG levels, dysmenorrheic women have higher levels of uterine activity during menstruation compared with asymptomatic women; basal or resting uterine tone (>10 mmHg), active intrauterine pressure (>120 mmHg), frequency of uterine contractions, and uncoordinated uterine contractions all are greater in dysmenorrheic women (Akerlund, 1979; Dawood, 1995, 2006; Hofmeyr, 1996). Furthermore, studies investigating uterine blood flow using Doppler ultrasonography have shown that the strong and abnormal uterine contractions in women with dysmenorrhea during menstruation are associated with reduced uterine blood flow and resultant myometrial ischemia, and hence pain (Altunyurt et al., 2005). Thus, during menstruation, excessive release of PGs by the endometrium results in hypercontractility of the uterus, and subsequent uterine muscle ischemia and hypoxia (Dawood, 1995; Hofmeyr, 1996). The contraction of the ischemic uterus therefore is the likely cause of dysmenorrheic pain.
In addition to PGs, vasopressin has been implicated in the etiology of primary dysmenorrhea, although the involvement of vasopressin remains controversial (Dawood, 2006). Limited studies have shown elevated circulating serum arginine vasopressin levels in women with primary dysmenorrhea during menstruation (Ekstrom et al., 1992; Akerlund, 2004). Higher arginine vasopressin levels result in dysrhythmical uterine contractions, which would ultimately contribute to the pain by causing further uterine hypoxia and ischemia (Akerlund, 1979). In contrast, other studies have not found higher plasma vasopressin levels in women with primary dysmenorrhea compared with controls (Baker et al., 1999; Valentin et al., 2000). Also, a vasopressin antagonist had no effect on menstrual pain, intrauterine pressure and uterine blood flow (Valentin et al., 2000).
Dysmenorrhea: not merely associated with menstruation?
While menstrual pain is clearly tied to the menstruation phase in women with primary dysmenorrhea, there is some evidence of physiological differences between women with and without dysmenorrhea during pain-free phases of the menstrual cycle. A recent study investigating cytokine gene expression profiles showed that, throughout the menstrual cycle, women with primary dysmenorrhea, compared with controls, exhibited a shift in the balance between expression patterns of pro-inflammatory cytokines and transforming growth factor-beta family member genes related to anti-inflammatory responses, with up-regulation of genes coding for pro-inflammatory cytokines and down-regulation of genes related to anti-inflammatory responses (Ma et al., 2013). This study suggests that underlying inflammatory responses differ in women with dysmenorrhea even in the absence of pain, which may play an etiological role in dysmenorrhea. In addition, some studies have found that dysmenorrheic women present with elevated levels of prolactin in the luteal phase (Litschgi and Glatthaar, 1978; Ylikorkala et al., 1979; Baker et al., 1999) compared with other phases of the menstrual cycle. Also, there are reports of higher nocturnal body temperatures and increased morning estrogen concentrations in women with primary dysmenorrhea compared with asymptomatic women, outside of the menstruation phase (Baker et al., 1999). Therefore, even in the absence of pain, women with dysmenorrhea may have distorted hormonal and cytokine profiles compared with women without menstrual-associated disorders. How these altered profiles contribute to the pathophysiology of primary dysmenorrhea is unknown. Below we will review another physiological abnormality that occurs outside of the menstruation phase: altered processing and perception of pain.
Pain sensitivity in women with dysmenorrhea
The first study alluding to a possible enhanced pain sensitivity across the menstrual cycle in women with dysmenorrhea compared with women without dysmenorrhea was conducted in 1944 (Haman, 1944). In this study, dysmenorrheic women had lower pain thresholds to a pressure stimulus applied to the thumb, compared with non-dysmenorrheic women, in all menstrual cycle phases (Haman, 1944). Since then, several studies have investigated pain sensitivity in dysmenorrhea, with mixed findings (Table I) partly due to differences in methodology.
Summary of human studies, in chronological order, investigating experimental pain responses in dysmenorrheic, compared with non-dysmenorrheic women across the menstrual cycle.
| Authors, year . | Number of women in each group . | Study results and outcome variable used . | Menstrual phases studied . | Body location . |
|---|---|---|---|---|
| Goolkasian (1983) | 12 dysmenorrheic, 12 non-dysmenorrheic. | Dysmenorrheic women had ↑ sensitivity to radiant heat stimulation (dolorimeter) across the menstrual cycle (no phase effect on pain discriminability)* |
| Right forearm |
| Aberger et al. (1983) | 19 spasmodic dysmenorrheic, 20 congestive dysmenorrheic, 17 combined dysmenorrheic 18 non-dysmenorrheic. | No difference in sensitivity to ischemia (threshold and tolerance) across groups• |
| Non-dominant arm and hand |
| Hapidou and De Catanzaro (1988) | 27 non-dysmenorrheic, 19 non-dysmenorrheic. | Dysmenorrheic women had ↓ sensitivity to cold pressor pain (VAS) compared with the non-dysmenorrheic women during the follicular phase.• |
| Arm |
| Amodei and Nelson-Grey (1989) | 12 spasmodic dysmenorrheic, 10 congestive dysmenorrheic, 13 combined dysmenorrheic, 12 non-dysmenorrheic. | No difference in sensitivity to muscle ischemia and pressure (Forgione Barber pressure device) pain (threshold and tolerance) between the different groups of women across the different phases of the menstrual cycle. |
| Arm (ischemia) and middle phalanx of index finger (pressure). |
| Giamberardino et al. (1997) | 10 dysmenorrheic, 10 non-dysmenorrheic. | Women with dysmenorrhea had ↓ sensitivity to subcutis and muscle electrical stimulation (thresholds) during the luteal phase. |
| Arm, leg and abdomen |
| Granot et al. (2001) | 22 dysmenorrheic, 31 non-dysmenorrheic. | Women with dysmenorrhea had ↑ sensitivity to heat (thermode) pain (supra-threshold magnitude estimations) and pain-evoked potentials by laser stimuli across the menstrual cycle.# |
| Thenar eminence of the non-dominant hand (heat) and dorsal superficial radial nerve of hand (laser) |
| Bajaj et al. (2002) | 20 dysmenorrheic, 15 non-dysmenorrheic. | Dysmenorrheic, compared with the non-dysmenorrheic, women had ↑ sensitivity to pressure and heat pain (thresholds) both within and outside areas of referred menstrual pain, during the menstrual phase.† |
| Arm, thigh, Abdomen, lower back |
| Brinkert et al. (2007) | 11 dysmenorrheic, 10 non-dysmenorrheic. | Dysmenorrheic women had ↑ intestinal sensitivity (decreased colonic distention volume thresholds). | Not accounted for. | Distal sigmoid colon |
| Vincent et al. (2011) | 12 dysmenorrheic, 12 non-dysmenorrheic. | Dysmenorrheic women had ↑ sensitivity to thermal pain, across the menstrual cycle at both sites (within and outside areas of referred menstrual pain).†,# |
| Lower abdomen and inner arm |
| Iacovides et al. (2013) | 12 dysmenorrheic, 9 non-dysmenorrheic. | Dysmenorrheic women had ↑ sensitivity (VAS) to muscle pain (hypertonic saline injections) across the menstrual cycle at both sites (within and outside areas of referred menstrual pain).†,# |
| Lower back and forearm |
| Iacovides et al. (2015) | 11 dysmenorrheic, 9 non-dysmenorrheic | Dysmenorrheic women had ↑ sensitivity to muscle ischemia (VAS) at both phases of the menstrual cycle.†,# |
| Arm |
| Arendt-Nielsen et al. (2014) | 10 dysmenorrheic, 10 non-dysmenorrheic | Dysmenorrheic women had ↑ sensitivity to cervical phasic and tonic distentions (electronic VAS) |
| Uterine cervix |
| Authors, year . | Number of women in each group . | Study results and outcome variable used . | Menstrual phases studied . | Body location . |
|---|---|---|---|---|
| Goolkasian (1983) | 12 dysmenorrheic, 12 non-dysmenorrheic. | Dysmenorrheic women had ↑ sensitivity to radiant heat stimulation (dolorimeter) across the menstrual cycle (no phase effect on pain discriminability)* |
| Right forearm |
| Aberger et al. (1983) | 19 spasmodic dysmenorrheic, 20 congestive dysmenorrheic, 17 combined dysmenorrheic 18 non-dysmenorrheic. | No difference in sensitivity to ischemia (threshold and tolerance) across groups• |
| Non-dominant arm and hand |
| Hapidou and De Catanzaro (1988) | 27 non-dysmenorrheic, 19 non-dysmenorrheic. | Dysmenorrheic women had ↓ sensitivity to cold pressor pain (VAS) compared with the non-dysmenorrheic women during the follicular phase.• |
| Arm |
| Amodei and Nelson-Grey (1989) | 12 spasmodic dysmenorrheic, 10 congestive dysmenorrheic, 13 combined dysmenorrheic, 12 non-dysmenorrheic. | No difference in sensitivity to muscle ischemia and pressure (Forgione Barber pressure device) pain (threshold and tolerance) between the different groups of women across the different phases of the menstrual cycle. |
| Arm (ischemia) and middle phalanx of index finger (pressure). |
| Giamberardino et al. (1997) | 10 dysmenorrheic, 10 non-dysmenorrheic. | Women with dysmenorrhea had ↓ sensitivity to subcutis and muscle electrical stimulation (thresholds) during the luteal phase. |
| Arm, leg and abdomen |
| Granot et al. (2001) | 22 dysmenorrheic, 31 non-dysmenorrheic. | Women with dysmenorrhea had ↑ sensitivity to heat (thermode) pain (supra-threshold magnitude estimations) and pain-evoked potentials by laser stimuli across the menstrual cycle.# |
| Thenar eminence of the non-dominant hand (heat) and dorsal superficial radial nerve of hand (laser) |
| Bajaj et al. (2002) | 20 dysmenorrheic, 15 non-dysmenorrheic. | Dysmenorrheic, compared with the non-dysmenorrheic, women had ↑ sensitivity to pressure and heat pain (thresholds) both within and outside areas of referred menstrual pain, during the menstrual phase.† |
| Arm, thigh, Abdomen, lower back |
| Brinkert et al. (2007) | 11 dysmenorrheic, 10 non-dysmenorrheic. | Dysmenorrheic women had ↑ intestinal sensitivity (decreased colonic distention volume thresholds). | Not accounted for. | Distal sigmoid colon |
| Vincent et al. (2011) | 12 dysmenorrheic, 12 non-dysmenorrheic. | Dysmenorrheic women had ↑ sensitivity to thermal pain, across the menstrual cycle at both sites (within and outside areas of referred menstrual pain).†,# |
| Lower abdomen and inner arm |
| Iacovides et al. (2013) | 12 dysmenorrheic, 9 non-dysmenorrheic. | Dysmenorrheic women had ↑ sensitivity (VAS) to muscle pain (hypertonic saline injections) across the menstrual cycle at both sites (within and outside areas of referred menstrual pain).†,# |
| Lower back and forearm |
| Iacovides et al. (2015) | 11 dysmenorrheic, 9 non-dysmenorrheic | Dysmenorrheic women had ↑ sensitivity to muscle ischemia (VAS) at both phases of the menstrual cycle.†,# |
| Arm |
| Arendt-Nielsen et al. (2014) | 10 dysmenorrheic, 10 non-dysmenorrheic | Dysmenorrheic women had ↑ sensitivity to cervical phasic and tonic distentions (electronic VAS) |
| Uterine cervix |
VAS, visual analogue scale.
•Between-subjects study design (no crossover).
Ovulation confirmed with body temperature.
†Ovulation confirmed by LH surge in urine.
#Menstrual phase confirmed with hormone assays.
Summary of human studies, in chronological order, investigating experimental pain responses in dysmenorrheic, compared with non-dysmenorrheic women across the menstrual cycle.
| Authors, year . | Number of women in each group . | Study results and outcome variable used . | Menstrual phases studied . | Body location . |
|---|---|---|---|---|
| Goolkasian (1983) | 12 dysmenorrheic, 12 non-dysmenorrheic. | Dysmenorrheic women had ↑ sensitivity to radiant heat stimulation (dolorimeter) across the menstrual cycle (no phase effect on pain discriminability)* |
| Right forearm |
| Aberger et al. (1983) | 19 spasmodic dysmenorrheic, 20 congestive dysmenorrheic, 17 combined dysmenorrheic 18 non-dysmenorrheic. | No difference in sensitivity to ischemia (threshold and tolerance) across groups• |
| Non-dominant arm and hand |
| Hapidou and De Catanzaro (1988) | 27 non-dysmenorrheic, 19 non-dysmenorrheic. | Dysmenorrheic women had ↓ sensitivity to cold pressor pain (VAS) compared with the non-dysmenorrheic women during the follicular phase.• |
| Arm |
| Amodei and Nelson-Grey (1989) | 12 spasmodic dysmenorrheic, 10 congestive dysmenorrheic, 13 combined dysmenorrheic, 12 non-dysmenorrheic. | No difference in sensitivity to muscle ischemia and pressure (Forgione Barber pressure device) pain (threshold and tolerance) between the different groups of women across the different phases of the menstrual cycle. |
| Arm (ischemia) and middle phalanx of index finger (pressure). |
| Giamberardino et al. (1997) | 10 dysmenorrheic, 10 non-dysmenorrheic. | Women with dysmenorrhea had ↓ sensitivity to subcutis and muscle electrical stimulation (thresholds) during the luteal phase. |
| Arm, leg and abdomen |
| Granot et al. (2001) | 22 dysmenorrheic, 31 non-dysmenorrheic. | Women with dysmenorrhea had ↑ sensitivity to heat (thermode) pain (supra-threshold magnitude estimations) and pain-evoked potentials by laser stimuli across the menstrual cycle.# |
| Thenar eminence of the non-dominant hand (heat) and dorsal superficial radial nerve of hand (laser) |
| Bajaj et al. (2002) | 20 dysmenorrheic, 15 non-dysmenorrheic. | Dysmenorrheic, compared with the non-dysmenorrheic, women had ↑ sensitivity to pressure and heat pain (thresholds) both within and outside areas of referred menstrual pain, during the menstrual phase.† |
| Arm, thigh, Abdomen, lower back |
| Brinkert et al. (2007) | 11 dysmenorrheic, 10 non-dysmenorrheic. | Dysmenorrheic women had ↑ intestinal sensitivity (decreased colonic distention volume thresholds). | Not accounted for. | Distal sigmoid colon |
| Vincent et al. (2011) | 12 dysmenorrheic, 12 non-dysmenorrheic. | Dysmenorrheic women had ↑ sensitivity to thermal pain, across the menstrual cycle at both sites (within and outside areas of referred menstrual pain).†,# |
| Lower abdomen and inner arm |
| Iacovides et al. (2013) | 12 dysmenorrheic, 9 non-dysmenorrheic. | Dysmenorrheic women had ↑ sensitivity (VAS) to muscle pain (hypertonic saline injections) across the menstrual cycle at both sites (within and outside areas of referred menstrual pain).†,# |
| Lower back and forearm |
| Iacovides et al. (2015) | 11 dysmenorrheic, 9 non-dysmenorrheic | Dysmenorrheic women had ↑ sensitivity to muscle ischemia (VAS) at both phases of the menstrual cycle.†,# |
| Arm |
| Arendt-Nielsen et al. (2014) | 10 dysmenorrheic, 10 non-dysmenorrheic | Dysmenorrheic women had ↑ sensitivity to cervical phasic and tonic distentions (electronic VAS) |
| Uterine cervix |
| Authors, year . | Number of women in each group . | Study results and outcome variable used . | Menstrual phases studied . | Body location . |
|---|---|---|---|---|
| Goolkasian (1983) | 12 dysmenorrheic, 12 non-dysmenorrheic. | Dysmenorrheic women had ↑ sensitivity to radiant heat stimulation (dolorimeter) across the menstrual cycle (no phase effect on pain discriminability)* |
| Right forearm |
| Aberger et al. (1983) | 19 spasmodic dysmenorrheic, 20 congestive dysmenorrheic, 17 combined dysmenorrheic 18 non-dysmenorrheic. | No difference in sensitivity to ischemia (threshold and tolerance) across groups• |
| Non-dominant arm and hand |
| Hapidou and De Catanzaro (1988) | 27 non-dysmenorrheic, 19 non-dysmenorrheic. | Dysmenorrheic women had ↓ sensitivity to cold pressor pain (VAS) compared with the non-dysmenorrheic women during the follicular phase.• |
| Arm |
| Amodei and Nelson-Grey (1989) | 12 spasmodic dysmenorrheic, 10 congestive dysmenorrheic, 13 combined dysmenorrheic, 12 non-dysmenorrheic. | No difference in sensitivity to muscle ischemia and pressure (Forgione Barber pressure device) pain (threshold and tolerance) between the different groups of women across the different phases of the menstrual cycle. |
| Arm (ischemia) and middle phalanx of index finger (pressure). |
| Giamberardino et al. (1997) | 10 dysmenorrheic, 10 non-dysmenorrheic. | Women with dysmenorrhea had ↓ sensitivity to subcutis and muscle electrical stimulation (thresholds) during the luteal phase. |
| Arm, leg and abdomen |
| Granot et al. (2001) | 22 dysmenorrheic, 31 non-dysmenorrheic. | Women with dysmenorrhea had ↑ sensitivity to heat (thermode) pain (supra-threshold magnitude estimations) and pain-evoked potentials by laser stimuli across the menstrual cycle.# |
| Thenar eminence of the non-dominant hand (heat) and dorsal superficial radial nerve of hand (laser) |
| Bajaj et al. (2002) | 20 dysmenorrheic, 15 non-dysmenorrheic. | Dysmenorrheic, compared with the non-dysmenorrheic, women had ↑ sensitivity to pressure and heat pain (thresholds) both within and outside areas of referred menstrual pain, during the menstrual phase.† |
| Arm, thigh, Abdomen, lower back |
| Brinkert et al. (2007) | 11 dysmenorrheic, 10 non-dysmenorrheic. | Dysmenorrheic women had ↑ intestinal sensitivity (decreased colonic distention volume thresholds). | Not accounted for. | Distal sigmoid colon |
| Vincent et al. (2011) | 12 dysmenorrheic, 12 non-dysmenorrheic. | Dysmenorrheic women had ↑ sensitivity to thermal pain, across the menstrual cycle at both sites (within and outside areas of referred menstrual pain).†,# |
| Lower abdomen and inner arm |
| Iacovides et al. (2013) | 12 dysmenorrheic, 9 non-dysmenorrheic. | Dysmenorrheic women had ↑ sensitivity (VAS) to muscle pain (hypertonic saline injections) across the menstrual cycle at both sites (within and outside areas of referred menstrual pain).†,# |
| Lower back and forearm |
| Iacovides et al. (2015) | 11 dysmenorrheic, 9 non-dysmenorrheic | Dysmenorrheic women had ↑ sensitivity to muscle ischemia (VAS) at both phases of the menstrual cycle.†,# |
| Arm |
| Arendt-Nielsen et al. (2014) | 10 dysmenorrheic, 10 non-dysmenorrheic | Dysmenorrheic women had ↑ sensitivity to cervical phasic and tonic distentions (electronic VAS) |
| Uterine cervix |
VAS, visual analogue scale.
•Between-subjects study design (no crossover).
Ovulation confirmed with body temperature.
†Ovulation confirmed by LH surge in urine.
#Menstrual phase confirmed with hormone assays.
Without taking menstrual cycle phase into consideration, studies have reported mixed findings with some showing no differences in the perception of experimental pain, including ischemic pain, heat pain, and electrical stimulation of the skin, between dysmenorrheic and non-dysmenorrheic women (Aberger et al., 1983; Amodei and Nelson-Gray, 1989; Brinkert et al., 2007), and others reporting that women with dysmenorrhea have an enhanced perception of laser pain-evoked potentials (Granot et al., 2001), heat pain (Goolkasian, 1983; Bajaj et al., 2002; Vincent et al., 2011), pressure pain (Bajaj et al., 2002), ischemic pain (Iacovides et al., 2015) and electrical pain stimuli (Giamberardino et al., 1997) applied to the abdomen, lower back and extremities, compared with non-dysmenorrheic women. It is, however, essential to consider menstrual cycle phase when investigating pain sensitivity in women with primary dysmenorrhea since the unique hormone environment of each menstrual phase may impact pain perception (Fillingim and Ness, 2000; Craft et al., 2004; Aloisi and Bonifazi, 2006). Also, menstrual cycle phase is of particular importance when investigating pain sensitivity in women with dysmenorrhea because the perception of pain may be altered in the presence of background dysmenorrheic pain (during menstruation) compared with pain-free phases of the menstrual cycle.
However, even when considering menstrual cycle phase, studies investigating pain sensitivity in women with dysmenorrhea have produced inconsistent results. Women with dysmenorrhea have been reported to have reduced cold pain thresholds during the luteal phase compared with the follicular phase (Hapidouand De Catanzaro, 1988), reduced heat and pressure pain thresholds during the menstrual phase compared with all other phases of the menstrual cycle (Bajaj et al., 2002), and heightened electrical pain thresholds during the late luteal phase (Giamberardino et al., 1997). Other studies, however, report that thermal (Granot et al., 2001; Vincent et al., 2011), ischemic (Amodei and Nelson-Gray, 1989), pressure (Amodei and Nelson-Gray, 1989), and deep-muscle (Iacovides, et al., 2013) pain perception do not vary according to menstrual cycle phase in women with dysmenorrhea.
The inconsistent findings in the current literature regarding pain sensitivity in women across the menstrual cycle have been attributed to various experimental methodological concerns including: differences in the choice of experimental pain stimuli; where, and at what tissue-depth, these stimuli are applied (Sherman and LeResche, 2006; Vincent and Tracey, 2010); different outcome measures (thresholds versus tolerance) used; the arbitrary division of the menstrual cycle into functionally distinct phases, based either on the ovarian or endometrial cycle (Sherman and LeResche, 2006; Vincent and Tracey, 2010; Pogatzki-Zahn, 2013); and lack of consideration of menstrual cycle phase or measurement of gonadal hormones (Hapidouand De Catanzaro, 1988; Amodei and Nelson-Gray, 1989; Giamberardino et al., 1997; Bajaj et al., 2002).
The range of stimulations to induce experimental pain in women with dysmenorrhea include: pressure (Amodei and Nelson-Gray, 1989; Bajaj et al., 2002), heat (Granot et al., 2001; Bajaj et al., 2002; Vincent et al., 2011), cold pressor stimulation (Hapidou and De Catanzaro, 1988), electrical stimulation (Giamberardino et al., 1997), ischemia (Aberger et al., 1983; Amodei and Nelson-Gray, 1989), pinch (Bajaj et al., 2002), tactile stimulation (Bajaj et al., 2002), pain-evoked potentials by laser stimuli (Granot et al., 2001), and deep-muscle pain by means of an i.m. injection of hypertonic saline (Iacovides et al., 2013) and ischemia (Iacovides et al., 2015). The choice of experimental pain stimuli used in studies is fundamental for several reasons: when assessing pain, the pain stimulus needs to be (i) reproducible, (ii) strong enough to elicit a measurable response, (iii) moderate enough to highlight individual differences and (iv) either meaningful enough to resemble a natural physiological or clinical pain, or precise enough to elucidate the basic mechanism of a response to pain (Sherman and LeResche, 2006). It is likely that each painful stimulus results in differential processing of nociceptive afferents (Lynn and Perl, 1977). Electrical stimuli, for example, activate all classes of afferent neurons (i.e. both nociceptive and non-nociceptive fibers), and hence produce both painful and non-painful sensations (Gracely, 1990; Keefe et al., 1991), while i.m. injection of hypertonic saline is believed to excite wide dynamic range neurons (Ro and Capra, 1999), possibly via activation of group III (thinly myelinated A-delta fibers) and group IV (unmyelinated C-fibers) muscle nociceptors to produce both a local area of transient pain and referred pain (Paintal, 1960; Iggo, 1961; Kumazawa and Mizumura, 1977; Graven-Nielsen et al., 1997, 2002). Theories on tourniquet-induced ischemic pain support the role of C-fibers in the transmission of pain, while A-fiber conduction is believed to be abolished during an ischemic event (Chabel et al., 1990; Loram et al., 2007). Thermal stimuli, in contrast, activate both A-delta and C-fibers (Keefe et al., 1991).
Type, location, and depth of experimental pain stimulation are important factors that can influence study results. The variety of sites that have been used to induce experimental pain in women with dysmenorrhea include: the thumb (Haman, 1944), index finger (Amodei and Nelson-Gray, 1989), forearm (Goolkasian, 1983; Iacovides et al., 2015), upper arm (Giamberardino et al., 1997; Bajaj et al., 2002), leg (Giamberardino et al., 1997; Bajaj et al., 2002), abdomen (Giamberardino et al., 1997; Bajaj et al., 2002) and lower back (Bajaj et al., 2002; Iacovides et al., 2013). Several aspects of pain assessment have been shown to vary according to body location; particularly with respect to the proximity to the reproductive organs (Robinson and Short, 1977; Klonoff et al., 1993; Giamberardino et al., 1997). Given that menstrual pain is referred to the abdomen in 70–90% of women (Montero et al., 1999), and to the lower back in 40% of women (Tissot and Messing, 1995), it is appropriate to use those sites as the referred site of experimental pain. Only recently have studies compared pain sensitivity both within and outside areas of referred menstrual pain (Giamberardino et al., 1997; Bajaj et al., 2002; Vincent et al., 2011; Iacovides et al., 2013). These studies show that women with dysmenorrhea have lower pain thresholds to experimental pain stimulations at both the abdomen or lower back (within the area of referred menstrual pain) and limb sites (outside the area of referred menstrual pain) (Giamberardino et al., 1997; Bajaj et al., 2002; Vincent et al., 2011; Iacovides et al., 2013). Two of these studies (Vincent et al., 2011; Iacovides et al., 2013) found that the greater pain sensitivity in women with dysmenorrhea was evident throughout the menstrual cycle: during the menstruation, follicular, and luteal phases of the menstrual cycle, as confirmed with hormonal assays.
The tissue depth at which pain is applied is also likely to play a role in the inconsistent findings since hyperalgesia has been reported to differ in the skin compared with subcutaneous tissue and compared with deep muscle tissue (Vecchiet et al., 1990; Giamberardino et al., 1993). There is also some evidence supporting that nociceptive activity arising from deep tissues, such as muscle, is under greater inhibitory influence than activity from cutaneous sites (Mense, 1990). Indeed, several studies show that women with dysmenorrhea have hyperalgesia to pain in deep tissues, such as muscle and subcutaneous tissue (Giamberardino et al., 1997; Iacovides et al., 2013), but not to pain at skin level (Giamberardino et al., 1997; Brinkert et al., 2007). Such findings are in agreement with others who report that structures that become hyperalgesic under recurrent visceral pain conditions are primarily muscles, with lesser influence on subcutaneous tissues and even less on the skin (Vecchiet et al., 1990; Giamberardino et al., 1993).
Taken together, the majority of recent studies that have considered tissue depth, areas within and outside of the referred area of pain and controlled for menstrual cycle phase have shown that across the menstrual cycle, dysmenorrheic women are hypersensitive to experimental pain compared with controls. Two features of this hyperalgesia in women with dysmenorrhea are of particular importance. First, a heightened sensitivity to experimental pain is present when the women are experiencing menstrual pain as well as during non-painful phases of the menstrual cycle and second, hyperalgesia is present in muscles within and outside the area of referred menstrual pain. Collectively, these findings are significant because they show that differences in pain sensitivity in dysmenorrheic women are widespread and long lasting, supporting the hypothesis that women with dysmenorrhea are sensitized to pain, particularly deep-muscle pain.
Primary dysmenorrhea: the case for central sensitization
Repeated monthly painful episodes may lead to the development of central sensitivity to pain (Yunus, 2007, 2008). Central sensitization is defined as an abnormal augmentation of pain by mechanisms within the central nervous system (CNS), and therefore represents a state where the response to normal peripheral inputs is greatly enhanced (Woolf, 2004, 2007). This heightened excitability of nociceptive projection neurons not only increases their sensitivity to inputs from afferents from damaged or inflamed sites, but also to other convergent inputs (Sessle, 2007). Primary dysmenorrhea has been classified as a member of the central sensitivity syndromes together with several other clinical conditions including fibromyalgia and tension-type headaches (Yunus, 2007, 2008). These syndromes are characterized by pain hypersensitivity in the absence of tissue injury, inflammation, or a lesion to the nervous system (Woolf, 2007; Yunus, 2007).
Evidence is growing that women with dysmenorrhea have a variation in the mode of systemic pain processing; where the peripheral nociceptive message generated by the reproductive organs during menstruation is amplified, thus causing an increased excitability of somatovisceral convergent neurons in the spinal cord, and ultimately, increased pain perception (Granot et al., 2001; Bajaj et al., 2002). Possible consequences of prolonged massive afferent visceral barrage and, hence, increased neuronal input into the CNS are functional and structural alterations throughout the CNS, including central sensitization to pain (Giamberardino, 1999; Granot et al., 2001; Bajaj et al., 2002).
Studies have demonstrated significant differences between the brains of otherwise healthy women who experience moderate-to-severe dysmenorrheic pain and those of non-dysmenorrheic women; including differences in central activity induced by noxious skin stimulation (Vincent et al., 2011), cerebral metabolism (Tu et al., 2009), and cerebral structure (Tu et al., 2010).
Functional magnetic resonance imaging (fMRI) of the brain has demonstrated that activity in the entorhinal cortex, a region implicated in enhanced pain perception mediated by anxiety and anticipation (Ploghaus et al., 2001; Fairhurst et al., 2007), may explain the increased response to thermal pain in women with dysmenorrhea, even in the absence of menstrual pain (i.e. during non-menstrual phases) (Vincent et al., 2011). Further, during menstruation, control, but not dysmenorrheic women, displayed deactivation of brain regions in response to experimental noxious thermal stimulation (Vincent et al., 2011).
Primary dysmenorrhea also is associated with abnormal metabolic changes in several areas of the brain involved in pain processing (Tu et al., 2009). When experiencing menstrual pain, compared with a pain-free phase of the menstrual cycle, women with dysmenorrhea showed increased regional glucose metabolism in thalamic, orbitofrontal and prefrontal areas, and decreased regional metabolism in lateral somatic sensorimotor areas (Tu et al., 2009). These presentations of hyper- and hypo-metabolic cerebral regions were found to be unique to menstrual pain; they were not evident in controls, and they differ from those observed in acute visceral pain conditions and other kinds of persistent pain (Derbyshire, 2003; Apkarian et al., 2005; Kupers and Kehlet, 2006; Kulkarni et al., 2007). The authors suggest that disinhibition of thalamo-orbitofrontal-prefrontal networks may contribute to the generation of pain and increased pain sensitivity in women with primary dysmenorrhea, possibly by maintaining spinal and thalamic sensitization (associated with chronic visceral pain) and by increasing negative emotion/affect (Tu et al., 2009). Interestingly, changes were found during menstruation compared with other phases of the menstrual cycle and compared with controls and were not evident across the menstrual cycle, in contrast to the fMRI studies (Vincent et al., 2011).
There is also emerging findings of altered brain structure in women with dysmenorrhea: women with primary dysmenorrhea have lower gray matter volume in brain regions involved in pain transmission and higher level sensory processing, and larger gray matter volume in regions involved in pain modulation and endocrine function regulation compared with controls (Tu et al., 2010). Although the functional consequences of this central reorganization remain to be established, these differences support a combination of impaired pain inhibition and amplified pain facilitation (Tu et al., 2010). Similar morphological brain changes, specifically lower gray matter volume, have been observed in other recurrent or chronic pain states, including chronic pelvic pain (CPP) associated with endometriosis (As-Sanie et al., 2012) and irritable bowel syndrome (IBS) (Davis et al., 2008; Blankstein et al., 2010). These findings support the theory that prolonged nociceptive input into the CNS generates functional and structural modifications, and can alter the processing of pain within the CNS (Marcus, 1995; Apkarian et al., 2005; Hermann et al., 2008). However, the possibility remains that altered brain structure and/or functionality in regions involved in pain processing may predate the development of primary dysmenorrhea.
Primary dysmenorrhea has recently been classified as a CPP syndrome (Baranowski et al., 2012), and it is also a frequent co-morbid symptom in women with other CPP (Zondervan et al., 2001). For example, a recent robust 10-year follow-up study concluded that women with IBS are more likely to experience dysmenorrhea compared with women without IBS (Olafsdottir et al., 2012). This finding is not surprising given that in the clinical setting, a painful condition of one organ can affect the reactivity to painful stimuli of other visceral areas, with at least partially overlapping sensory projection (Giamberardino, 2000; Giamberardino et al., 2001). Further, other studies report that women who experience both dysmenorrhea and IBS, or dysmenorrhea and urinary calculosis, have more menstrual pain, IBS pain and abdominal muscle hyperalgesia (Giamberardino et al., 2010), or more menstrual pain, urinary pain and lumbar and abdominal muscle hyperalgesia (Giamberardino et al., 2010) compared with women with only one of these painful conditions. The mechanisms underlying this phenomenon, termed ‘viscero-visceral hyperalgesia’ or ‘cross-organ sensitization’ are not entirely understood, however, a plausible explanation is that increased nociceptive input to the CNS from one visceral domain (e.g. the reproductive organs), sensitizes or increases the excitability of viscero-visceral convergent neurons in the spinal cord. As a result, the central effect of the input from the second visceral location (e.g. the urinary tract) is amplified (Giamberardino, 2000) (see review (Brumovsky and Gebhart, 2010). Regardless of the exact mechanisms involved in producing this visceral interaction, remarkably, effective treatment of one painful condition in one organ decreases pain and symptoms from the other organ. For example, effective treatment of dysmenorrhea has been shown to significantly decrease pain reactivity from the urinary tract (Giamberardino, 2000), as well as IBS and urinary calculosis symptoms (Giamberardino et al., 2010).
Taken together, the evidence of structural and functional modifications within the CNS suggest that women with primary dysmenorrhea have central changes that persist beyond the time of menstruation, possibly due to the recurrent nociceptive input into the CNS (Marcus, 1995; Apkarian et al., 2005; Hermann et al., 2008). Recently, researchers have even suggested that dysmenorrhea may predispose women to a chronic pain state (As-Sanie et al., 2012). Indeed, there is evidence that previous pain predicts future pain (Hunter, 2001; Katz and Seltzer, 2009), and that increased sensitivity to experimental pain is a risk factor for developing chronic pain (Carli et al., 2002; Staud et al., 2003). Why some women with dysmenorrhea undergo a transition to a chronic pain state while others do not, remains unclear. However, it has been hypothesized that central pain modulation (amplification or inhibition) may account for this phenomenon. It is also interesting to speculate whether such central changes contribute to the gender difference in chronic pain conditions (Berkley, 1997; Pogatzki-Zahn, 2013). Without a longitudinal study in dysmenorrheic women, however, it is not possible to discern whether the increased sensitivity to muscle pain is the cause or the effect of recurrent menstrual pain.
Leyendecker and colleagues have recently proposed that the high intrauterine pressure, peristalsis, and myometrial contractions that account for primary dysmenorrheic pain, result in mechanical strain and injury of the uterus, and ultimately, may drive the development of adenomyosis (Leyendecker et al., 2009, 2015). The authors believe that endometrial-derived oxytocin is responsible for the hypercontractility of the uterus; a hypothesis substantiated by a recent report that oxytocin receptor over-expression in myometrial smooth muscle cells may be responsible for increased uterine contractility and adenomyosis-associated dysmenorrhea (Guo et al., 2013). The proposed link between adenomyosis and primary dysmenorrhea by this group is derived primarily from results of a recent prospective study showing that a particular form of adenomyosis was observed exclusively in women with severe primary dysmenorrhea, and that ±80% of patients complained of a history of primary dysmenorrhea (Leyendecker et al., 2015). However, whether the primary dysmenorrheic pain in fact leads to the development of secondary dysmenorrhea (adenomyosis), or whether adenomyosis was already present but undiagnosed, remains to be established. At present, it appears that there are cases of adenomyosis in adolescents who present with primary dysmenorrheic pain that is unresponsive to traditional medical treatment (Dietrich, 2010).
While one potential long-term outcome of primary dysmenorrhea may be heightened risk for developing other painful conditions later on in life, recurrent menstrual pain also has immediate impact on the daily lives of women due to the effect of pain on daily functioning, QoL, mood and sleep.
Impact of primary dysmenorrhea on QoL
Daytime functioning, QoL and mood
The painful menstrual cramps experienced by women with dysmenorrhea can be considerably disabling, having been likened to renal colic pain (Ayan et al., 2012). In various large cross-sectional studies conducted worldwide involving hundreds-to-thousands of women and/or female adolescents, menstrual pain has a negative impact on multiple aspects of the personal lives of those affected, including: family relationships, friendships, school/work performance, and social and recreational activities (Ortiz et al., 2009; Eryilmaz et al., 2010; Wong and Khoo, 2010; Pitangui et al., 2013). The intense cyclic pain is associated with a restriction of physical activity (Dawood, 1995; Chen et al., 2006; Patel et al., 2006; Chantler et al., 2009a,b), and dysmenorrheic pain has been reported to be the primary cause of recurrent short-term school or work absenteeism among young women of child-bearing age. Several longitudinal studies on young dysmenorrheic women have revealed that rates of absenteeism in these women range from 34 to 50% (Andersch and Milsom, 1982; Sundell et al., 1990) with an estimated 10–30% of all working or studying women with dysmenorrhea losing 1–2 working days per month. This amounts to an annual loss of ∼600 million working hours or up to $2 billion annually in the USA (Dawood, 1988). In a Swedish population of only 4 million, primary dysmenorrhea has been reported as the cause of 230 000 lost working days, with >50% of women claiming absenteeism from work or school on at least one occasion due to dysmenorrhea. Thus, given the significant impact of dysmenorrhea on productivity, it ultimately can have severe worldwide economic consequences (Hofmeyr, 1996; Jones, 2004). Given that most women do not seek medical attention for their pain, and that most cases go undocumented because many women still believe that menstrual pain is a normal part of the female menstrual cycle (Ortiz et al., 2009; Ortiz, 2010; Wong, 2010; Wong and Khoo, 2010), these numbers may even be underestimates.
Chronic pain is a major contributor to a reduced QoL (Skevington, 1998; Laursen et al., 2005; O'Connor, 2009; Matusiak et al., 2010; Souza et al., 2011; Langley, 2012). Primary dysmenorrhea presents features of both chronic and acute pain syndromes; it is a recurring pain with a regular onset, however it is of short duration (Baker et al., 1999). Women with dysmenorrhea score significantly lower in the domains of physical and social functioning, physical role functioning, bodily pain and general health perceptions, and have an overall lower QoL during menstruation, compared with women who do not report dysmenorrhea (Barnard et al., 2003; Unsal et al., 2010; Iacovides et al., 2014b). It would therefore appear that, each month, dysmenorrheic pain has an immediate negative impact on QoL, specifically during menstruation.
Given that pain not only is a sensory experience, but also an emotional event (IASP, 1979; Bromm, 1995), the effects of dysmenorrheic pain on psychological distress and affective states, such as mood, also need to be considered. Pain exacerbates psychological distress (Von Korff and Simon, 1996; Bair et al., 2003) and psychological distress can exacerbate pain (Von Korff and Simon, 1996; Bair et al., 2003). Numerous studies on pain-free male and female participants have also shown that affective processes can modulate pain; arousing positive emotions and mood are able to reduce pain perception, while arousing negative emotions and mood induces pain facilitation (Weisenberg et al., 1984, 1998; Zelman et al., 1991; Zillmann et al., 1996; Rhudy and Meagher, 2000; Meagher et al., 2001; Wunsch et al., 2003; Rainville et al., 2005; Rhudy et al., 2005; Rhudy and Bartley, 2010).
The few studies that have evaluated emotional distress in women who experience cyclical primary dysmenorrheic pain have reported that women with primary dysmenorrhea are significantly more agitated (Baker et al., 1999) and have a poorer mood state during menstruation compared with their follicular phase, and compared with the menstruation phase of pain-free control women (Iacovides et al., 2009, 2013). Also, depression and anxiety are strongly associated with menstrual pain (in both primary and secondary dysmenorrhea) (Alonso and Coe, 2001; Dorn et al., 2009).
Sleep
There is a large body of literature, including experimental (Drewes et al., 1997; Lavigne et al., 2000), and epidemiological (Pilowsky et al., 1985; Gislason and Almqvist, 1987; Moffitt et al., 1991; Lavigne et al., 2005; Goodin et al., 2011) studies, supporting the existence of an intricate relationship between pain and sleep. Most studies investigating sleep and pain have been conducted in patients with long-term chronic pain such as fibromyalgia and rheumatoid arthritis (Drewes, 1999; Lavigne et al., 2005; Wolfe et al., 2006). Pain is believed to be the primary cause of insomnia in patients with various medical conditions (Moffitt et al., 1991; Drewesand Arendt-Nielsen, 2001), and there is evidence showing that pain is associated with poorer subjective (Moffitt et al., 1991; Lavigne et al., 2005; Kelly et al., 2012; Nicassio et al., 2012) and objective (Jennum et al., 1993; Drewes et al., 1998; Drewesand Arendt-Nielsen, 2001; Onen et al., 2005; Blagestad et al., 2012) measures of sleep. Evidence from surveys suggests that dysmenorrhoeic pain similarly disrupts sleep. The National Sleep Foundation's Women and Sleep Poll (1998) found that women reported more disturbed sleep during the first few days of menstruation than at other times of the menstrual cycle and that 28% of the sample reported that their sleep was disturbed by menstrual cramps or pain (NSF, 1998). Moreover, in association with their painful uterine cramps, women with dysmenorrhea frequently complain of daytime fatigue and sleepiness; which further is suggestive of disrupted sleep (Delgado et al., 1994; Chen and Chen, 2005; El-Gilany et al., 2005; Ohde et al., 2008).
Three studies have investigated in detail the extent to which dysmenorrheic pain disturbs subjective and objective measures of sleep (Baker et al., 1999; Iacovides et al., 2009; Araujo et al., 2011). Baker et al. (1999) investigated the sleep architecture of 10 women with unmedicated severe primary dysmenorrhea and 8 women free from any menstrual-associated disorders on the first night of menstruation as well as during the mid-follicular and mid-luteal phases of their menstrual cycles. In association with their pain, the dysmenorrheic women rated their sleep quality as significantly worse than controls during menstruation, and compared with their own pain-free follicular and luteal phases (Baker et al., 1999). Sleep disturbances also were evident in the polysomnographic recordings; women with dysmenorrhea had significantly reduced sleep efficiency during menstruation, with an extended combined time spent awake, moving and in light Stage 1 sleep, compared with both the pain-free phases of their menstrual cycle, and with controls. While experiencing pain, women with dysmenorrhea had significantly less rapid-eye movement (REM) sleep than when they were pain-free, however dysmenorrheic pain had no significant effect on slow wave sleep. Similarly, the second study reported that compared with a pain-free phase of the menstrual cycle, when experiencing menstrual pain, women with severe primary dysmenorrhea had reduced sleep efficiency, reduced REM sleep, and increased Stage 1 sleep (Iacovides et al., 2009). In contrast, another study reported that overnight polysomnographic measures were similar during menstruation in women experiencing untreated menstrual pain (n = 8) compared with women without menstrual pain (n = 8) and women taking medication to relieve their menstrual pain (n = 8) (Araujo et al., 2011).
However, the women in the latter study (Araujo et al., 2011) reported less severe menstrual pain, and were older (mean ± SD of women without (35 ± 7 years) and with (37 ± 7 years) medication) compared with participants in the studies of Baker et al. (23 ± 5 years) and Iacovides et al. (21 ± 1 year) (Baker et al., 1999; Iacovides et al., 2009). This age difference is likely to be significant as only 5% of women above 35 years of age have been shown to experience severe menstrual pain (Polat et al., 2009). Therefore, it is likely that the women included in the latter described recent study (Araujo et al., 2011) did not experience menstrual pain severe enough to disrupt their sleep. Indeed, no woman reported being awakened during the night due to the pain (Araujo et al., 2011). In addition, Araujo et al. (2011) made no distinction between primary and secondary dysmenorrhea, and women were only assessed once in a random menstrual cycle phase, with approximately 6% of women being assessed during the ovulatory phase, 38% during the follicular phase, 21% during the luteal phase, and 35% in an anovulatory menstrual cycle. Thus comparisons could not be made within the same women, with and without pain (Araujo et al., 2011).
Importantly, the relationship between sleep and pain is interactive and bidirectional, such that pain disrupts sleep and disturbances in sleep modify pain perception (Gislason and Almqvist, 1987; Belza, 1995; Onen et al., 2001; Ohayon, 2006; Stanton et al., 2006; Takahashi et al., 2006; Wolfe et al., 2006; Bennett et al., 2007). A poorer sleep quality, for example, has been associated with pain catastrophization of the cold pressor task (Goodin et al., 2011). Further, a recent study of a population of chronic pain patients found that actigraphy-based measures of sleep, particularly total sleep time and wake after sleep onset, predicted pain severity the following day (Tang et al., 2012).
In the context of dysmenorrhea, painful uterine cramps may be the cause of a vicious cycle of negative events; menstrual pain reduces sleep quality and efficiency, and the consequent fatigue experienced by these women is likely to intensify the negative effect of the pain on daytime functioning and mood (Driver and Baker, 1998). Indeed, preliminary findings show that young women with insomnia experience more severe menstrual pain compared with young women who do not have insomnia (Woosley and Lichstein, 2013). It is possible, therefore, that disturbed sleep observed in women with severe primary dysmenorrhea during menstruation (Baker et al., 1999; Iacovides et al., 2009), may heighten their sensitivity to pain.
Treatment of primary dysmenorrhea
On account of the PG-based etiology of primary dysmenorrhea, the current most common pharmacological treatment for dysmenorrhea is non-steroidal anti-inflammatory drugs (NSAIDs) (Harel, 2004; Zahradnik et al., 2010). NSAIDs are classified as prostaglandin synthetase inhibitors and are, on a global scale, among the most frequently prescribed group of drugs (Frolich, 1997; Warner et al., 1999; Bianchi, 2004). The various formulations of NSAIDs have comparable efficacy for dysmenorrhea, and pain relief is successfully achieved in 64–100% of women (Smith, 1993; Marjoribanks et al., 2003; Proctor and Farquhar, 2006).
However, ∼15% of women across the age range who suffer from dysmenorrhea do not respond to, or are intolerant to PG-inhibitors (Rauh et al., 1985; Campbell and McGrath, 1999). In these women, oral contraceptives often are used as second-line therapy. The synthetic hormones in oral contraceptives suppress ovulation and reduce the thickness of the endometrial lining of the uterus, thereby reducing the volume of menstrual fluid, PG synthesis and dysmenorrheic pain (Dawood, 1995; Proctor et al., 2001; Ruoff and Lema, 2003; Strowitzki et al., 2012). However, a recent meta-analysis has confirmed the long-suspected association between oral contraceptive use and the risk of venous thromboembolism (Manzoli et al., 2012) and use in some women may therefore be contra-indicated. Hormonal intrauterine devices, which typically reduce bleeding, have also been shown to reduce the severity of menstrual pain (Suhonen et al., 2004; Lindh and Milsom, 2013). However, the use of hormonal intrauterine devices in nulliparous women is still relatively low (Lindh and Milsom, 2013; Ekelund et al., 2014).
Other currently available therapeutic approaches for the management of dysmenorrheic pain include: transcutaneous electric nerve stimulation, which alters the body's ability to receive or perceive pain signals; transdermal nitroglycerin patches, which inhibit uterine contractions; acupuncture/acupressure; and surgical interventions such as laparoscopic uterosacral nerve ablation surgery (Ruoff and Lema, 2003; Jones, 2004; Proctor and Farquhar, 2006; Cho and Hwang, 2010; Gharloghi et al., 2012). Such therapeutical approaches, however, are not considered to be effective enough to be widely used in clinical practice (Khan et al., 2012), and RCTs showing efficacy of such approaches are limited (Proctor and Farquhar, 2006).
Many women also resort to alternative non-pharmacologic therapies to manage their menstrual discomfort, although these often are ineffective. Alternative approaches include heating pads for cramps, extra bed rest or sleep, physical exercise, meditation, aromatic oils, ginger root tea, salt water, increased calcium intake, increased vitamin D intake and various food sources such as beans, tofu and salmon (Campbell and McGrath, 1999; Ogunfowokan and Babatunde, 2010; Lasco et al., 2012; Ou et al., 2012).
While ∼47–70% of university students use analgesics for pain relief (Cronje and Kritzinger, 1991; Polat et al., 2009; Ortiz, 2010), ∼30% of adolescents do not use over-the-counter medications to treat their menstrual pain and only ∼18% use prescription medication (Wenzloff and Shimp, 1984; Campbell and McGrath, 1997; O'Connell et al., 2006), although the perceived effectiveness of pharmacological methods in the treatment of menstrual discomfort is superior to that of non-pharmacologic methods. In a questionnaire-based study of 289 female adolescents, 98% reported using no less than one non-pharmacologic method to control menstrual discomfort. However, the mean perceived effectiveness of most non-pharmacologic methods was reported to be below 40% (Campbell and McGrath, 1999).
The large variability in the perceived efficacy of the various non-pharmacologic strategies suggests that the efficacy of such methods is personal; one technique may provide relative pain relief for one adolescent, but may not provide the same perceived pain relief for others. However, some studies indicate that methods with a direct physiological impact, such as heat and exercise, are more effective than psychological-based methods, such as distraction (Campbell and McGrath, 1999) and may be as effective as some NSAIDs. For example, an abdominal heat wrap was found to be as effective as ibuprofen, and more effective than acetaminophen in relieving dysmenorrheic pain (Akin et al., 2001, 2004). The reader is referred to more detailed reviews about the treatment of primary dysmenorrhea (Dawood, 2006; French, 2008).
Dysmenorrhea and NSAIDs
NSAIDs act by inhibiting the enzyme that catalyzes the conversion of arachidonic acid to cyclic endoperoxides, namely cyclo-oxygenase (COX) (Fig. 3), which in turn inhibits the production of PGs (Warner et al., 1999; Ruoff and Lema, 2003). Given that suppression of PG formation results in a reduction in uterine PG secretion and thus less vigorous uterine contractions, many NSAIDs provide effective relief from dysmenorrheic pain. Thus, NSAIDs alleviate primary dysmenorrheic pain predominantly through the suppression of endometrial PG synthesis (Dawood, 1995). This was indeed confirmed in an early study in which COX inhibitors reduced both the levels of PGF2α and pain in small numbers of dysmenorrheic women (Chan and Dawood, 1980).
Numerous randomized, placebo-controlled studies have investigated the efficacy and safety of NSAIDs in treating dysmenorrhea, and have shown that several NSAID formulations, including naproxen sodium, zomepirac sodium, mefenamic acid, ketoprofen, ibuprofen, and diclofenac, provide effective pain relief in women with primary dysmenorrhea (Hanson et al., 1978; Ingemanson et al., 1981; Riihiluoma et al., 1981; Budoff, 1982; Mehlisch, 1988, 1990; Marchini et al., 1995; Facchinetti et al., 2002; Milsom et al., 2002; Letzel et al., 2006; Chantler et al., 2008, 2009a, b; Iacovides et al., 2014a). Further, a meta-analysis of 31 studies on the efficacy of NSAIDs in primary dysmenorrhea revealed that compared with placebo, naproxen, ibuprofen and mefenamic acid all provided significant pain relief (Zhang and Li Wan Po, 1998). The various formulations of NSAIDs have, in fact, been reported to have comparable efficacy for dysmenorrhea (Zahradnik et al., 2010), and pain relief is successfully achieved in 64–100% of women (Smith, 1993; Marjoribanks et al., 2003; Proctor and Farquhar, 2006). Only one randomized, double-blind, crossover trial found an advantage of one NSAID over another. In this report, rofecoxib and diclofenac potassium were found to be statistically superior to meloxicam (Chantler et al., 2008).
NSAIDs, specifically diclofenac potassium, have also been shown to be effective in restoring dysmenorrheic pain-induced reduction in physical activities (Chantler et al., 2009a, b) and disruption of objective and subjective sleep (Iacovides et al., 2009). Effects of NSAIDs are generally tolerable, and gastrointestinal safety issues are generally of less concern in acute use of NSAIDs compared with chronic use (Langford and Evans, 2002).
Research agenda
We suggest future research using longitudinal studies be directed at determining whether recurrent severe primary dysmenorrheic pain does indeed lead to central sensitization, or whether primary dysmenorrhea is a precursor to other chronic pain conditions or secondary dysmenorrhea, particularly adenomyosis. Given that genetic factors have a significant etiological contribution to both chronic pain conditions (Kato et al., 2006) and experimentally-induced pain (Norbury et al., 2007; Williams et al., 2012), future studies should also investigate pain sensitivity in female adolescents with primary dysmenorrhea soon after menarche or in female adolescents at high risk for developing dysmenorrhea, to determine whether they have an underlying sensitivity to pain that predates their development of primary dysmenorrhea. More studies are required to further our understanding of environmental and genetic risk factors for primary dysmenorrhea. Studies are further needed in mid-life women to determine the progression of repetitive acute dysmenorrheic pain into chronic pain conditions. Using advanced imaging techniques, future studies on primary dysmenorrhea, particularly investigations on severe dysmenorrhea of long duration (Kissler et al., 2008) that is unresponsive to traditional treatment (Dietrich, 2010), should exclude a diagnosis of adenomyosis as a possible source of pain. New prospective data regarding the prevalence of adenomyosis in women with primary dysmenorrhea are required. Given the reciprocal relationship between sleep and pain (Woosley and Lichstein, 2014) more research is required to determine the interaction between sleep disruption and pain sensitivity in women with primary dysmenorrhea.
Longitudinal studies suggest that the central changes in chronic pain states are dynamic and therefore reversible after removal of the nociceptive source (Teutsch et al., 2008; Gwilym et al., 2010), although such investigations have not yet been conducted in women with dysmenorrhea. Future studies should investigate whether long-term effective treatment of moderate-to-severe menstrual pain is able to reverse any behavioral signs of central sensitization in women with primary dysmenorrhea, and whether any changes in cerebral structure and metabolism, as seen using brain imagery techniques, can be restored to normal. With a clearer understanding of the etiology of primary dysmenorrhea, treatment options may be expanded from only being able to manage pain to preventing the development of pain to begin with.
Conclusion
The prevalence of dysmenorrhea is high and its consequences are extensive, as illustrated schematically in Fig. 1. However, as recently stated, there is an ‘appalling’ disregard for dysmenorrhea in the pain community (Berkleyand McAllister, 2011; Berkley, 2013). Surprisingly little scientific attention has been given to primary dysmenorrhea, even with regards to available research funding (Berkley, 2013). Consequently, primary dysmenorrhea remains a poorly understood disorder that many women simply accept as a normal part of their menstrual cycle (Reddish, 2006). In this review we illustrate the extensive negative consequences of cyclic menstrual pain in the lives of women with primary dysmenorrhea. Further, we highlight evidence suggesting that dysmenorrhea is not merely a disorder noticeable during menstruation, particularly with regard to pain processing and the perception of pain. It is feasible that recurrent menstrual pain not only is associated with central sensitization, but also may predispose women with primary dysmenorrhea to other chronic painful conditions. Limiting the noxious input into the CNS of dysmenorrheic women therefore seems imperative to prevent the possible development of central sensitization, as well as any potential progression of repetitive dysmenorrhea into other chronic pain conditions. As outlined above, further research is required to advance understanding of primary dysmenorrhea and its long-term consequences. With a clearer understanding of primary dysmenorrhea, treatment options may be expanded from only being able to manage pain to preventing the development of pain.
Authors' roles
S.I. and F.C.B. conceptualized the rationale and design of the review article. S.I. drafted and edited the manuscript. All authors read, revised and approved the final manuscript.
Funding
No funding was received for this review.
Conflict of interest
None of the authors have conflicts of interest with respect to this work.



