-
PDF
- Split View
-
Views
-
Cite
Cite
Qin Li, Cuishan Chen, Jiaming Wu, Liona C Poon, Chi Chiu Wang, Tin Chiu Li, Tao Zhang, Xianghao Guo, Liang Song, Xia Wang, Qian Zhang, Ziying Ye, Yongkang Yang, Jing Lu, Jianyu Yao, Dewei Ye, Yao Wang, Decreased serum soluble programmed cell death ligand-1 level as a potential biomarker for missed miscarriage, Human Reproduction, Volume 38, Issue 11, November 2023, Pages 2128–2136, https://doi.org/10.1093/humrep/dead178
Close - Share Icon Share
Abstract
Can maternal serum levels of soluble programmed cell death-1 (sPD-1) and its ligand (sPD-L1) serve as biomarkers for missed miscarriage (MM)?
Serum sPD-L1 levels are significantly decreased in MM patients and may serve as a potential predictive biomarker for miscarriage.
Programmed cell death-1 (PD-1) and its ligand (PD-L1) comprise important immune inhibitory checkpoint signaling to maintain pregnancy. Their soluble forms are detectable in human circulation and are associated with immunosuppression.
Three independent cohorts attending tertiary referral hospitals were studied. The first (discovery) cohort was cross-sectional and included MM patients and healthy pregnant (HP) women matched on BMI. The second validation cohort contained MM patients and women with legally induced abortion (IA). The third prospective observational study recruited subjects requiring IVF treatment.
In the discovery cohort, we enrolled 108 MM patients and 115 HP women who had a full-term pregnancy at 6–14 weeks of gestation. In the validation cohort, we recruited 25 MM patients and 25 women with IA. Blood samples were collected at the first prenatal visit for HP women or on the day of dilatation and curettage surgery (D&C) for MM and IA subjects to determine serum sPD-1 and sPD-L1 levels. Placenta samples were harvested during the D&C within the validation cohort to measure gene and protein expression. The prospective cohort collected serial blood samples weekly from 75 volunteers with embryo transfer (ET) after IVF.
Circulating sPD-L1 levels were reduced by 50% in patients with MM (55.7 ± 16.04 pg/ml) compared to HP controls (106.7 ± 58.46 pg/ml, P < 0.001) and the difference remained significant after adjusting for maternal age and gestational age, whereas no significant differences in sPD-1 level were observed. Likewise, serum sPD-L1 was lower in MM patients than in IA subjects and accompanied by downregulated PD-L1-related gene expression levels in the placenta. In the IVF cohort, applying the changing rate of sPD-L1 level after ET achieved a predictive performance for miscarriage with receiver operating characteristics = 0.73 (95% CI: 0.57–0.88, P < 0.01).
The study was mainly confined to East Asian pregnant women. Further large prospective pregnancy cohorts are required to validate the predictive performance of sPD-L1 on miscarriage.
Reduced circulating sPD-L1 level and downregulated placental PD-L1 expression in miscarriage indicate that dysfunction in PD-L1 signals is a potential underlying mechanism for pregnancy loss. Our findings further extend the importance of the PD-L1 axis in pregnancy maintenance in early pregnancy.
This study was financially supported by grants from the Subject Innovation Team of Shaanxi University of Chinese Medicine (2019-Y502), General Research Fund (14122021), and Key Laboratory of Model Animal Phenotyping and Basic Research in Metabolic Diseases (2018KSYS003). The authors declare that they have no competing interests to be disclosed.
N/A.
Introduction
Missed miscarriage (MM), also known as silent miscarriage, is a type of spontaneous miscarriage that occurs without obvious clinical symptoms which affects pregnancies worldwide (Weeks and Danielsson, 2006). Though several risk factors including genetic, endocrinological, and immunological abnormality contribute to the development of MM (Woolner et al., 2020), the underlying mechanisms remain to be investigated. Owing to the significant risk of MM on pregnancy health, it is imperative to identify novel biomarkers for MM, which will further provide a deeper understanding of its underlying etiologies and raise the possibility for early diagnosis and timely interventions (Sonalkar et al., 2020).
Programmed cell death-1 (PD-1) and its ligand (PD-L1) comprise an important inhibitory checkpoint signaling system for immune regulation (Cha et al., 2019). In the context of tumors and cancers, PD-L1 is highly expressed to induce immune tolerance (Cha et al., 2019). Similarly, the PD-1/PD-L1 axis plays a crucial role in promoting immune tolerance to support the survival and development of the semi-allogeneic fetus during pregnancy (PrabhuDas et al., 2015). PD-L1 is highly expressed in the trophoblasts of placenta (Veras et al., 2017). Reduced placental PD-L1 expression has been observed in an autoimmune disease mice model and is associated with the risk of miscarriage (Chen et al., 2019). Furthermore, depleting PD-L1 expression or blocking PD-L1 signaling significantly exacerbates embryo resorption and diminishes fetomaternal tolerance in mice (Guleria et al., 2005). PD-1 is detectable in various immune cells residing in decidua during pregnancy. Decreased PD-1+ T cells in decidua have been shown to be associated with miscarriage in humans (Wang et al., 2016), which is corroborated by animal studies that anti-PD-1 antibody treatment causes fetal loss (Wang et al., 2015, 2016). Collectively, the PD-1/PD-L1 axis orchestrates the immunotolerance of pregnancy, and disturbance of this axis in the fetomaternal interface is likely to be an important factor in the pathogenesis of pregnancy loss.
Notably, apart from the classic membrane-bound form of PD-1 and PD-L1, their soluble forms are detectable in human sera. Soluble PD-1 (sPD-1) and soluble PD-L1 (sPD-L1) are produced either by proteolytic cleavage from the cell membrane or transcription of mRNA splice variants with a functional domain (Daassi et al., 2020). They are all biologically active and able to regulate the immune system. Circulating levels of sPD-L1 and sPD-1 have been applied as predictive or prognostic biomarkers for cancers. Elevation in sPD-L1 and sPD-1 levels have been linked with severe tumor burdens, poor prognosis, and reduced overall survival in patients with various cancers (Zhou et al., 2017; Buderath et al., 2019; Shigemori et al., 2019). Importantly, the increase in serum sPD-1 and sPD-L1 levels is in parallel with the PD-1 and PD-L1 expression in immune cells and tumor cells (Okla et al., 2020), respectively. Therefore, it raises the diagnostic potential to monitor the PD-1/PD-L1 axis by measuring their soluble forms.
During pregnancy, serum sPD-L1 level is increased, while serum sPD-1 level is reduced when compared to non-pregnant women (Okuyama et al., 2019). A study has observed that serum sPD-L1 levels gradually increase throughout the first trimester (Zhang et al., 2020). Therefore, the elevation of sPD-L1 and sPD-1 may reflect the tolerogenic immune status during pregnancy. However, the perturbation of their serum levels in MM is still under-investigated. Considering the importance of the PD-1/PD-L1 axis in pregnancy maintenance, we reason that the measurement of their soluble forms will provide an alternative approach to detecting MM.
Herein, we organized a cross-sectional study to measure the serum levels of sPD-L1 and sPD-1 in patients with MM and healthy pregnancies at 6–14 weeks of gestation. We validated our findings in an independent cohort with MM patients and control subjects who underwent artificially induced abortion (IA) and further investigated the PD-1/PD-L1-related gene expression in their placental samples. Moreover, the predictive value of sPD-L1 for miscarriage was evaluated in a prospective cohort of IVF patients after embryo transfer (ET).
Materials and methods
Study design
The study protocol was approved by the human research ethics committee of the Second Affiliated Hospital of Shaanxi University of Chinese Medicine (IRB Ref. No.: KY201913) and conducted in the Department of Obstetrics and Gynaecology, Shaanxi University of Chinese Medicine, China. All participants signed informed consent before recruitment.
In the discovery cohort, we enrolled 108 patients with confirmed MM and 115 healthy pregnant (HP) women at 6–14 weeks of gestation from our established pregnancy biobank (Yang et al., 2021). The MM patients and HP group were matched for BMI. Of note, all control cases had a full-term delivery without any major pregnancy-associated complications, such as gestational diabetes, preeclampsia, and preterm birth. In the validation cohort, we enrolled another 25 patients with MM and 25 BMI-matched cases who underwent IA legally for non-medical reasons. The diagnostic criteria of MM are defined as (i) B-transvaginal ultrasonography of empty gestational sac (mean sac diameter ≥25 mm without obvious yolk sac) or embryonic crown rump length ≥7 mm in the absence of cardiac activity and (ii) no obvious clinical symptoms of miscarriage including vaginal bleeding, abdominal pain, etc. (Jurkovic et al., 2013; Preisler et al., 2015). The gestational stage was estimated according to the first date of the last menstrual period and ultrasonographic examination. To evaluate the predictive value of sPD-L1 on miscarriage, we reanalyzed a prospective cohort in subjects with ET at the Assisted Reproductive Technology Unit, Prince of Wales Hospital, the Chinese University of Hong Kong from 2018 to 2020. A total of 75 women with longitudinal measurements of serum sPD-L1 levels at 0, 9, 16, 23, and 30 days after ET were enrolled. Of these participants, 16 of them experienced miscarriage while 59 subjects had successful live birth. The details of recruitment, treatment, and clinical information have been described in our previous publication (Zhang et al., 2022b).
Human specimen collection and preparation
Blood samples were collected at the first prenatal visit for HP women, whereas it was collected on the day of Dilation and Curettage (D&C) surgery for MM patients and IA subjects. After centrifugation (800g × 15 min, 4°C), serum was collected from the supernatant and stored at −80°C. Placental samples were collected during the D&C surgery within the validation cohort. They were aliquoted and preserved immediately at −80°C or processed in 10% formalin (v/v) and embedded as a paraffin section with routine procedures.
Immunoassay of serum sPD-1 and sPD-L1
Human serum soluble PD-1 (Cat. BMS2214, Thermo, Massachusetts, USA) and PD-L1 (Cat.DB7H10, R&D, Minnesota, USA) levels were measured using commercial kits by enzyme-linked immunosorbent assay (ELISA) methods according to the manufacturer’s instructions. The sensitivity, limit of detection, and inter- and intra-assay coefficient of variation (COV) of these two kits are shown in Supplementary Table S1. The intra-plate variations of our ELISA measurement were controlled by 15%.
Western blot analysis
Total placental proteins were isolated using RIPA buffer containing protease inhibitor cocktail (Roche, 11873580001, Switzerland). The protein concentration was determined using Pierce Rapid Gold BCA Protein Assay Kit (Thermo, A53226, Massachusetts, USA). Forty micrograms of proteins per sample were loaded and resolved by SDS–polyacrylamide gel electrophoresis and then transferred to polyvinylidene difluoride membranes. The membrane was blocked in 5% Tris-buffered saline with Tween 20 (TBST)-milk (w/v) for 1 h and probed with PD-L1 (1:1000; ab213524, Abcam, Cambridge, UK) or GAPDH (1:2000; # 2118S, CST, Massachusetts, USA) for overnight. Membranes were washed in TBST and incubated with horse radish peroxidase (HRP)-linked goat anti-rabbit secondary antibody (1:2000, # 7074S, CST, Massachusetts, USA). Protein bands were visualized with clarity ECL substrate (Bio-Rad, 170-5061, California, USA). The band intensity of PD-L1 was quantified by grayscale analysis using NIH ImageJ software, with normalization to glyceraldehyde 3-phosphate dehydrogenase (GAPDH).
RNA extraction and q-PCR analysis
mRNA of placenta samples was extracted using TRIzol (Invitrogen, California, USA) and reverse transcribed into cDNA. 50 ng cDNA was used for RT-PCR quantitation with the SYBR Green Premix Pro Taq (Accurate Biology, A3A1357, Hunan, China) by the real-time PCR detection system (LightCycler® 480 Instrument II, Roche, Basel, Switzerland). The relative expression level of targeted genes was normalized by the cycle threshold (CT) value of human GAPDH (ΔCT) and calculated by the 2−ΔΔCt method. The primer sequences are presented in Supplementary Table S2 and were synthesized by Sangon Biotech (Shanghai, China).
Immunohistochemistry staining
Five-millimeter sections from formalin-fixed paraffin-embedded placenta were dewaxed and rehydrated following routine procedures. For immunohistochemistry (IHC) staining, slides were incubated with an anti-human PD-L1 antibody (5 μg/ml; Cat # ab205921, Abcam), followed by a secondary antibody conjugated with HRP (Cell Signalling Technology, # 7074S, Massachusetts, USA). Images were visualized and captured using a color digital camera (Olympus, DP80, Tokyo, Japan). The abundance of PD-L1-positive signals was semi-quantified using the software ImageJ (version 1.53q, NIH) with a published protocol (Crowe and Yue, 2019).
Sample size calculation
The sample size for the discovery cohort was estimated based on the expected mean and standard deviation (SD) of serum concentration of sPD-L1 in pregnant women at 8 weeks of gestation (Enninga et al., 2018). To achieve the power (1-β) of 0.8 and 0.05 significance (α), At least 22 patients with MM and 22 HP women, with a ratio of 1:1, were required. The online Sample Size Calculator for Clinical Study by Cleveland Clinic was employed for this calculation (Wang and Ji, 2020).
Statistical analysis
The continuous variables and categorical variables were shown as mean ± SD or N (%), respectively. The normality of variable distributions was examined with the Kolmogorov–Smirnov test. Two-tailed Student’s t-test or Mann–Whitney test were used to calculate the differences in continuous data between groups. Categorical variables were compared by the Chi-square test or Fisher’s exact test. Levene's test was performed to adjust for the impacts of maternal age and gestational age on the serum sPD-1 and sPD-L1 levels. The potential impact of multiple covariances, such as maternal age and gestational age, on sPD-L1 and sPD-1 levels, was adjusted using the analysis of covariance method by fitting the general linear model (Egbewale et al., 2014). The odds ratio between serum levels of sPD-L1 and PD-1 and miscarriage was estimated by binary logistic regression analysis. The diagnostic performance was analyzed by the receiver operating characteristics (ROC) analysis and the area under the ROC curve (AUROC). The optimal cut-off value of ROC was estimated using Youden’s index. The relative change in sPD-L1 after ET between successive samples was calculated by dividing the difference in sPD-L1 level between two by the sPD-L1 level at the earlier time point. Statistical analyses were performed with SPSS (version 26, IBM) and data were visualized by Prism software (version 8.0, GraphPad).
Results
Cohort demographics
The clinical characteristics of the discovery cohort are described in Table 1. There were no significant differences in maternal height, weight, BMI, pregnancy history, and history of miscarriage and IA between HP women and MM patients. However, the mean maternal age of the HP group (28.17 ± 4.11 years) was younger than the MM group (30.25 ± 4.54, P < 0.001). In addition, since the control subjects were enrolled in their first prenatal visit (10.99 ± 2.24 weeks), their gestational week was significantly greater than the MM group (8.93 ± 1.45 weeks, P < 0.001). The stratification details of the two variables are presented in Table 1.
Demographic and clinical characteristics between healthy pregnancies and patients with miscarriage.
| Variables . | Healthy pregnancy (n = 115) . | Missed miscarriage (n = 108) . | P-value . |
|---|---|---|---|
| Maternal age, years | 28.2 ± 4.11 | 30.3 ± 4.54 | <0.001 |
| <25, N (%) | 25 (21.7) | 7 (6.5) | |
| 25–29, N (%) | 50 (43.5) | 48 (44.4) | |
| 30–35, N (%) | 35 (30.4) | 38 (35.2) | |
| >35, N (%) | 4 (3.5) | 14 (13.0) | |
| Height, cm | 160.8 ± 4.80 | 160.4 ± 4.45 | 0.595 |
| Weight, kg | 56.3 ± 11.76 | 55.3 ± 7.25 | 0.455 |
| BMI, kg/m2 | 21.8 ± 4.25 | 21.5 ± 3.42 | 0.682 |
| Racial origin, N (%) | |||
| East Asian | 115 (100.0) | 108 (100.0) | 1.000 |
| Smoking, N (%) | 3 (2.6) | 2 (1.8) | 0.703 |
| Gestational age, weeks | 11.0 ± 2.24 | 8.9 ± 1.45 | <0.001 |
| <7, N (%) | 0 (0.0) | 7 (6.5) | |
| 7–9, N (%) | 26 (22.6) | 65 (61.9) | |
| 10–12, N (%) | 62 (53.9) | 34 (31.5) | |
| >12, N (%) | 26 (22.6) | 0 (0) | |
| Parity, N | 0.98 ± 1.22 | 1.23 ± 1.22 | 0.145 |
| Nulliparous, N (%) | 43 (37.4) | 29 (26.9) | 0.093 |
| Parous, N (%) | 72 (62.6) | 79 (73.1) | 0.093 |
| Number of miscarriages | 0.6 ± 0.92 | 0.7 ± 0.93 | 0.344 |
| Number of artificial abortions | 0.7 ± 0.92 | 0.7 ± 0.98 | 0.407 |
| Cytokines levels | |||
| sPD-1 (ng/ml) | 31.5 ± 13.72 | 33.6 ± 15.73 | 0.291 |
| sPD-L1 (pg/ml) | 107.3 ± 58.35 | 55.1 ± 15.42 | <0.001 |
| Adjusted sPD-1 (ng/ml) | 32.5 ± 15.58 | 33.0 ± 15.70 | 0.644 |
| Adjusted sPD-L1 (pg/ml) | 100.5 ± 44.79 | 62.7 ± 45.10 | <0.001 |
| Variables . | Healthy pregnancy (n = 115) . | Missed miscarriage (n = 108) . | P-value . |
|---|---|---|---|
| Maternal age, years | 28.2 ± 4.11 | 30.3 ± 4.54 | <0.001 |
| <25, N (%) | 25 (21.7) | 7 (6.5) | |
| 25–29, N (%) | 50 (43.5) | 48 (44.4) | |
| 30–35, N (%) | 35 (30.4) | 38 (35.2) | |
| >35, N (%) | 4 (3.5) | 14 (13.0) | |
| Height, cm | 160.8 ± 4.80 | 160.4 ± 4.45 | 0.595 |
| Weight, kg | 56.3 ± 11.76 | 55.3 ± 7.25 | 0.455 |
| BMI, kg/m2 | 21.8 ± 4.25 | 21.5 ± 3.42 | 0.682 |
| Racial origin, N (%) | |||
| East Asian | 115 (100.0) | 108 (100.0) | 1.000 |
| Smoking, N (%) | 3 (2.6) | 2 (1.8) | 0.703 |
| Gestational age, weeks | 11.0 ± 2.24 | 8.9 ± 1.45 | <0.001 |
| <7, N (%) | 0 (0.0) | 7 (6.5) | |
| 7–9, N (%) | 26 (22.6) | 65 (61.9) | |
| 10–12, N (%) | 62 (53.9) | 34 (31.5) | |
| >12, N (%) | 26 (22.6) | 0 (0) | |
| Parity, N | 0.98 ± 1.22 | 1.23 ± 1.22 | 0.145 |
| Nulliparous, N (%) | 43 (37.4) | 29 (26.9) | 0.093 |
| Parous, N (%) | 72 (62.6) | 79 (73.1) | 0.093 |
| Number of miscarriages | 0.6 ± 0.92 | 0.7 ± 0.93 | 0.344 |
| Number of artificial abortions | 0.7 ± 0.92 | 0.7 ± 0.98 | 0.407 |
| Cytokines levels | |||
| sPD-1 (ng/ml) | 31.5 ± 13.72 | 33.6 ± 15.73 | 0.291 |
| sPD-L1 (pg/ml) | 107.3 ± 58.35 | 55.1 ± 15.42 | <0.001 |
| Adjusted sPD-1 (ng/ml) | 32.5 ± 15.58 | 33.0 ± 15.70 | 0.644 |
| Adjusted sPD-L1 (pg/ml) | 100.5 ± 44.79 | 62.7 ± 45.10 | <0.001 |
Data are shown as mean ± SD unless shown otherwise. sPD-1, soluble program cell death-1; sPD-L1, soluble program cell death ligand-1. sPD-1 and sPD-L1 levels were adjusted for maternal age and gestational age.
Demographic and clinical characteristics between healthy pregnancies and patients with miscarriage.
| Variables . | Healthy pregnancy (n = 115) . | Missed miscarriage (n = 108) . | P-value . |
|---|---|---|---|
| Maternal age, years | 28.2 ± 4.11 | 30.3 ± 4.54 | <0.001 |
| <25, N (%) | 25 (21.7) | 7 (6.5) | |
| 25–29, N (%) | 50 (43.5) | 48 (44.4) | |
| 30–35, N (%) | 35 (30.4) | 38 (35.2) | |
| >35, N (%) | 4 (3.5) | 14 (13.0) | |
| Height, cm | 160.8 ± 4.80 | 160.4 ± 4.45 | 0.595 |
| Weight, kg | 56.3 ± 11.76 | 55.3 ± 7.25 | 0.455 |
| BMI, kg/m2 | 21.8 ± 4.25 | 21.5 ± 3.42 | 0.682 |
| Racial origin, N (%) | |||
| East Asian | 115 (100.0) | 108 (100.0) | 1.000 |
| Smoking, N (%) | 3 (2.6) | 2 (1.8) | 0.703 |
| Gestational age, weeks | 11.0 ± 2.24 | 8.9 ± 1.45 | <0.001 |
| <7, N (%) | 0 (0.0) | 7 (6.5) | |
| 7–9, N (%) | 26 (22.6) | 65 (61.9) | |
| 10–12, N (%) | 62 (53.9) | 34 (31.5) | |
| >12, N (%) | 26 (22.6) | 0 (0) | |
| Parity, N | 0.98 ± 1.22 | 1.23 ± 1.22 | 0.145 |
| Nulliparous, N (%) | 43 (37.4) | 29 (26.9) | 0.093 |
| Parous, N (%) | 72 (62.6) | 79 (73.1) | 0.093 |
| Number of miscarriages | 0.6 ± 0.92 | 0.7 ± 0.93 | 0.344 |
| Number of artificial abortions | 0.7 ± 0.92 | 0.7 ± 0.98 | 0.407 |
| Cytokines levels | |||
| sPD-1 (ng/ml) | 31.5 ± 13.72 | 33.6 ± 15.73 | 0.291 |
| sPD-L1 (pg/ml) | 107.3 ± 58.35 | 55.1 ± 15.42 | <0.001 |
| Adjusted sPD-1 (ng/ml) | 32.5 ± 15.58 | 33.0 ± 15.70 | 0.644 |
| Adjusted sPD-L1 (pg/ml) | 100.5 ± 44.79 | 62.7 ± 45.10 | <0.001 |
| Variables . | Healthy pregnancy (n = 115) . | Missed miscarriage (n = 108) . | P-value . |
|---|---|---|---|
| Maternal age, years | 28.2 ± 4.11 | 30.3 ± 4.54 | <0.001 |
| <25, N (%) | 25 (21.7) | 7 (6.5) | |
| 25–29, N (%) | 50 (43.5) | 48 (44.4) | |
| 30–35, N (%) | 35 (30.4) | 38 (35.2) | |
| >35, N (%) | 4 (3.5) | 14 (13.0) | |
| Height, cm | 160.8 ± 4.80 | 160.4 ± 4.45 | 0.595 |
| Weight, kg | 56.3 ± 11.76 | 55.3 ± 7.25 | 0.455 |
| BMI, kg/m2 | 21.8 ± 4.25 | 21.5 ± 3.42 | 0.682 |
| Racial origin, N (%) | |||
| East Asian | 115 (100.0) | 108 (100.0) | 1.000 |
| Smoking, N (%) | 3 (2.6) | 2 (1.8) | 0.703 |
| Gestational age, weeks | 11.0 ± 2.24 | 8.9 ± 1.45 | <0.001 |
| <7, N (%) | 0 (0.0) | 7 (6.5) | |
| 7–9, N (%) | 26 (22.6) | 65 (61.9) | |
| 10–12, N (%) | 62 (53.9) | 34 (31.5) | |
| >12, N (%) | 26 (22.6) | 0 (0) | |
| Parity, N | 0.98 ± 1.22 | 1.23 ± 1.22 | 0.145 |
| Nulliparous, N (%) | 43 (37.4) | 29 (26.9) | 0.093 |
| Parous, N (%) | 72 (62.6) | 79 (73.1) | 0.093 |
| Number of miscarriages | 0.6 ± 0.92 | 0.7 ± 0.93 | 0.344 |
| Number of artificial abortions | 0.7 ± 0.92 | 0.7 ± 0.98 | 0.407 |
| Cytokines levels | |||
| sPD-1 (ng/ml) | 31.5 ± 13.72 | 33.6 ± 15.73 | 0.291 |
| sPD-L1 (pg/ml) | 107.3 ± 58.35 | 55.1 ± 15.42 | <0.001 |
| Adjusted sPD-1 (ng/ml) | 32.5 ± 15.58 | 33.0 ± 15.70 | 0.644 |
| Adjusted sPD-L1 (pg/ml) | 100.5 ± 44.79 | 62.7 ± 45.10 | <0.001 |
Data are shown as mean ± SD unless shown otherwise. sPD-1, soluble program cell death-1; sPD-L1, soluble program cell death ligand-1. sPD-1 and sPD-L1 levels were adjusted for maternal age and gestational age.
The serum sPD-L1 level is significantly decreased in MM patients compared to HP women
Circulating sPD-1 levels between MM patients and HP women were quite similar (HP: 31.5 ± 13.72 pg/ml; MM: 33.6 ± 15.73 pg/ml, P = 0.291). In contrast, the serum sPD-L1 level was nearly 50% lower in the MM patients compared to HP women (HP: 107.3 ± 58.09 pg/ml; MM: 55.7 ± 16.04 pg/ml, P < 0.001) (Table 1). Since both the maternal age and gestational stage were significantly different in the two groups, we first conducted a subgroup analysis based on gestational age. In the HP control, sPD-L1 was linearly increased with the gestational stage (r = 0.36, P < 0.0001) (Supplementary Fig. S1A and B), which was in line with previous studies that maternal sPD-L1 level is evaluated during pregnancy (Enninga et al., 2018). By contrast, serum sPD-L1 levels remained constant with the progression of gestation in the MM group (Supplementary Fig. S1C and D). Moreover, even for HP controls with gestational ages <12 weeks, the mean serum sPD-L1 level (n = 42, sPD-L1: 76.3 pg/ml, P < 0.001) was still higher than in MM patients (Supplementary Fig. S1E). We then adjusted their potential impact on sPD-L1 and sPD-1 measuring. After adjustment, the serum sPD-L1 level was significantly lower in MM patients compared to HP controls, whereas sPD-1 remained unchanged (Table 1). Therefore, serum sPD-L1 level was independently decreased in MM patients.
We next estimated the association of serum sPD-1 and sPD-L1 with the risk of miscarriage. The odds ratio (OR) between sPD-L1 and miscarriage was 0.951 (95% CI: 0.937–0.965, P < 0.0001), indicating that a reduction of every unit of serum sPD-L1 levels was associated with a 5% increased odds of miscarriage. In contrast, no association between serum sPD-1 and the risk of miscarriage was observed (Supplementary Table S3).
Circulating sPD-L1 levels effectively classify patients with MM
We then evaluated the performance of circulating sPD-L1 in identifying MM patients. Applying the serum sPD-1 level discriminated MM patients from control cases with an AUROC of 0.56 (95% CI: 0.48–0.63). Using serum sPD-L1 level achieved a greater diagnostic ability with an AUROC of 0.83 (95% CI: 0.77–0.88, P < 0.001). The optimal cut-off value of sPD-L1 was 81.5 pg/ml with 92.59% sensitivity and 65.79% specificity (Fig. 1A). We also evaluate the performance of the ratio of sPD-L1:sPD-1, which presented an AUROC of 0.77 (95% CI: 0.70–0.83) (Fig. 1A). Taken together, our results suggested that a single measurement of serum sPD-L1 level enabled a moderately effective identification of patients with MM.
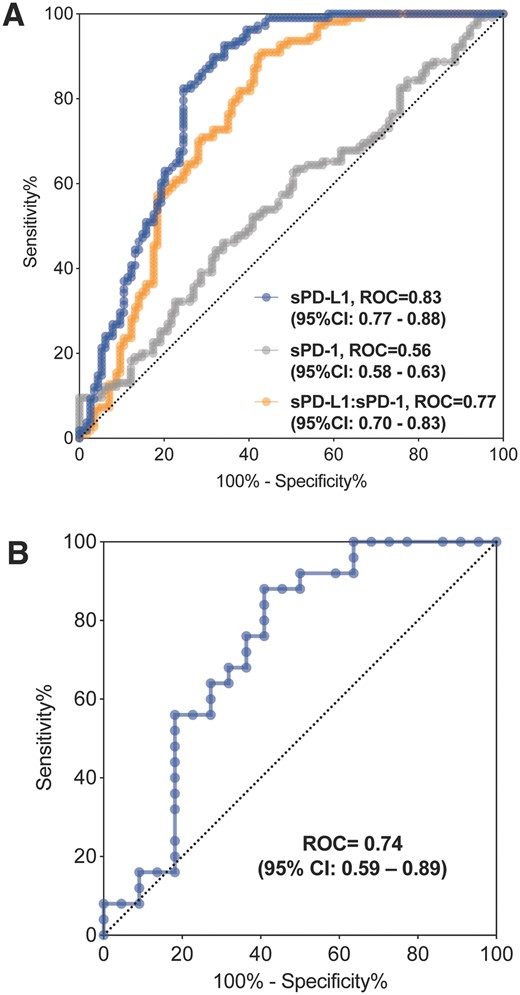
The use of Serum sPD-L1 level as a potential biomarker for identifying patients with miscarriage. (A) Discriminatory performance of serum sPD-L1, sPD-1 level for diagnosing miscarriage by comparison of their ROC curves in the discovery cohort. (B) ROC curves for identification of miscarriage patients with serum sPD-L1 value in the validation cohort. ROC, receiver operating characteristics.
To further validate the performance of sPD-L1 on identifying miscarriage, we recruited an independent cohort with MM patients and healthy women who underwent legally IA for non-medication reasons. The maternal age was comparable between the two groups (Table 2), while the gestational age was lower in the IA group (6.9 ± 0.95 weeks) than in the MM group (9.5 ± 1.16 weeks, P < 0.001). As expected, circulating sPD-L1 levels in serum were significantly lower in the MM group (IA: 62.1 ± 15.53 pg/ml versus MM: 52.7 ± 10.82 pg/ml, P < 0.01). After covariates adjustment, the sPD-L1 levels were still 20% decreased in the MM group (Table 2). We subsequently estimated the distinguishing capacity of sPD-L1 for miscarriage in this independent cohort. Likewise, the diagnostic performance of sPD-L1 to identify miscarriage patients was also considerable (AUROC of 0.74, 95% CI: 0.59–0.89) (Fig. 1B). Of note, the 95% CI of AUROC in the validation cohort overlapped with the discovery cohort (95% CI: 0.77–0.88), indicating that there was unlikely to be a statistical difference between the two AUCs.
Demographic and clinical characteristics of subjects with induced abortion and missed miscarriage.
| Variables . | Induced abortion (n = 25) . | Missed miscarriage (n = 25) . | P-value . |
|---|---|---|---|
| Maternal age, years | 32.2 ± 3.41 | 29.6 ± 4.32 | .024 |
| <25, N (%) | 0 (0) | 2 (8.0) | |
| 25–29, N (%) | 7 (28.0) | 12 (48.0) | |
| 30–35, N (%) | 11 (44.0) | 8 (32.0) | |
| >35, N (%) | 7 (28.0) | 3 (12.0) | |
| Height, cm | 161.1 ± 4.88 | 159.3 ± 4.48 | .229 |
| Weight, kg | 54.4 ± 6.16 | 58.0 ± 9.60 | .160 |
| BMI, kg/m2 | 21.0 ± 2.11 | 23.0 ± 4.48 | .122 |
| Racial origin, N (%) | |||
| East Asian | 25 (100) | 25 (100) | 1.000 |
| Smoking, N (%) | 0 (0.0) | 0 (0.0) | 1.000 |
| Gestational age, weeks | 6.9 ± 0.95 | 9.5 ± 1.16 | <.001 |
| <7, N (%) | 17 (68.0) | 0 (0.0) | |
| 7–9, N (%) | 8 (32.0) | 15 (60.0) | |
| 10–12, N (%) | 0 (0.0) | 10 (40.0) | |
| Parity, number of pregnancies | 1.2 ± 0.78 | 1.1 ± 1.05 | .649 |
| Nulliparous, N (%) | 4 (16.0) | 9 (36.0) | |
| Parous, N (%) | 21 (84.0) | 16 (64.0) | |
| Number of artificial abortions | 1.1 ± 0.78 | 0.4 ± 0.50 | .003 |
| sPD-L1 (pg/ml) | 62.1 ± 15.53 | 52.7 ± 10.82 | .019 |
| Adjusted sPD-L1 (pg/ml) | 64.1 ± 18.76 | 50.7 ± 18.22 | .040 |
| Variables . | Induced abortion (n = 25) . | Missed miscarriage (n = 25) . | P-value . |
|---|---|---|---|
| Maternal age, years | 32.2 ± 3.41 | 29.6 ± 4.32 | .024 |
| <25, N (%) | 0 (0) | 2 (8.0) | |
| 25–29, N (%) | 7 (28.0) | 12 (48.0) | |
| 30–35, N (%) | 11 (44.0) | 8 (32.0) | |
| >35, N (%) | 7 (28.0) | 3 (12.0) | |
| Height, cm | 161.1 ± 4.88 | 159.3 ± 4.48 | .229 |
| Weight, kg | 54.4 ± 6.16 | 58.0 ± 9.60 | .160 |
| BMI, kg/m2 | 21.0 ± 2.11 | 23.0 ± 4.48 | .122 |
| Racial origin, N (%) | |||
| East Asian | 25 (100) | 25 (100) | 1.000 |
| Smoking, N (%) | 0 (0.0) | 0 (0.0) | 1.000 |
| Gestational age, weeks | 6.9 ± 0.95 | 9.5 ± 1.16 | <.001 |
| <7, N (%) | 17 (68.0) | 0 (0.0) | |
| 7–9, N (%) | 8 (32.0) | 15 (60.0) | |
| 10–12, N (%) | 0 (0.0) | 10 (40.0) | |
| Parity, number of pregnancies | 1.2 ± 0.78 | 1.1 ± 1.05 | .649 |
| Nulliparous, N (%) | 4 (16.0) | 9 (36.0) | |
| Parous, N (%) | 21 (84.0) | 16 (64.0) | |
| Number of artificial abortions | 1.1 ± 0.78 | 0.4 ± 0.50 | .003 |
| sPD-L1 (pg/ml) | 62.1 ± 15.53 | 52.7 ± 10.82 | .019 |
| Adjusted sPD-L1 (pg/ml) | 64.1 ± 18.76 | 50.7 ± 18.22 | .040 |
Data are shown as mean ± SD unless stated otherwise. sPD-L1, soluble program cell death ligand-1. sPD-L1 levels were adjusted for maternal age, gestational age, and history of artificial abortion.
Demographic and clinical characteristics of subjects with induced abortion and missed miscarriage.
| Variables . | Induced abortion (n = 25) . | Missed miscarriage (n = 25) . | P-value . |
|---|---|---|---|
| Maternal age, years | 32.2 ± 3.41 | 29.6 ± 4.32 | .024 |
| <25, N (%) | 0 (0) | 2 (8.0) | |
| 25–29, N (%) | 7 (28.0) | 12 (48.0) | |
| 30–35, N (%) | 11 (44.0) | 8 (32.0) | |
| >35, N (%) | 7 (28.0) | 3 (12.0) | |
| Height, cm | 161.1 ± 4.88 | 159.3 ± 4.48 | .229 |
| Weight, kg | 54.4 ± 6.16 | 58.0 ± 9.60 | .160 |
| BMI, kg/m2 | 21.0 ± 2.11 | 23.0 ± 4.48 | .122 |
| Racial origin, N (%) | |||
| East Asian | 25 (100) | 25 (100) | 1.000 |
| Smoking, N (%) | 0 (0.0) | 0 (0.0) | 1.000 |
| Gestational age, weeks | 6.9 ± 0.95 | 9.5 ± 1.16 | <.001 |
| <7, N (%) | 17 (68.0) | 0 (0.0) | |
| 7–9, N (%) | 8 (32.0) | 15 (60.0) | |
| 10–12, N (%) | 0 (0.0) | 10 (40.0) | |
| Parity, number of pregnancies | 1.2 ± 0.78 | 1.1 ± 1.05 | .649 |
| Nulliparous, N (%) | 4 (16.0) | 9 (36.0) | |
| Parous, N (%) | 21 (84.0) | 16 (64.0) | |
| Number of artificial abortions | 1.1 ± 0.78 | 0.4 ± 0.50 | .003 |
| sPD-L1 (pg/ml) | 62.1 ± 15.53 | 52.7 ± 10.82 | .019 |
| Adjusted sPD-L1 (pg/ml) | 64.1 ± 18.76 | 50.7 ± 18.22 | .040 |
| Variables . | Induced abortion (n = 25) . | Missed miscarriage (n = 25) . | P-value . |
|---|---|---|---|
| Maternal age, years | 32.2 ± 3.41 | 29.6 ± 4.32 | .024 |
| <25, N (%) | 0 (0) | 2 (8.0) | |
| 25–29, N (%) | 7 (28.0) | 12 (48.0) | |
| 30–35, N (%) | 11 (44.0) | 8 (32.0) | |
| >35, N (%) | 7 (28.0) | 3 (12.0) | |
| Height, cm | 161.1 ± 4.88 | 159.3 ± 4.48 | .229 |
| Weight, kg | 54.4 ± 6.16 | 58.0 ± 9.60 | .160 |
| BMI, kg/m2 | 21.0 ± 2.11 | 23.0 ± 4.48 | .122 |
| Racial origin, N (%) | |||
| East Asian | 25 (100) | 25 (100) | 1.000 |
| Smoking, N (%) | 0 (0.0) | 0 (0.0) | 1.000 |
| Gestational age, weeks | 6.9 ± 0.95 | 9.5 ± 1.16 | <.001 |
| <7, N (%) | 17 (68.0) | 0 (0.0) | |
| 7–9, N (%) | 8 (32.0) | 15 (60.0) | |
| 10–12, N (%) | 0 (0.0) | 10 (40.0) | |
| Parity, number of pregnancies | 1.2 ± 0.78 | 1.1 ± 1.05 | .649 |
| Nulliparous, N (%) | 4 (16.0) | 9 (36.0) | |
| Parous, N (%) | 21 (84.0) | 16 (64.0) | |
| Number of artificial abortions | 1.1 ± 0.78 | 0.4 ± 0.50 | .003 |
| sPD-L1 (pg/ml) | 62.1 ± 15.53 | 52.7 ± 10.82 | .019 |
| Adjusted sPD-L1 (pg/ml) | 64.1 ± 18.76 | 50.7 ± 18.22 | .040 |
Data are shown as mean ± SD unless stated otherwise. sPD-L1, soluble program cell death ligand-1. sPD-L1 levels were adjusted for maternal age, gestational age, and history of artificial abortion.
The placental gene expression level of PD-L1 is significantly decreased in MM cases
During pregnancy, PD-L1 is highly expressed in the placenta to promote an immune-deprived environment, thereby protecting the fetus from immune attacks from the mother (PrabhuDas et al., 2015; Ander et al., 2019). Hence, we interrogated whether the expression of placental PD-L1 was distinct between miscarriage patients and healthy gravida. mRNA expression levels of PD-L1 were reduced in the MM group by 3.5-fold, compared to the IA subjects (Fig. 2A). Meanwhile, the protein expression level of placental PD-L1 was also dramatically decreased in the MM group. (Fig. 2B), which was consistent with the IHC staining results (Fig. 2C and D). Our IHC data further demonstrated that PD-L1 was mainly expressed in the outer layer of chorionic villi, which is in line with previous observations that PD-L1 was enriched in placental syncytiotrophoblasts (Veras et al., 2017).
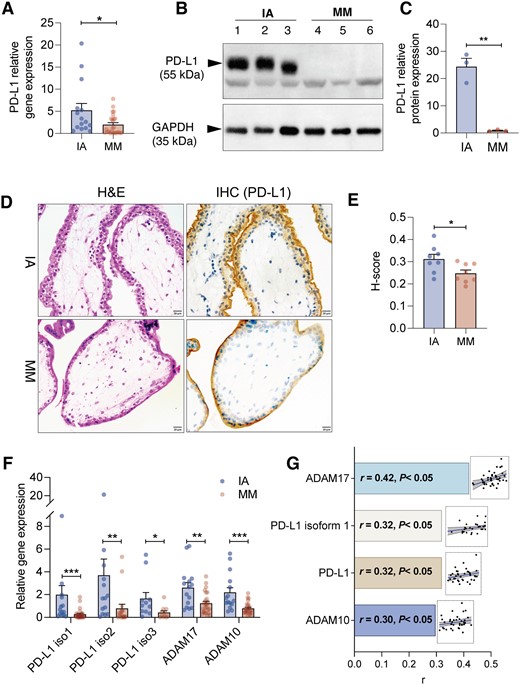
Placental PD-L1 expression is significantly attenuated in patients with miscarriage. (A) The relative gene expression level of PD-L1 in placenta in the induced abortion (IA) group (n = 15) and missed miscarriage (MM) group (n = 25). (B) Western blot analysis and (C) grayscale analysis of PD-L1 expression in IA and MM groups. The placenta samples were collected from three different individuals in each group. (D) Representative image of immunohistochemistry (IHC) staining of PD-L1 expression in placenta samples from two groups. (E) Semi-quantified analysis of PD-L1 expression by IHC staining. The staining intensities were calculated as the ratio of the average sizes of the positive staining area and the count of nuclei from eight different images in each group. (F) The relative gene expression level of PD-L1 isoforms 1–3 and ADAM protease (ADAM17 and ADAM10) in the placenta (n = 15 in the IA group, n = 25 in the MM group). (G) Correlation analysis between serum sPD-L1 level (pg/ml) and log-transformed ΔCt value of PD-L1, PD-L1 isoform-1, ADAM17, and ADAM10. The relative expression level of PD-L1 genes was normalized by β-actin with 2ΔΔCt method). r was determined by Pearson correlation. *P < 0.05, **P < 0.01, ***P < 0.001 by two-tail unpaired Student’s t-test or Mann–Whitney test. Blue or brown dots indicated data from individual subjects in the IA or MM groups, respectively.
Accumulating evidence suggested that sPD-L1 secretion is mainly modulated by isoforms of PD-L1 mRNA splice variants (Gong et al., 2019), or through the process of proteolytic cleave mediated by a disintegrin and metalloproteases 10 and 17 (ADAM10 and ADAM17) (Orme et al., 2020b; Romero et al., 2020). We found gene expression levels of all three isoforms of PD-L1, ADAM17, and ADAM10 were significantly reduced in patients with MM compared to the IA subjects (Fig. 2E). Interestingly, serum concentration of sPD-L1 was positively correlated with placental gene expression (log transformation) of PD-L1 (r = 0.32, P < 0.05), PD-L1 isoform-1 (r = 0.320, P < 0.05), ADAM17 (r = 0.420, P < 0.05), and ADAM10 (r = 0.297, P = 0.045) (Fig. 2F), suggesting placenta is a potential contributor to the circulating sPD-L1 during the gestation period.
Serum sPD-L1 levels predict miscarriage in subjects who underwent IVF
To evaluate the predictive value of serum sPD-L1 level for miscarriage, we reanalyzed changes of sPD-L1 level in a prospective cohort of subjects undergoing IVF. In this study, the serum sPD-L1 level was longitudinally measured before and after ET. The subjects with unexplained infertility cause and incomplete sPD-L1 serum data were excluded from the analysis. We included 16 cases who suffered a miscarriage by Days 30 (ET-30) and 59 subjects who went on to have a live birth.
For women with live birth, the relative change of sPD-L1 between samples was increased at ET-16 and ET-23 (the equivalent of 5 and 6 weeks of gestation) compared to their own baseline, whereas no significant difference was observed in miscarriage cases (Supplementary Fig. S2.A). Although the relative change sPD-L1 between samples in both groups was comparable at ET-9 (the equivalent of 4 weeks of gestation) and ET-16 it was 16.4% reduced at ET-23 in the miscarriage group compared to the live birth group (Supplementary Fig. S2B). We then applied the relative change of sPD-L1 from ET-16 to ET-23 to predict miscarriage outcomes and achieved performance with ROC = 0.73 (95% CI: 0.57–0.88, P < 0.01) (Fig. 3). Therefore, our results suggested that sPD-L1 is a potential biomarker for miscarriage, which predicts the occurrence of miscarriage 2 weeks in advance.

The relative change of sPD-L1 level between samples after embryo transplantation (ET) predicates miscarriage. Applying the relative change between samples of sPD-L1 at ET-16 to ET-23 for early detection of miscarriage. The sample size of women with miscarriage and live birth is 16 and 59, respectively.
Discussion
sPD-L1 has been applied as a predictive or prognostic biomarker for types of cancer (Daassi et al., 2020; Orme et al., 2020a; Han et al., 2021). During gestation, accumulating evidence has shown that serum sPD-L1 levels progressively increase with maternal age and gestational weeks (Enninga et al., 2018; Chen et al., 2011). In our cross-sectional study, we found that serum sPD-L1 was 50% decreased in MM patients compared to HP women and remain distinctly lower after adjustment of maternal and gestational age. Moreover, our data in the prospective study further exhibited that the relative change between day 16 and day 23 samples of sPD-L1 after IVF was significantly reduced in miscarriage cases compared to those with live birth, which agreed with the observation that serum sPD-L1 level was gradually elevated after a successful ET in humans (Zhang et al., 2022b). Intriguingly, growing evidence has proven that sPD-L1 is a general immunomodulatory molecule regulating the holistic immune (Xu et al., 2020; Zhang et al., 2020). sPD-L1 secreted from trophoblast cells promoted decidual macrophage polarization toward an anti-inflammatory status (Zhang et al., 2020). Okuyama et al. showed that inflammatory cytokines production from human peripheral blood mononuclear cells was inhibited when co-incubated with the serum of pregnant women (Okuyama et al., 2019). Therefore, our results suggest that the deprivation of immune suppression as evidenced by lower sPD-L1 level contributes to the development of miscarriage.
The success of pregnancy requires finely adapted immune homeostasis, which heavily relies on the immunologic privilege of the placenta. Substantial evidence has proved that PD-L1 is highly expressed in the placenta's trophoblast cells and gradually rises throughout gestation to promote the immunosuppression microenvironment (Holets et al., 2006). By contrast, blocking the PD-L1 signal or depleting PD-L1 in mice significantly exacerbated embryo resorption and decreased the fetomaternal tolerance (Guleria et al., 2005). Except for immunoregulation effects of PD-L1 in placenta, an in vitro study demonstrated that blocking PD-L1 significantly impaired migration and invasion abilities in HTR8/SVneo cell line (Zhang et al., 2022a). In our study, we identified placenta in MM patients exhibited downregulated gene expression of PD-L1 and gene-regulated sPD-L1 production. In conjunction with our findings, women with recurrent miscarriage have decreased PD-L1 expression at both mRNA and protein levels in placental villi (Li et al., 2015). Interestingly, our data in prospective IVF cohort revealed that the relative change of sPD-L1 between samples was dramatic reduced from ET-16 to ET-23, which is a critical time for trophoblasts migration and invasion and largely regulated by placental PD-L1 (Knofler et al., 2019; Zhang et al., 2022a). Considering our findings showed that serum sPD-L1 level is closely associated with expression levels of PD-L1-related genes in the placenta, therefore, these results indicate that aberrance in placental PD-L1 expression may function as potentially relevant pathogenesis for pregnancy loss, of which underlying mechanisms warrant further explorations.
Strengths and limitations
This is the first study to have comprehensively evaluated the soluble form of PD-1 and PD-L1 in MM. A key strength of this study is to compare serum sPD-L1 and sPD-1 concentrations in two independent cohorts with considerable sample sizes. Moreover, previous studies on miscarriage mostly focused on the alternations of PD-1/PD-L1 expression in immune cells (Morita et al., 2020; Wang et al., 2020), whereas the PD-L1 expression in the placenta has been under-investigated. Our study examined the placental PD-L1 expression at both gene and protein levels. Another advantage is that we have revealed the correlation between the serum sPD-L1 level and placental PD-L1-related gene expression level using matched samples. Importantly, we further evaluated the prediction value of maternal sPD-L1 levels on miscarriage using a prospective cohort of IVF, which highlighted sPD-L1 is a promising biomarker for the early detection of miscarriage.
Several limitations of our study need to be acknowledged. First, the study was confined to East Asian pregnant women, which may limit the generalizability of the study conclusions. Further studies are required for the evaluation of the serum sPD-L1 levels in different ethnic groups. Secondly, our study indicates the need to further explore the dynamic changes of circulating sPD-L1 between healthy pregnancies and patients with other types of pregnancy loss. Moreover, the prediction value of serum sPD-L1 on miscarriage outcomes was evaluated in women subjected to IVF, which is different from nature conception. Hence, our current conclusions still should be cautiously reviewed. It is imperative to evaluate the predictive performance of sPD-L1 on miscarriage in large prospective pregnant cohorts. In addition, though we observed a reduction in placental gene expression levels of PD-L1, PD-L1 mRNA splice variants, and ADAM protein in miscarriage, however, mechanistic studies are needed to unearth the pathway for the downregulation of placental PD-L1 expression and attenuation of sPD-L1 production in miscarriage.
Conclusion
In summary, circulating sPD-L1 level is significantly and independently decreased in patients with miscarriage compared to healthy pregnancies. By employing a prospective cohort of IVF, we identified that serum sPD-L1 level at the early stage of pregnancy is a potential predictive biomarker for the early detection of miscarriage. Our results further imply that monitoring the dynamic alternations in maternal sPD-L1 levels at early gestation may serve as a possible aid for miscarriage management, which would enable the provision of timely medical treatment and appropriate psychotherapy support for patients in clinical implementation. Moreover, placental PD-L1 and relative gene expression levels are downregulated upon miscarriage, indicating that dysfunction in PD-L1 signal is a potential pathogen to trigger pregnancy loss, raising the possibility of developing therapeutic strategies to prevent pregnancy loss by targeting PD-L1. Our findings suggest that PD-L1 not only sustains immunotolerance at the fetomaternal interface but also suppresses the holistic immune response through the secretion of sPD-L1. Importantly, our results reveal the importance of the PD-L1 signals in sustaining immunotolerance for pregnancy maintenance, which extends the current understanding of PD-L1 from the field of immune oncology to pregnancy complications and confers a novel perspective to investigating gestational immune regulation.
Data availability
The data will be available and shared upon reasonable request to a corresponding author.
Acknowledgements
We appreciate all participants who enrolled in this study.
Authors’ roles
Q.L. designed the study and organized the cohort. C.C. conducted experiments to determine the serum PD-L1 concentration and placenta PD-L1 expression. J.W. interpreted the results and prepared tables. L.C.P. and C.C.W. supervised the statistical analysis and reviewed the manuscript. T.C.L. and T.Z. provided the data of the prospective cohort of IVF. X.G., X.W., Z.Y., Q.Z., and Y.Y. helped with the recruitment of volunteers and collected and processed clinical samples. L.S. helped with the ELISA. J.L. and J.Y. performed IHC staining. D.Y. supervised the biochemistry experiments and prepared the manuscript. Y.W. conceptualized and designed the project, interpreted the results, prepared figures, and wrote and edited the manuscript.
Funding
This study was financially supported by grants from the Subject Innovation Team of Shaanxi University of Chinese Medicine (2019-Y502), General Research Fund (14122021), and Key Laboratory of Model Animal Phenotyping and Basic Research in Metabolic Diseases (2018KSYS003).
Conflict of interest
The authors have nothing to declare.
References
Author notes
Qin Li and Cuishan Chen contributed equally to this work.
- apoptosis
- pregnancy
- body mass index procedure
- signal transduction
- gene expression
- fertilization in vitro
- missed abortion
- abortion, spontaneous
- biological markers
- embryo transfer
- genes
- gestational age
- ligands
- maternal age
- medicine, chinese traditional
- mothers
- pregnancy maintenance
- therapeutic immunosuppression
- placenta
- prenatal care
- pregnancy loss
- intravenous fluid
- programmed cell death 1 ligand 1
- east asian people



