-
PDF
- Split View
-
Views
-
Cite
Cite
A Rehfeld, H Frederiksen, R H Rasmussen, A David, J Chaker, B S Nielsen, J E Nielsen, A Juul, N E Skakkebæk, D M Kristensen, Human sperm cells can form paracetamol metabolite AM404 that directly interferes with sperm calcium signalling and function through a CatSper-dependent mechanism, Human Reproduction, Volume 37, Issue 5, May 2022, Pages 922–935, https://doi.org/10.1093/humrep/deac042
Close - Share Icon Share
Abstract
Do paracetamol (N-acetyl-para-aminophenol (APAP) or acetaminophen) and/or its metabolites affect human sperm Ca2+-signalling and function?
While APAP itself does not interact with Ca2+-signalling in human sperm, its metabolite N-arachidonoyl phenolamine (AM404), produced via fatty acid amide hydrolase (FAAH), interferes with human sperm Ca2+-signalling and function through a suggested CatSper channel-dependent action.
Studies have shown that adult men with high urinary levels of over-the-counter mild analgesic APAP have impaired sperm motility and increased time-to-pregnancy.
This study consists of (i) an in vivo human pharmaceutical APAP exposure experiment to understand to what degree APAP reaches the sperm cells in the seminal fluid; (ii) in vitro calcium imaging and functional experiments in freshly donated human sperm cells to investigate CatSper channel-dependent activation by APAP and its metabolites; and (iii) experiments to understand the in situ capabilities of human sperm cells to form APAP metabolite AM404.
Three healthy young males participated in the in vivo human exposure experiment after prior consent. Human semen samples were provided by healthy young volunteer donors after prior consent on the day of the in vitro experiments.
Pharmaceutical APAP exposure reaches the seminal plasma in high micromolar concentrations and accumulates in the seminal plasma between 3 and 5 days of exposure (P-value 0.023). APAP and its primary metabolite 4-aminophenol (4AP) do not interact with human sperm Ca2+-signalling. Instead, the APAP metabolite AM404 produced via FAAH interferes with human sperm Ca2+-signalling through a CatSper-dependent action. Also, AM404 significantly increases sperm cell penetration into viscous mucous (P-value of 0.003). FAAH is functionally expressed in human sperm cells in the neck/midpiece region, as evidenced by immunohistochemical staining and the ability of human sperm cells to hydrolyse the fluorogenic FAAH substrate arachidonyl 7-amino, 4-methyl coumarin amide in an FAAH-dependent manner. Importantly, human sperm cells have the capacity to form AM404 in situ after exposure to 4AP (P-value 0.0402 compared to vehicle-treated sperm cells).
The experiments were conducted largely in vitro. Future studies are needed to test whether APAP can disrupt human sperm function in vivo through the action of AM404.
We hypothesize that these observations could, at least in part, be responsible for the negative association between male urinary APAP concentrations, sperm motility and time-to-pregnancy.
D.M.K. is funded by the Lundbeck Foundation, grant number R324-2019-1881, and the Svend Andersen Foundation. A.R. is funded by a BRIDGE—Translational Excellence Programme grant funded by the Novo Nordisk Foundation, grant agreement number: NNF18SA0034956. All authors declare no competing interests.
N/A.
Introduction
Concern has been raised over decreased male reproductive health in the Western world (Hull et al., 1985; Brown et al., 2019). The exact mechanisms are not well understood, but evidence suggests that exposure to endocrine disrupters both during foetal and postnatal life can result in decreased sperm counts (Skakkebaek et al., 2016). Furthermore, dysfunction of mature sperm cells, especially impaired motility, has been shown to be a significant contributing factor to male infertility (Brown et al., 2019). The CatSper Ca2+-channel regulates important sperm cell functions including sperm motility, is essential for male fertility (Brown et al., 2019; Wang et al., 2021) and has been shown to be promiscuously activated by a plethora of structurally diverse endocrine-disrupting chemicals (Schiffer et al., 2014). This suggests that exposure of human sperm cells to endocrine disrupters could lead to impaired sperm function.
Studies have shown that adult men with high urinary levels of paracetamol (N-acetyl-para-aminophenol (APAP) or acetaminophen) experience increased time-to-pregnancy, impaired sperm motility and increased sperm DNA fragmentation (Smarr et al., 2016, 2017). APAP is one of the most commonly used and environmentally released pharmaceutical drugs, and biomonitoring studies have indicated ubiquitous human exposure to APAP either through direct exposure to APAP or through endogenous conversion of aniline, a major industrial chemical that is converted in vivo into APAP (Modick et al., 2014; Holm et al., 2015; Kristensen et al., 2016; David et al., 2021). Nonetheless, no in-depth studies have investigated the possible mode of action by which APAP exposure in men may interfere with human sperm function and thereby male fertility.
Therefore, in this study, we examined how APAP and its active metabolites affect human sperm cell function. Because of the intrinsic great challenge in identifying effects of xenobiotics in the adult human, we utilized a combination of three interconnected approaches: (i) an invivo human exposure experiment to understand to what degree environmental and pharmaceutical exposure to APAP reach the sperm cells in the seminal fluid; (ii) invitro calcium imaging and functional experiments in freshly donated live human sperm cells to investigate CatSper-dependent activation by APAP and its metabolites; and (iii) experiments to understand the insitu capabilities of the human sperm cell to form the APAP metabolite AM404. The collective results revealed that while APAP itself does not interact with the sperm cell function per se, its metabolite AM404 produced via enzymes in the sperm cell interfered with human sperm cell Ca2+-signalling and function through a CatSper-dependent action.
Materials and methods
Reagents and chemicals
APAP, 4-aminophenol (4AP), arachidonic acid (AA) and PF750 were purchased from Sigma-Aldrich (MO, USA) and dissolved in dimethyl sulfoxide (DMSO) at a stock concentration of 10 mM. AM404 was purchased from Sigma-Aldrich (MO, USA), the ethanol was evaporated under a gentle stream of nitrogen and DMSO was immediately added to yield a stock concentration of 10 mM. These stock solutions were aliquoted into small plastic tubes and stored at −20°C until use. Importantly, 4AP was found not to be stable in DMSO at −20°C for longer periods of time. The 4AP stock solution slowly changed to a darker colour and started interfering with calcium signalling in human sperm (data not shown), possibly due to novel molecules formed through auto-oxidation, as described before (Hegedus and Nayak, 1991). Because of this, we only used 4AP dissolved on the same day, just prior to the experiments. Arachidonyl 7-amino, 4-methyl coumarin amide (AAMCA) was purchased from Cayman Chemicals (MI, USA) and dissolved in DMSO at a stock concentration of 10 mM. AAMCA was aliquoted into small plastic tubes and stored at -80°C until use. Methyl arachidonyl fluorophosphonate (MAFP) was purchased from Cayman Chemicals (MI, USA), the methyl acetate solvent was evaporated under a gentle stream of nitrogen, and DMSO was immediately added to yield a stock concentration of 10 mM. The MAFP was aliquoted into small plastic tubes and stored at -20°C until use. Progesterone was obtained from Sigma-Aldrich (MO, USA), dissolved in DMSO at stock concentrations of 20 mM, aliquoted into small plastic tubes and stored at −20°C until use. RU1968 was obtained from Prof. Timo Strünker upon request, dissolved in DMSO at a stock concentration of 10 mM, aliquoted into small plastic tubes and stored at −20°C until use. Fluo-4, Acetoxymethyl Ester (AM) and 2′,7′-Bis-(2-Carboxyethyl)-5-(and-6)-Carboxyfluorescein, AM (BCECF) were purchased from Invitrogen (CA, USA). Fluo-4, AM, and BCECF, AM, were stored at −20°C until use and freshly dissolved just prior to experiments. Human serum albumin (HSA) was obtained from Irvine Scientific (CA, USA) and stored at 4°C. For ultra-high-performance liquid chromatography coupled to electrospray ionization high-resolution mass spectrometry (UHPLC-ESI-HRMS) experiments, all solvents were high-performance liquid chromatography grade, purchased from Biosolve Chime (Dieuze, France). Strata-X Polymeric Reversed-Phase cartridges (200 mg, 3 ml) were supplied by Phenomenex (Le Pecq, France).
Semen, blood and urine samples for liquid chromatography–mass spectrometry measurements of APAP and metabolites
The invivo study was designed as a 5-day exposure study with blood, semen and urine collected before, during and after the exposure. Three healthy male volunteers were included in the study and instructed to avoid using APAP before entering the study. The three male volunteers visited the Department of Growth and Reproduction, Rigshospitalet, on Day 1 (Monday morning) to deliver a semen, urine and venous blood sample. After delivering the samples, the volunteers were instructed to take orally APAP 1 g × 4 per day (Panodil®, GlaxoSmithKline, UK) following the maximally recommended dose. The volunteers then visited the clinic on Day 3 (Wednesday morning) and Day 5 (Friday morning) to deliver semen, urine and venous blood samples. After the last sample on Day 5, the volunteers were advised to stop using APAP. Semen samples were produced by masturbation and ejaculated into wide-mouthed plastic containers. Urine samples were collected in plastic containers. Blood samples were drawn by an experienced laboratory technician. After separation of sperm cells from seminal fluid and of blood cells from serum using centrifugation, the seminal fluid, serum and urine samples were stored at −20°C until chemical analysis. No subjects reported any adverse signs of taking the medication nor did any blood parameters indicate or suggest adverse effects (data not shown).
Semen samples and sperm purification for sperm cell-based laboratory experiments
Healthy male volunteer donors donated semen samples at the Department of Growth and Reproduction, Rigshospitalet, in the morning before an experiment. After allowing the samples to liquefy for 15–30 min at 37°C, the motile sperm cell fraction was isolated from the semen by the swim-up method in human tubular fluid (HTF+) with the composition: 97.8 mM NaCl, 4.69 mM KCl, 0.2 mM MgSO4, 0.37 mM KH2PO4, 2.04 mM CaCl2, 0.33 mM Na-pyruvate, 21.4 mM Na-lactate, 2.78 mM glucose, 21 mM HEPES and 4 mM NaHCO3, adjusted to pH 7.3–7.4 with NaOH, as described (Rehfeld et al., 2019). Hereafter, the sperm cell sample was adjusted to 10 × 106 sperm cells/ml in HTF+-medium and allowed to incubate for at least 1 h with 3 mg/ml HSA added to the HTF+-medium.
Measurement of changes in [Ca2+]i
Changes in the free intracellular Ca2+ concentration [Ca2+]i in a population of human sperm cells were measured in 384 multi-well plates in a fluorescence plate reader (Fluostar Omega, BMG Labtech, Germany) at 30°C as described in Rehfeld et al. (2019). Briefly, an aliquot of sperm cells (10 × 106 sperm cells/ml in HTF+-medium with 3 mg/ml HSA) was incubated with the fluorescent Ca2+ indicator Fluo-4, AM (10 μM) for 45 min at 37°C and excess dye was removed by centrifugation (700g, 10 min, RT). The sperm pellet was then re-suspended in HTF+-medium to 5 × 106 sperm cells/ml. Aliquots of 50 μl were pipetted into the wells of the multi-well plate. Fluorescence was excited at 480 nm and emission was recorded at 520 nm with bottom optics. Fluorescence was recorded before and after addition of compounds and controls to duplicate wells. Changes in Fluo-4 fluorescence are shown as ΔF/F0 (%), indicating the percentage change in fluorescence (ΔF) with respect to the mean basal fluorescence (F0) before addition of compounds and controls. For estimation of dose–response relationships, 10 compound dilutions with a fixed 1:2 dilution ratio were made from a high starting concentration, known to induce a maximal response in the assay.
Measurement of fatty acid amide hydrolase-activity
Fatty acid amide hydrolase (FAAH)-activity in a population of human sperm cells was measured in 384 multi-well plates in a fluorescence plate reader (Fluostar Omega, BMG Labtech, Germany) at 30°C using AAMCA, a fluorogenic substrate for FAAH (Ramarao et al., 2005). An aliquot of sperm cells (10 × 106 sperm cells/ml in HTF+-medium with 3 mg/ml HSA) was mixed with AAMCA (40 μM) and aliquots of 50 μl were loaded to the wells of the multi-well plate. Fluorescence was excited at 355 nm and emission was recorded at 460 nm with bottom optics. Fluorescence was recorded before and after addition of compounds and controls to duplicate wells. Changes in AAMCA fluorescence are shown as ΔF/F0 (%), indicating the percentage change in fluorescence (ΔF) with respect to the mean basal fluorescence (F0) before addition of compounds and controls.
Measurement of changes in pHi
Changes in pHi in human sperm cells were measured in 384 multi-well plates in a fluorescence plate reader (Fluostar Omega, BMG Labtech, Germany) at 30°C as in Schiffer et al. (2014). An aliquot of sperm cells (10 × 106 sperm cells/ml in HTF+-medium with 3 mg/ml HSA) was mixed with the fluorescent pH indicator BCECF, AM (10 μM) for 15 min at 37°C. Fluorescence was excited at 440 and 480 nm (dual excitation) and emission was recorded at 520 nm with bottom optics. Fluorescence was recorded before and after addition of compounds and controls. Changes in the ratio of BCECF fluorescence between the 440 and 480 nm excitation are shown as ΔR/R0 (%) and indicate the percentage change in the ratio of fluorescence between the two modes of excitation (ΔR) with respect to the mean basal ratio of fluorescence between the two modes of excitation (R0) before addition of compounds and controls.
Penetration into viscous medium
Sperm penetration into viscous medium was assessed with 4000 cP methylcellulose (1% w/v) as an artificial viscous medium as in Rehfeld et al. (2018). The 4000 cP methylcellulose was prepared by adding 10 mg/ml to HTF+ and mixing it by rotation overnight at RT. The methylcellulose (1% w/v) medium was hereafter introduced into glass capillary tubes (borosilicate microslides (VitroTubes) 0.20 mm × 2.0 mm × 10 cm (VitroCom, USA)) by capillary forces. Care was taken to prevent air bubbles from entering the glass tubes. The filled end of the glass was sealed with wax (Hounisens laboratorieudstyr A/S, Denmark) and the other end was cut within the part filled with methylcellulose, just before the methylcellulose-air transition. Hereafter additional wax was added to force out a small droplet of methylcellulose at the cut end. This cut end was then placed vertically in a 1.4-ml tube (Eppendorf, Germany) containing 200 μl non-capacitated sperm sample (10 × 106/ml in HTF+-medium with 3 mg/ml HSA). Just prior to the insertion of the glass tubes, AM404 (10 µM), 5 µM progesterone (positive control) or 0.1% DMSO (negative vehicle control) were added to the sample. We chose to test AM404 at a dose of 10 µM as this is above the observed EC50, but still low enough to avoid other putative unwanted and unspecific effects on sperm motility. The sperm cells were allowed to penetrate into the methylcellulose (1% w/v) for 60 min at 37°C. The glass tubes were then removed, wiped to remove residual sperm cells from the surface of the glass, placed under a UV lamp (302 nm) in a BIO-RAD Universal Hood III (BIO-RAD, CA, USA) for 3 min to paralyze the sperm cells (Rehfeld et al., 2020), and hereafter examined using phase-contrast optics on an Olympus BX45 microscope at a total magnification of ×200 (Olympus, Denmark). The amount of sperm cells was counted at 2-cm distance from the opening of the tube, with three fields in each of four planes counted. Throughout the study, all samples were counted by the same observer.
Liquid chromatography–mass spectrometry measurements of APAP and metabolites
Human urine, blood plasma and seminal plasma from the three male volunteers were analysed for the total (free and conjugated) content of three aniline metabolites: N-acetyl-4-aminophenol (APAP), CAS No. 103-90-2; N-acetyl-2-aminophenol (NA2AP), CAS No. 614-80-2; Acetanilide (ACA), CAS No. 103-84-4. For analysis of aniline metabolites, we developed and validated a new method based on previously published methods (Modick et al., 2013; Dierkes et al., 2014) using isotope diluted online-TurboFlow-liquid chromatography mass spectrometry (LC-MS/MS) equipped with a probe for heated electrospray ionization running in positive mode and with prior enzymatic de-conjugation. The preceding enzymatic de-conjugation was done by a mixture of ß-glucuronidase (Escherichia coli K12) and sulfatase from Aerobacter Aerogenes. Method validation and limit of detection (LOD) were determined by using the approach described by the International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) guidelines (ICH, 2005). Limit of detection for the three aniline metabolites in urine was: NA4AP, 0.48 µg/l; NA2AP, 2.96 µg/l; and ACA, 0.08 µg/l. The same LODs were used for measurement of the metabolites in blood plasma and seminal plasma. The samples were analysed for the three metabolites in one batch, also including standards for calibration curves, three blanks and three times three urine pool controls, where one of them was spiked with a mixture of native standards, ending up with three urine control pools with aniline metabolites in three different concentration levels. For all three metabolites, the relative standard deviation in all three control levels ranged from 3.9% to 11%. All chemical analyses were performed at the Department of Growth and Reproduction, Rigshospitalet.
UHPLC-ESI-HRMS identification of AM404
Aliquots of 300 µl of swim-up purified human sperm cells (10 × 106/ml in HTF+-medium with 3 mg/ml HSA) were treated with either 10 µM 4AP, 10 µM 4AP+AA, 100 µM APAP or vehicle control at 37°C for 240 min. The aliquots were centrifuged for 10 min at 3000g, and hereafter the top 250 µl of the supernatant was removed, flash frozen in liquid nitrogen and kept at -80°C until analysis. The supernatants from three male volunteer semen donors were analysed using the same method as described previously (David et al., 2021). Briefly, the samples (100 µl) were diluted to 4 ml in HPLC grade water, acidified with 1% formic acid and extracted using Strata-X solid-phase extraction (SPE). A mix of internal standards was added to the sample prior to the SPE. Cartridges were conditioned with 4 ml methanol and 4 ml of HPLC grade water. Strata-X cartridges were then loaded with the 4 ml of diluted supernatant and washed with 2 × 3 ml of 95/5 HPLC grade water/methanol (v/v). SPE phases were dried under vacuum and elution was performed with 4 ml of methanol. The eluate was evaporated to dryness, reconstituted in 50 µl 80/20 water/acetonitrile (v/v) and stored at -80°C until analysis. Extracts were profiled using an Exion UHPLC system (AB Sciex, USA) coupled to an AB Sciex X500R Q-TOF-MS system (Sciex technologies, Canada), equipped with a DuoSpray ion source. Extracts of 2 µl were loaded and separated on an Acquity UHPLC HSS-T3 column, 1.0 mm × 150 mm × 1.8 µm, maintained at 40°C (Waters Technologies, Saint Quentin, France). HPLC grade water was used as solvent A and acetonitrile was used as solvent B. The Q-TOF was recalibrated automatically after each sample using an ESI positive/negative calibration solution via a calibration delivery system. As a first step, all the samples were analysed in full scan experiment (50–1100 Da) in both − and + ESI modes. MS/MS mass fragmentation information for chemical elucidation was obtained by further analysis of selected samples in sequential window acquisition of theoretical mass spectrum (SWATH) and a data-dependent acquisition method using the molecular ion of AM404 as the precursor. One workup blank sample (i.e. extraction with HPLC grade water instead of sample) per analytical batch was prepared to monitor for background contaminants. Quality control samples comprising a composite sample were prepared in order to monitor for UHPLC-ESI-TOF-MS repeatability and sensitivity during analysis of a sample run. Solvent blank samples (acetonitrile/H2O (20:80)) were also injected to ensure that there was no carryover in the UHPLC system that might affect adjacent results in analytical runs. Positive control samples consisting of supernatant spiked with AM404 were used to ensure that the sample preparation method could recover the chemical. Annotation of AM404 was determined from its accurate mass (<5 ppm), isotopic fit, and from comparison with the Retention time (Rt) from the standard compounds (<0.1 min; Supplementary Fig. S1). Additionally, MS/MS fragmentation pattern of AM404 as standard was collected and was consistent with MS/MS patterns observed in the literature (Muramatsu et al., 2016) as well as with an experimental MS/MS profile provided by Metlin (Guijas et al., 2018). Relevant MS/MS patterns could be observed for the standard AM404 but only at concentrations higher than the ones observed in the supernatant samples. Nevertheless, the accurate mass (<5 ppm), isotopic fit and the match with the Rt from the standard compounds, as well as the context of the study (i.e. exposure to AM404 precursors is associated with relevant increases of the signals corresponding to the annotated AM404), provided confidence in the AM404 annotation. Peak integration and normalization were carried out to study the formation of AM404 within the different groups. Peaks corresponding to AM404 were manually integrated using the Sciex OS Analytics tool. The integrated peak area of individual markers was normalized to the closest internal standard (i.e. diazinon-d10, Rt = 45.4 min vs. 46.2 min for AM404).
Immunohistochemical staining of FAAH in human sperm cells and the epididymis
The FAAH reactions on cytospin of human sperm cells and human epididymis sections are surplus material from a previous study where the antibody underwent extensive validation (Nielsen et al., 2019). Methods are as described in Nielsen et al. (2019). Briefly, cytospin of human semen samples was obtained as previously described (Hoei-Hansen et al., 2007). Formalin-fixed semen cytospin was stored over night at 5°C before immunohistochemistry. Antigen retrieval was performed in TEG buffer (Tris 6.96 g, EGTA 0.959 g in 5 L, pH 8.5) in a microwave oven 1 min at 750 W followed by 15 min at 350 W. Endogenous peroxidase was blocked with 0.5% (v/v) H2O2 in water for 30 min. Blockade for unspecific antibody binding was accomplished with 5% BSA (w/v) in horse serum (20% v/v) ImmPRESS (Vector Laboratories, Burlingame, CA, USA) and Tris-buffered saline (80% v/v) for 30 min. Primary antibody against FAAH (Sigma HPA007425) 1:150 in TBS was applied to the cytospins overnight at 5°C and 1 h at RT next morning. Secondary antibody ImmPRESS (MP-7401, Vector Laboratories, Burlingame, CA, USA) was applied for 30 min before development with 3-amino-9-ethylcarbazole. Thorough washing in TBS was carried out between all steps except blockade for unspecific antibody binding and primary antibody. Controls were performed on separate cytospins by omission of primary antibody from the protocol and the spermatozoa were here negative. Counterstaining was done using Meyers haematoxylin.
After deparaffination, human epididymal sections were demasked in Citrate buffer in a pressure cooker for 10 min at 110°C, blocked for endogen peroxidase 1% (v/v) in methanol for 30 min and rinsed in running tab water and TBS before blockade against unspecific binding with 0.5% skimmed milk (w/v)/TBS for 30 min. FAAH antibody 1:100 in 0.5% skimmed milk/TBS was applied to the sections and left over night at room temperature. After TBS wash, the section was exposed for secondary antibody (MP-7401, ImmPRESS HRP reagent, 30 Ingold Road Burlingame, CA, USA) for 1 h, then washes in TBS and stained with 3-amino-9-ethylcarbazole. Sections treated as above but without FAAH antibody treatment were negative.
Ethical approval
Semen, blood and urine samples for LC-MS/MS measurements of APAP and metabolites were donated by healthy human volunteers after their prior consent. No data on the fertility status or the general health of donors were provided. Each donor received a compensation of 500 DKK (about 75 US dollars) per visit to the clinic for delivery of samples for their inconvenience. The study was approved by the regional scientific ethical committee of the Capital Region of Denmark with permit number H-19037055 and journal nr. protocol nr.: 17003845.
Semen samples for sperm cell-based laboratory experiments were donated by healthy human volunteers after their prior consent. No data on the fertility status or the general health of donors is provided. Each donor received a compensation of 500 DKK (about 75 US dollars) per sample for their inconvenience. All samples were used for experiments on the day of delivery and destroyed immediately after the laboratory analyses were conducted. The study was approved by the regional scientific ethical committee of the Capital Region of Denmark with permit number H-19089581.
Human epididymal samples were obtained from testis cancer patients (orchiectomy specimens) at Copenhagen University Hospital. All patients have given informed content for donating the residual tissues for research and the permit number from the research ethics committee of the Capital region of Copenhagen is H-16019636. Further details can be found in Nielsen et al. (2019).
Statistical analysis
APAP levels in seminal plasma and blood plasma, as well as in seminal plasma from Day 3 and Day 5, were compared using a ratio paired two-tailed T-test. Data from the sperm penetration into viscous medium assay were transformed with the natural logarithm to avoid variance heterogeneity and to obtain approximate normality of model residuals. The data on AM404 normalized area in sperm sample supernatants were used untransformed. These data were then analysed using repeated measures one-way ANOVA with Geissner–Greenhouse correction. P-values were corrected for multiple comparison type I error inflation by Dunnett’s method. Statistical analyses were performed using GraphPad Prism 9.2.0 (GraphPad Software Inc., USA).
Results
APAP is transferred to the seminal fluid at high micromolar concentrations
We conducted a longitudinal 5-day in vivo exposure study with three young healthy men using the maximally recommended APAP dosing regimen of 1 g four times per day to identify levels in urine, blood plasma and seminal plasma. Before administration of APAP, baseline values showed that the men had an average background exposure of 1.1 µg/ml (7.6 µM) in urine, 5.8 ng/ml (38.3 nM) in blood plasma and 10 496 ng/ml (69.7 nM) in seminal plasma (Fig. 1). Similarly, related NA2AP was found as a background exposure in all three men in approximately the same concentrations as APAP, while metabolite ACA was not detected above the limit of detection in any of the samples. After daily APAP pharmaceutical exposure of 1 g × 4, the levels in urine increased to an average of 1.55 mg/ml (10 mM) at Day 3 (Fig. 1), while levels of NA2AP levels had not increased. Correspondingly, the levels of APAP in blood plasma and seminal plasma had increased to 16.9 µg/ml (111.9 µM) and 36.5 µg/ml (241.7 µM), respectively (Fig. 1). While the APAP levels did not increase in urine and blood plasma between Days 3 and 5 of the oral exposure regimen, the seminal plasma concentration increased significantly (P-value 0.023) by ∼30% to 45.8 µg/ml (303.3 µM; Fig. 1). Despite this increase, the APAP levels in seminal plasma were not significantly higher than in blood plasma on Day 5 (P-value 0.203). Taken together, these data show a background environmental exposure to APAP in urine, blood plasma and seminal plasma and that during oral pharmaceutical exposure, APAP accumulates in the seminal plasma to high micromolar levels.

Paracetamol levels after oral intake. Levels of paracetamol (N-acetyl-para-aminophenol (APAP)) in the urine (a), blood (b) and seminal plasma (c) in three adult men before, during and after 5 days intake of APAP 4 g/day. All men had a background exposure to APAP on Day 1, before initiating the oral intake of APAP 4 g/day (a–c). While concentrations reached a steady level in blood plasma on Day 3 (b), APAP significantly accumulated in seminal plasma during the 5 days of exposure, (*) P-value of 0.023 from a ratio paired t-test between Days 3 and 5 APAP levels (c).
APAP metabolite AM404 induces a dose-dependent increase in intracellular calcium through activation of the CatSper channel
Since APAP is transferred from the blood plasma to the seminal plasma in high levels, we next investigated whether sperm cells reacted to APAP. Exposing human sperm cells to increasing concentrations of APAP with a maximal concentration of 1000 µM only induced a very modest calcium influx at the two highest concentrations (500 and 1000 µM) and did not inhibit the calcium influx induced subsequently by the endogenous CatSper agonist progesterone (Fig. 2a). APAP is metabolized in the body (mainly by the liver) through a variety of biotransformation processes, which include the formation of the primary amine 4AP by de-acetylation (Högestätt et al., 2005). As with APAP, 4AP only resulted in minor changes in the calcium influx in the sperm cells at the highest concentrations and did not inhibit the calcium influx induced subsequently by the endogenous CatSper agonist progesterone (Fig. 2b).
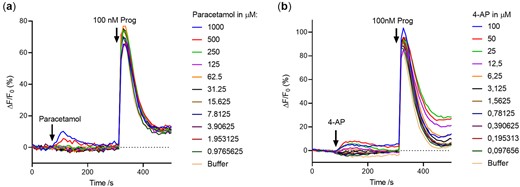
Effect of paracetamol and 4-aminophenol (4AP) on Ca2+-signalling in human sperm cells. Paracetamol (N-acetyl-para-aminophenol (APAP)) (a) and metabolite 4AP (b) only induce a very modest calcium influx at the highest concentrations and exert a very modest effect on the subsequent endogenous ligand progesterone (Prog) induced calcium influx. Representative graphs from a single experiment (n = 3).
4AP can be further conjugated with AA by the enzyme FAAH to form the bioactive fatty acid amide N-arachidonoyl phenolamine (AM404; Högestätt et al., 2005). Exposing the sperm cells to AM404 resulted in a dose-dependent induction of calcium influx with a mean EC50 of 3.9 µM and a mean EC02 (estimate of lowest effective dose) of 25.77 nM. Furthermore, a weak dose-dependent inhibition of the subsequent progesterone-induced peak calcium influx was observed (Fig. 3a and b). Compared to the calcium influx induced by the endogenous CatSper agonist progesterone, which is rapid and transient, the calcium influx induced by AM404 was sustained. Moreover, the effect of AM404 on the sperm cells did not resemble that by AA alone, which induced a calcium influx into the cells at a mean EC50 of 1.8 µM. The AA induced calcium influx had kinetics similar to the calcium influx induced by progesterone (Fig. 3c and d). These data show that while APAP does not by itself interact with calcium signalling in sperm cells, its metabolite AM404 does. To understand whether the AA and AM404 mediated influx of calcium in the sperm cells was dependent of CatSper, we stimulated the sperm cells with near-EC100 doses of AA and AM404 with or without pre-incubation with the specific CatSper inhibitor RU1968 at 30 µM. Although the kinetics of the calcium influxes induced by AA and AM404 do not at all resemble each other, RU1968 pre-incubation was found to fully block the induced calcium influx for both (Fig. 4a and b), although a minor slowly rising increase in [Ca2+]i remained in the presence of RU1968 for both AM404 and AA. A similar full inhibitory effect of RU1968 was observed for the endogenous CatSper-ligand, progesterone (Fig. 4c). As human CatSper can be activated by intracellular alkalization, we examined the effect of AM404 near the EC100 dose on pH(i) in human sperm cells. AM404 was found to slowly increase pH(i) in human sperm cells as compared to positive control NH4Cl (Fig. 4d). However, this increase was observed at a slower timeframe than the induced calcium influxes. This suggests that AM404 activates CatSper directly through another mode of action, while the sustained part of the calcium signal induced by AM404 may be, at least in part, due to its effects on pH(i).
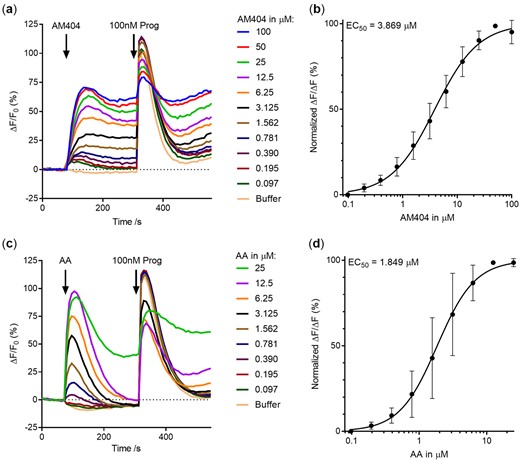
Effect of N-arachidonoyl phenolamine (AM404) and arachidonic acid (AA) on Ca2+-signalling in human sperm cells. Paracetamol metabolite N-arachidonoyl phenolamine (AM404) formed from conjugation of 4-aminophenol (4AP) and AA induced a dose-dependent induction of calcium influx in human sperm cells and subsequent weak inhibition of endogenous ligand progesterone (Prog) mediated influx (a). (b) The dose–response relationship yielded a mean EC50 value of 3.869 µM, and a mean EC02 value of 25.77 nM for the calcium influx inducing effect of AM4040 (n = 5). The AM404 induced calcium influx did not resemble induction by AA (c). (d) The dose–response relationship yielded a mean EC50 value of 1.8 µM for the calcium influx inducing effect of AA (n = 3).
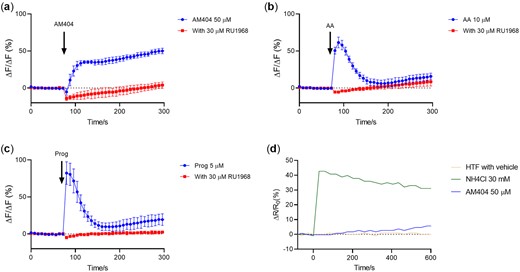
Involvement of CatSper. The specific CatSper inhibitor RU1968 blocked calcium influxes induced by N-arachidonoyl phenolamine (AM404) (a), arachidonic acid (AA) (b) and progesterone (Prog) (c). Values are mean ± SD (n = 3). AM404 was found to slowly increase pH(i) in human sperm cells as compared to positive control NH4Cl. HTF+-medium (HTF) represents the negative control (d). Representative graph from a single experiment (n = 3). HTF, human tubular fluid.
Exposure of sperm cells to AM404 interferes with CatSper-dependent sperm motility
Next, we examined whether AM404 exposure could alter motility of sperm cells in a CatSper-dependent manner. When added, 10 µM AM404 significantly (P-value of 0.003) affected sperm cell penetration into viscous mucous; i.e. increased the number of sperm cells penetrating the viscous mucous. The observed effect mimicked the effect of endogenous CatSper-ligand progesterone, although to a much lower extent (Fig. 5). Together, these data suggest that the calcium influx induced by AM404 dependents on CatSper and results in a CatSper-dependent change in motility.
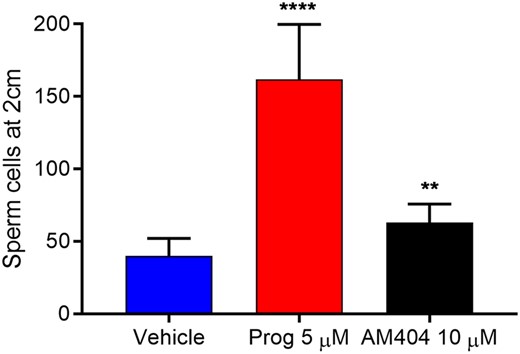
Effect of N-arachidonoyl phenolamine (AM404) on human sperm penetration into viscous mucous. Paracetamol metabolite N-arachidonoyl phenolamine (AM404) changed the penetration of cells into the viscous mucous mimicking the effect of endogenous ligand progesterone (Prog). Values are mean ± SEM of fourteen independent experiments. **** signifies an adjusted P-value <0.0001, ** signifies an adjusted P-value <0.01. Statistics from repeated measures one-way ANOVA on ln-transformed raw values with Geissner–Greenhouse correction and Dunnett’s correction for multiple comparisons. Mean values are 40 (vehicle), 161.7 (Prog) and 62.9 (AM404).
FAAH is expressed during spermatogenesis, in the epididymis and in mature human sperm cells
Conjugation of APAP metabolite 4AP with AA to form AM404 is dependent on FAAH (Högestätt et al., 2005). As previously reported (Nielsen et al., 2019), FAAH is highly expressed during spermatogenesis. In addition, we here observed a strong expression in the epididymal epithelium (Fig. 6a). To investigate whether this enzyme was present in mature human sperm, we stained human sperm cells immunohistochemically for FAAH and found strong expression in all sperm cells in the neck/midpiece region (Fig. 6b). To test the functionality of FAAH in human sperm cells, we measured the FAAH-activity using the FAAH fluorogenic substrate AAMCA that is hydrolysed to AA and a fluorescent 7-amino 4-methyl coumarin (AMC) metabolite (Ramarao et al., 2005). Data showed that the sperm cell FAAH was functional and that conversion of AAMCA to AA and AMC could be blocked by both an FAAH specific (PF750) and an unspecific general inhibitor of membrane serine hydrolases (MAFP; Fig. 6c and d). These data show that sperm cells have functional FAAH expressed in the neck/midpiece region.
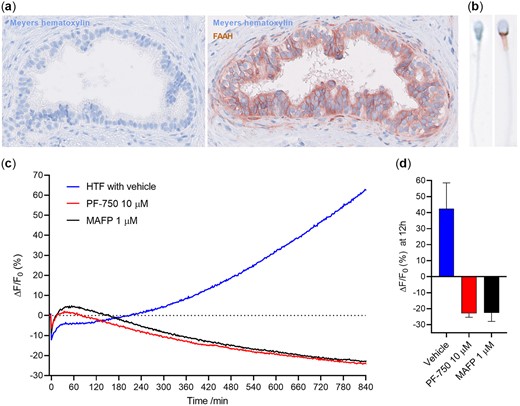
Fatty acid amide hydrolase (FAAH) expression and activity. Human epididymis (×40 magnification) (a) and sperm cells (×80 magnification) (b) have FAAH expression. (c, d) Human sperm cells are capable of hydrolyzing 4-methyl coumarin amide (AAMCA), a fluorogenic substrate for FAAH, with an activity that can be inhibited by both an FAAH specific (PF750) inhibitor and an unspecific inhibitor of membrane serine hydrolases (Methyl arachidonyl fluorophosphonate, MAFP). Data in (c) are representative graphs from a single experiment. Data in (d) are shown after 12 h and values are mean ± SD (n = 3).
Sperm cells can form APAP metabolite AM404
To understand whether sperm cells can form AM404 from its precursors, we next exposed aliquots of 3 million human sperm cells to 10 µM 4AP, 10 µM 4AP+AA, 100 µM APAP, or vehicle control at 37°C for 240 min and flash froze the supernatant. In two of three experiments, we found a background level of AM404 in vehicle controls (Fig. 7). The workup blank performed along the sample preparation did not show contamination of AM404 during the procedure, strongly suggesting that AM404 background in controls originated from the sperm cells. Exposing the cells to 4AP resulted in a significant increase in AM404 formation (P-value 0.0402). Addition of AA to the 4AP preparation also resulted in a significant increase (P-value 0.0267), but did not further increase AM404 as compared to 4AP alone, suggesting that endogenous AA was not a rate-limiting step in the AM404 formation. Exposing cells to 100 µM APAP did not result in increased levels of AM404, as compared to the vehicle exposed cells. This suggests that the sperm cells do not have a capacity to deacetylate APAP to 4AP. Taken together, these data suggest that human sperm cells have the capacity to generate AM404 from the primary APAP metabolite 4AP de novo.
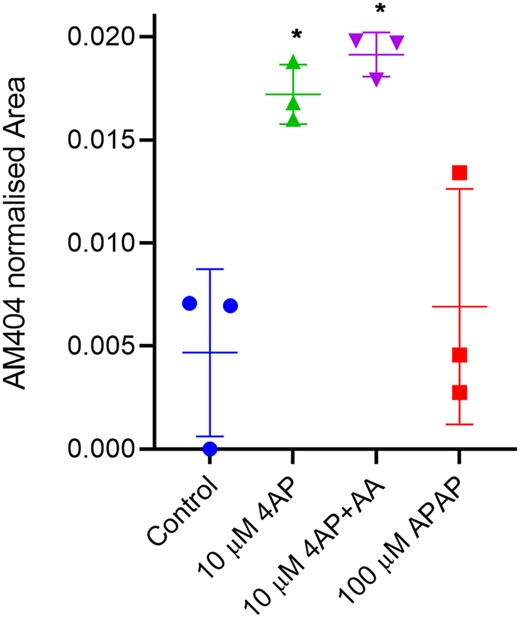
In situ formation of N-arachidonoyl phenolamine (AM404) in human sperm cells. Human sperm cells form N-arachidonoyl phenolamine (AM404) from its precursors 4-aminophenol (4AP) and arachidonic acid (AA). Paracetamol (N-acetyl-para-aminophenol (APAP)) resulted in minor non-significant increase in AM404. Vehicle versus 4AP (*P-value = 0.0402); Vehicle versus 4AP+AA (*P-value = 0.0267); and Vehicle versus APAP (P-value = 0.75). Statistics from repeated measures one-way ANOVA on untransformed raw values with Geissner–Greenhouse correction and Dunnett’s correction for multiple comparisons. Data are after exposure for 240 min at 37°C and values represents means ± SD (n = 3).
Discussion
In this study, we observed that APAP is transferred into the seminal fluid in healthy men after oral exposure and that sperm cells have a metabolizing capacity to form the APAP metabolite AM404 from the primary APAP amine metabolite 4AP. We found AM404 to have direct effects on CatSper-mediated calcium influx and sperm function (Fig. 8). A previous study suggested that adult men with high levels of urinary APAP have increased time-to-pregnancy (Smarr et al., 2016). During pharmaceutical exposure, high levels of APAP in blood are transferred to the seminal fluid reaching high micromolar concentrations. During 5 days of APAP exposure, we observed an accumulation of APAP in the seminal fluid with a maximal concentration of 70.3 µg/ml (466 µM) (Fig. 1). Previous studies have shown that the liver is likely the primary de-acetylation site of APAP to 4AP, whereas the subsequent conjugation of 4AP with AA leading to the formation of AM404 is taking place in tissues where FAAH is expressed (Högestätt et al., 2005). However, evidence also suggests it is possible that FAAH directly participates in the de-acetylation of APAP, as FAAH inhibition partially blocks in vivo formation of 4AP in the rat brain, and there is a tendency towards reduced levels of 4AP in liver homogenates from FAAH deficient mice as compared with control littermates (Högestätt et al., 2005). These data might explain why we observed background formation of AM404 by sperm cells in both control experiments and in experiments with exposure to APAP (Fig. 7).
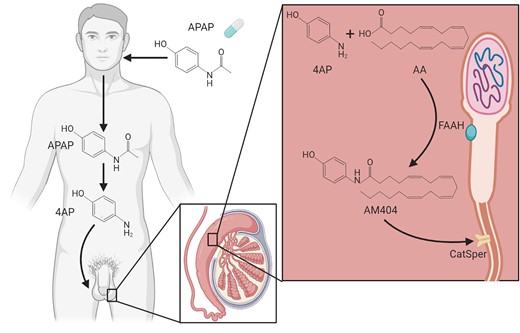
Summary figure. Schematic representation of de-acetylation of paracetamol (N-acetyl-para-aminophenol (APAP)) to 4-amoniphenol (4AP) and subsequent conjugation with arachidonic acid (AA) to form AM404. In contrast to APAP and 4AP, AM404 directly interferes with CatSper-mediated calcium influx and thereby potentially sperm function and male fertility. Created using BioRender.com.
Neither APAP (Fig. 2a) nor its primary metabolite 4AP (Fig. 2b) had an effect on calcium signalling in human sperm cells at physiologically relevant concentrations, whereas the second APAP metabolite AM404 was found to induce a dose-dependent and sustained increase in [Ca2+]i in human sperm cells with an EC50 of 3.9 µM and EC02 of 25.77 nM (Fig. 3a). Importantly, an effect on calcium influx was observed for AM404 even in low nanomolar concentrations, comparable to the low nanomolar levels of AM404 reported in human cerebrospinal fluid, i.e. 5–40 nM (Sharma et al., 2017). Our data also strongly suggest that sperm cells themselves have the capability to form AM404 from the primary APAP metabolite 4AP. This is highly concerning as it indicates that high local levels of AM404 exist in the near vicinity of the sperm cells. However, while relevant MS/MS patterns could be observed for the standard AM404, it was not possible to trigger MS/MS for AM404 in the samples as the signal here was too low. Hence, the exact concentrations of AM404 in the local environment surrounding the sperm cells remain unknown and cannot be related to our observed dose–response data (Fig. 3b). Future human biomonitoring studies using more sensitive tandem MS/MS equipment are required to reveal the exact concentrations of AM404 formed by the sperm cells. Moreover, we observed a background exposure to both APAP and NA2AP in urine, blood plasma and seminal plasma in the baseline samples. It is therefore clear from these data that human sperm cells may be exposed to APAP both through high acute doses after oral pharmaceutical use and via low chronic exposure from the environment. It has been suggested that aniline may be the origin of this environmental exposure through a rapid acetylation to intermediate ACA and subsequent hydroxylation to APAP (Modick et al., 2014; Holm et al., 2015). However, ACA was consistently not observed in our samples, suggesting that sources other than aniline are responsible for the background APAP exposure.
Our data using the specific CatSper inhibitor RU1968 (Rennhack et al., 2018) suggest that AM404 induces a calcium influx in human sperm cell through CatSper, similar to the endogenous ligand progesterone (Fig. 4a and c). AM404 also induced an increase in sperm penetration into viscous media, although at a lower level as compared to progesterone. As sperm penetration into viscous mucous is known to be CatSper dependent (Alasmari et al., 2013; Williams et al., 2015), this again indicates that the calcium influx induced by AM404 dependents on CatSper and results in a CatSper-dependent change in sperm function. Furthermore, we observed that AM404 did not activate CatSper via intracellular alkalization (Fig. 4s) within the time window where the peak calcium signals were observed (∼56 s after addition of AM404), indicating that AM404 primarily activates CatSper directly through a mode of action not involving intracellular alkalization. However, the slowly increasing and sustained component of the calcium signals induced by AM404 may in part be due to the observed effect on pH(i), although a minor sustained increase in [Ca2+]i remained even in the presence of RU1968 (Fig. 4a), which indicates that AM404 to a minor degree also affects Ca2+-signalling in human sperm in a non-CatSper-dependent manner. The exact mode of action by which AM404 activates CatSper remains unknown.
We also observed that AA induces a calcium influx in human sperm cells in a CatSper-dependant fashion (Figs 3c and 4b). This observed agonistic action on CatSper is in line with the findings of a previous study (Miller et al., 2016). The Ca2+-signal induced by AA is transient, similar to the signal induced by progesterone and does not resemble that induced by AM404 (Fig. 3a). Furthermore, AA with a mean EC50 of 1.8 µM is a more potent inducer of Ca2+-signals than AM404 with a mean EC50 of 3.869 µM. Together, this indicates that AA and AM404 interacts with their putative receptor leading to CatSper activation in quite different ways.
Regardless of its mode of action, the fact that AM404 can induce calcium influxes through CatSper and affect sperm function in a CatSper-dependent manner indicates that exposure of sperm cells to this metabolite could interfere with normal human sperm calcium signalling. Calcium signalling controls important human sperm functions, including changes in sperm motility, that must be triggered in the correct time and place during the journey of the sperm cells through the female reproductive tract before a successful fertilization can take place (Publicover et al., 2007; Wang et al., 2021). In contrast to the events during fertilization, the role of CatSper during the development, maturation and storage of sperm cells within the male reproductive tract remains largely unknown. It has been shown that progesterone can increase CatSper currents even in human elongated spermatids, showing that functional and responsive CatSper channels are at least present at this stage (Smith et al., 2013). FAAH is expressed already from the pachytene spermatocyte stage during spermatogenesis (Nielsen et al., 2019). Here, we further show that FAAH is expressed in the epididymal epithelium and in the neck/midpiece region of mature human sperm cells (Fig. 6a and b) and that FAAH in human sperm cells is functional (Fig. 6c and d). These findings are in line with previous findings for FAAH in human sperm cells (Francavilla et al., 2009). Together, this indicates that human sperm cells have the capability to produce AM404 from 4AP already from the pachytene spermatocyte stage during spermatogenesis and that the epididymal epithelium may similarly be able to produce AM404. Thereby the sperm cells may be exposed to high local AM404 levels throughout the male reproductive tract, especially since APAP can readily cross blood-tissue barriers and should thus be able to reach both the lumen of the seminiferous tubules and epididymis (Ghanem et al., 2016). It has recently been shown that pharmacological inhibition of CatSper in mouse sperm cells extracted from the epididymis could block sperm fertilizing ability in vivo after subsequent injection into the female reproductive tract, indicating that pharmacologically induced actions on CatSper may lead to irreversible and long-lasting functional effects in the transcriptionally silent sperm cells (Curci et al., 2021). Although AM404 act agonistically on human CatSper, it could thus still be speculated that exposure of human sperm cells to AM404 within the male reproductive tract could similarly lead to irreversible and long-lasting effects in the ejaculated sperm cells. This would support the observations from previous studies that have shown that adult men with high urinary levels experienced increased time-to-pregnancy and impaired sperm motility (Smarr et al., 2016, 2017).
The results here would similarly suggest that female exposure to APAP might lead to sperm-mediated generation of AM404 that could interfere acutely with the function of human sperm cells within the female reproductive tract. However, several publications have failed to identify an association between APAP exposure in women and time-to-pregnancy (Smarr et al., 2016; Mcinerney et al., 2017). Importantly, the exact concentrations of AM404 in the local environment surrounding the sperm cells remain unknown. Regardless, even low contractions may interact alone or more likely together with other xenobiotics that interfere with CatSper-mediated calcium signalling in human sperm cells in an additive (Schiffer et al., 2014) or even synergistic manner (Brenker et al., 2018). Although it is reasonable to suspect that any xenobiotic present in the male and/or female reproductive tract with the ability to affect human sperm Ca2+-signalling in human sperm cells are more likely to disturb sperm function than to increase the chances of achieving pregnancy, this can only be answered through future in vivo studies.
Conclusion
The present data show that while APAP itself does not interact with the sperm cell function per se, its metabolite AM404, produced by the sperm cells themselves via FAAH-mediated conjugation of 4AP with AA, can interfere with human sperm cell Ca2+-signalling and function through a CatSper-dependent action. This is concerning, as both environmental and pharmaceutical APAP exposures reach the seminal plasma and because functional CatSper is critical for sperm function and male fertility. We hypothesize that this could, at least in part, be responsible for the observed negative association between male urinary APAP levels, sperm motility and time-to-pregnancy.
Supplementary data
Supplementary data are available at Human Reproduction online.
Data availability
The data underlying this article will be shared on reasonable request to the corresponding authors.
Authors’ roles
D.M.K. and A.R. conceived and designed the study. A.R., H.F. A.D., J.C., J.E.N. and D.M.K. performed the experiments. D.M.K., A.R., A.D., J.C., J.E.N., A.J., N.E.S., R.H.R. and B.S.N. analysed and interpreted the data. Drafting the article was performed by D.M.K., A.R., B.S.N., R.H.R. and A.D. All authors have read and approved the final version of this manuscript and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Funding
D.M.K. is funded by the Lundbeck Foundation, grant number R324-2019-1881, and the Svend Andersen Foundation. A.R. is funded by a BRIDGE—Translational Excellence Programme grant funded by the Novo Nordisk Foundation, grant agreement number: NNF18SA0034956.
Conflict of interest
The authors declare they have no competing interests.



