-
PDF
- Split View
-
Views
-
Cite
Cite
Danilo Cimadomo, Antonio Capalbo, Lisa Dovere, Luisa Tacconi, Daria Soscia, Adriano Giancani, Emiliano Scepi, Roberta Maggiulli, Alberto Vaiarelli, Laura Rienzi, Filippo Maria Ubaldi, Leave the past behind: women’s reproductive history shows no association with blastocysts’ euploidy and limited association with live birth rates after euploid embryo transfers, Human Reproduction, Volume 36, Issue 4, April 2021, Pages 929–940, https://doi.org/10.1093/humrep/deab014
Close - Share Icon Share
Abstract
Is there an association between patients’ reproductive history and the mean euploidy rates per biopsied blastocysts (m-ER) or the live birth rates (LBRs) per first single vitrified-warmed euploid blastocyst transfers?
Patients’ reproductive history (as annotated during counselling) showed no association with the m-ER, but a lower LBR was reported after euploid blastocyst transfer in women with a history of repeated implantation failure (RIF).
Several studies have investigated the association between the m-ER and (i) patients’ basal characteristics, (ii) ovarian stimulation strategy and dosage, (iii) culture media and conditions, and (iv) embryo morphology and day of full blastocyst development. Conversely, the expected m-ER due to women’s reproductive history (previous live births (LBs), miscarriages, failed IVF cycles and transfers, and lack of euploid blastocysts among prior cohorts of biopsied embryos) still needs investigations. Yet, this information is critical to counsel new patients about a first cycle with preimplantation genetic testing for aneuploidy (PGT-A), but even more so after former adverse outcomes to prevent treatment drop-out.
This observational study included all patients undergoing a comprehensive chromosome testing (CCT)-based PGT-A cycle with at least one biopsied blastocyst in the period April 2013-December 2019 at a private IVF clinic (n = 2676 patients undergoing 2676 treatments and producing and 8151 blastocysts). m-ER were investigated according to women’s reproductive history of LBs: no/≥1, miscarriages: no/1/>1; failed IVF cycles: no/1/2/>2, and implantation failures after previous transfers: no/1/2/>2. Among the 2676 patients included in this study, 440 (16%) had already undergone PGT-A before the study period; the data from these patients were further clustered according to the presence or absence of euploid embryo(s) in their previous cohort of biopsied blastocysts. The clinical outcomes per first single vitrified-warmed euploid blastocyst transfers (n =1580) were investigated according to the number of patients’ previous miscarriages and implantation failures.
The procedures involved in this study included ICSI, blastocyst culture, trophectoderm biopsy without hatching in Day 3, CCT-based PGT-A without reporting segmental and/or putative mitotic (or mosaic) aneuploidies and single vitrified-warmed euploid blastocyst transfer. For statistical analysis, Mann–Whitney U or Kruskal–Wallis tests, as well as linear regressions and generalised linear models among ranges of maternal age at oocyte retrieval were performed to identify significant differences for continuous variables. Fisher’s exact tests and multivariate logistic regression analyses were instead used for categorical variables.
Maternal age at oocyte retrieval was the only variable significantly associated with the m-ER. We defined five clusters (<35 years: 66 ± 31%; 35–37 years: 58 ± 33%; 38–40 years: 43 ± 35%; 40–42 years: 28 ± 34%; and >42 years: 17 ± 31%) and all analyses were conducted among them. The m-ER did not show any association with the number of previous LBs, miscarriages, failed IVF cycles or implantation failures. Among patients who had already undergone PGT-A before the study period, the m-ER did not associate with the absence (or presence) of euploid blastocysts in their former cohort of biopsied embryos. Regarding clinical outcomes of the first single vitrified-warmed euploid blastocyst transfer, the implantation rate was 51%, the miscarriage rate was 14% and the LBR was 44%. This LBR was independent of the number of previous miscarriages, but showed a decreasing trend depending on the number of previous implantation failures, reaching statistical significance when comparing patients with >2 failures and patients with no prior failure (36% versus 47%, P < 0.01; multivariate-OR adjusted for embryo quality and day of full blastocyst development: 0.64, 95% CI 0.48–0.86, P < 0.01). No such differences were shown for previous miscarriage rates.
The sample size for treatments following a former completed PGT-A cycle should be larger in future studies. The data should be confirmed from a multicentre perspective. The analysis should be performed also in non-PGT cycles and/or including patients who did not produce blastocysts, in order to investigate a putative association between women’s reproductive history with outcomes other than euploidy and LBRs.
These data are critical to counsel infertile couples before, during and after a PGT-A cycle, especially to prevent treatment discontinuation due to previous adverse reproductive events. Beyond the ‘maternal age effect’, the causes of idiopathic recurrent pregnancy loss (RPL) and RIF are likely to be endometrial receptivity and selectivity issues; transferring euploid blastocysts might reduce the risk of a further miscarriage, but more information beyond euploidy are required to improve the prognosis in case of RIF.
No funding was received and there are no competing interests.
N/A.
Introduction
All of the new procedures implemented across recent decades, from blastocyst culture to vitrification, PGT-A and cycle segmentation have led to the key breakthrough of defining cumulative live birth rate per intention to treat (CLBR per ITT) as the main outcome of modern IVF (Maheshwari et al., 2015). Patient counselling has profoundly changed accordingly. At present, our aim is not performing an embryo transfer (ET), but identifying at least one embryo with high chance of resulting in a healthy live birth with the lowest possible risk of complications (Ubaldi et al., 2019). Nowadays, patient counselling has become a time-consuming but crucial phase of the treatment, which involves a multidisciplinary team of experts. Before, during and even after a treatment, the counselling will certainly affect couples’ choices and ultimately their chance to conceive. The definition of their estimated risk of producing aneuploid blastocysts and of the variables which might associate with such a risk is therefore essential. In this regard, the typical indications to PGT-A are mainly three: advanced maternal age (AMA), recurrent pregnancy loss (RPL) and repeated implantation failure (RIF) (Harper et al., 2012; Coonen et al., 2020). Although the lower threshold to define AMA has been finally set as 35 years (Zegers-Hochschild et al., 2017a, b; Coonen et al., 2020) and RPL is commonly defined as ’the loss of two or more pregnancies’ (Atik et al., 2018), a consensus is missing for the definition of RIF on the basis of the number of previous failed transfers, previous embryos unsuccessfully transferred, their stage, morphological quality and chromosomal constitution (an international survey of the ESHRE ‘special interest group in implantation and early pregnancy’ focused on this topic has been recently published (Cimadomo et al., 2020)). A consensus for severe male factor (SMF) as an additional indication to PGT-A is also not defined at present (Mazzilli et al., 2018).
Several studies have investigated the association between euploidy rates assessed at the blastocyst stage with maternal age (e.g. Franasiak et al., 2014; Capalbo et al., 2017), ovarian stimulation and ovarian response to stimulation (e.g. Labarta et al., 2012; Hodes-Wertz et al., 2015; Barash et al., 2017; Cimadomo et al., 2018c; Morin et al., 2018; Wu et al., 2018; Irani et al., 2020), semen characteristics (e.g. Mazzilli et al., 2017), culture conditions (e.g. Werner et al., 2016; Cimadomo et al., 2018b), or blastocysts’ features (e.g. Capalbo et al., 2014; Tiegs et al., 2019); conversely, little information has been produced on the association between the patients’ reproductive history with PGT-A diagnoses or with implantation after euploid blastocyst transfer, which is also of critical concern for the couples (Sato et al., 2019; Wang et al., 2019; Bilibio et al., 2020; Liu et al., 2020).
In this observational study, we included all patients undergoing a PGT-A cycle at our private IVF centre in Rome between April 2013 and December 2019, and inspected the association between women’s reproductive history with (i) the mean euploidy rate of each cohort of biopsied blastocysts (m-ER) and (ii) the live birth rate (LBR) of first vitrified-warmed euploid single blastocyst transfers. We aimed at producing reliable data for honest and evidence-based counselling before, during and after a PGT-A cycle. Treatment discontinuation is indeed amongst the most important hurdles for a couples and for clinics to meet their expected CLBR per ITT (Gameiro et al., 2012); it is an issue relevant for new patients, but even more for patients who have already experienced previous IVF failures and/or miscarriages. These data are therefore required to inform patients about their intrinsic risks and chances and to help IVF practitioners in limiting treatment drop-out after failures.
Material and methods
Study design
This observational study included all patients undergoing a PGT-A cycle with at least one biopsied blastocyst between April 2013 and December 2019 at the Genera IVF centre in Rome (n =2676 patients undergoing 2676 cycles and obtaining 8151 blastocysts, on average 3 ± 2 blastocyst/cycle; maternal age: 39.3 ± 3.3 years, BMI: 21.9 ± 3.4, FSH: 8.1 ± 3.3 IU/ml and AMH: 1.9 ± 1.3 ng/ml). All patients had a normal karyotype. Cycles including preimplantation genetic testing for structural rearrangements (PGT-SR) or monogenic disorders (PGT-M) and oocyte donation cycles were not included. Comprehensive chromosome testing (CCT) was conducted at Igenomix (Marostica, Italy). The m-ER was investigated according to women’s reproductive history defined as (i) previous live births (LBs) achieved (both spontaneous or after IVF): no/≥1; (ii) previous miscarriages (both spontaneous of after IVF): no/1/>1; (iii) previous failed IVF cycles: no/1/2/>2; (iv) implantation failures after previous transfers: no/1/2/>2. Among the 2676 patients included in this study, 440 (16%) had already undergone PGT-A cycles before the study period. For these patients, the m-ER was further investigated in two groups clustered according to the presence or absence of euploid blastocyst(s) in their former cohort of biopsied embryos.
The main characteristics of the women included in the study (age, BMI, FSH, AMH) were investigated through regression analyses to identify putative associations with the m-ER. Maternal age at oocyte retrieval was the only variable significantly associated with the m-ER. Figure 1 is a dispersion plot portraying how the m-ER decreases as the maternal age increases. In the same figure, we defined five clusters of maternal age with the related boxplots to summarise the m-ER within them (<35 years: N = 208, 66 ± 31%; 35–37 years: N = 533, 58 ± 33%; 38–40 years: N = 909, 43 ± 35%; 40–42 years: N =574, 28 ± 34%; and >42 years: N =452, 17 ± 31%). All analyses were conducted among PGT-A cycles grouped within these clusters.
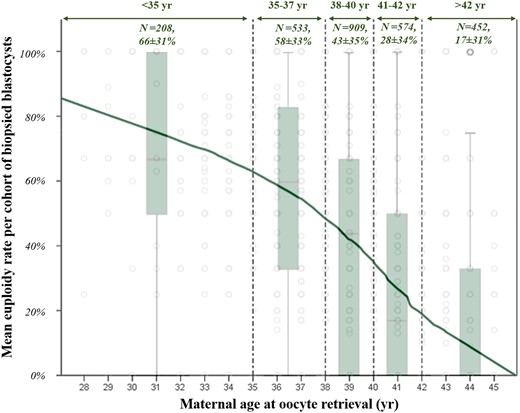
Euploidy rate per cohort of biopsied blastocysts from each patient among ranges of maternal age at oocyte retrieval. Grey dots identify each treatment. Dotted green lines and double headed arrows identify ranges of maternal age, whose absolute numbers of cycles and mean euploidy rate ± SD are also shown. The boxplots display the euploidy rate per cohort of biopsied blastocysts from each patient among different ranges of maternal age.
We also investigated the clinical outcomes after single embryo transfer (SET) according to the number of previous miscarriages (both spontaneous and after IVF) and number of implantation failures after previous transfers. We included only the first SET of vitrified-warmed euploid blastocysts from all cycles where at least one transferable embryo was identified. The clinical outcomes investigated were: implantation rate per SET, miscarriage rate per clinical pregnancy and LBR per SET. The odds-ratios (OR) were adjusted for blastocyst morphological quality (as defined previously (Capalbo et al., 2014), in an adapted method (Gardner and Schoolcraft 1999)) and for day of full blastocyst development up to day7 (Supplementary Tables SI and SII).
Institutional review board (IRB) approval was obtained from Clinica Valle Giulia for this study.
Statistical analysis
The m-ER followed a distribution different from a Gaussian (Kolgomorov–Smirnov and Shapiro-Wilk tests < 0.01). Therefore non-parametric tests were adopted for the investigations (i.e. Mann–Whitney U or Kruskal–Wallis tests). Linear regressions and generalised linear models among ranges of maternal age at oocyte retrieval were conducted to confirm the absence/presence of associations with the primary outcome under investigation (i.e. the m-ER). Fisher’s exact tests were conducted to outline putative differences among categorical variables. The results were confirmed through multivariate logistic regression analyses. All statistical analyses were conducted with SPSS (IBM, USA).
IVF procedures
During the counselling before the treatment, the clinicians interviewed each couple about their reproductive history, reviewed all their documentation, requested further tests, and performed transvaginal ultrasound and basal assessment of the ovaries. All information was noted in a relational database (Fertilab, Italy). Specifically, for the female partner, we requested peripheral blood test and Rhesus (RH) factors, thyroid function (TSH, FT3 and FT4), karyotyping, haemoglobin electrophoresis, coagulation screening (protein C, protein S, ATIII, homocysteine, factor V of Leiden and prothrombin), immunological screening (anti-cardiolipin, lupus anticoagulant, anti-nucleus antibodies, smooth anti-muscular antibodies), cardiological evaluation (electrocardiogram), gynaecologic evaluation (pap test, vaginal and cervical swabs), breast examination (ultrasound and/or mammography), infectious disease tests (hepatitis B, hepatitis C, HIV, VDRL/TPHA, HBcAb IgM-IgG) and TORCH screening (toxoplasmosis, rubella, cytomegalovirus). For the male partner, we requested semen culture analysis, karyotyping, haemoglobin electrophoresis, glucose-6-phosphate dehydrogenase, infectious disease tests (hepatitis B, hepatitis C, HIV, VDRL/TPHA, HBcAb IgM-IgG) and cytomegalovirus IgM-IgG.
The protocol and starting dose of the medications was chosen according to patients’ characteristics and gynaecologists’ judgement (Rienzi et al., 2008; Ubaldi et al., 2015). Oocyte retrieval was conducted 35 h after the ovulation trigger. ICSI was performed as detailed in (Maggiulli et al., 2020). Embryos were cultured in a controlled humidified atmosphere (37 °C, 6%CO2 and 5%O2) up to Day 5–7. No assisted hatching was performed on day3 and the blastocysts were biopsied whenever they reached full expansion as described previously (Capalbo et al., 2014; Maggiulli et al., 2019). Vitrification was performed soon after the biopsy (Cimadomo et al., 2018a). Only CCT techniques were adopted (Treff et al., 2012; Capalbo et al., 2015; Garcia-Pascual et al., 2020) to identify full chromosome meiotic aneuploidies. ‘Embryos with a PGT-A result falling in the mosaic range’ (Forman 2019) were not reported as, at present, the risk of false positive calls (Goodrich et al., 2016; Girardi et al., 2020) is deemed too high to justify a clinical implementation of such policy (Popovic et al., 2019, 2020; Capalbo et al., 2020a). Accordingly, CCT profiles from trophectoderm biopsies consistent with less than 50% aneuploid cells were reported as euploid, whereas chromosome copy number variations above this threshold were reported aneuploid.
In case of at least one euploid blastocyst identified, embryo replacement was performed in a subsequent menstrual cycle. After warming, only SETs were performed. Endometrial preparation and transfer procedures were chosen according to patients’ characteristics and gynaecologists’ judgement and conducted as described earlier (Vaiarelli et al., 2018) in the absence of endometrial fluid in the uterine cavity, hydrosalpinx and/or TSH levels ≥2.5 IU/l. In case of artificial cycle protocol, all patients had a transvaginal ultrasound between Day 2–3 of the menstrual cycle to evaluate the endometrial thickness and basal state of the ovaries. After the scan, oral estradiol valerate (Progynova; Bayer, Germany) was administered as follows: 2 mg twice a day for the first 4 days, then 2 mg three times a day for the rest of the therapy. When the endometrial thickness reached at least 7–8 mm and the aspect was trilaminar, vaginal micronised progesterone was administrated (Progeffik 200 mg, Effik, Italy) as follows: 400 mg on Day 0 and then 600 mg/day to support the luteal phase from Day 1. After six days of micronised progesterone, we planned embryo transfer. The hormone replacement was administered until the 10th week of gestation in case of pregnancy. In case of a modified natural cycle protocol, instead, a single intramuscular dose of 10 000 IU hCG was administered when the leading follicle was >17 mm and the endometrium measured >7–8 mm with a trilaminar aspect, along with 400 mg/day of micronised vaginal progesterone (Progeffik; Effik, Italy) for luteal phase support starting 36–40 h post-hCG administration (Day 0). The transfer was performed 7 days after hCG injection.
Results
Among the 2676 patients, 720 produced one blastocyst (27%), 616 produced a cohort of two blastocysts (23%), 450 produced a cohort of three blastocysts (17%), 352 cycles produced a cohort of four blastocysts (13%), 202 produced a cohort of five blastocysts (8%), 154 produced a cohort of six blastocysts (6%), and 182 produced a cohort of seven or more blastocysts (7%). Therefore, we adopted a generalised linear model adjusted for maternal age at oocyte retrieval to investigate whether the number of biopsied blastocysts from each patient was associated with the m-ER. No association was reported (partial eta-squared: 0.002, power: 0.36 and P = 0.5).
We then analysed whether a previous LB was associated with the m-ER per cohort of biopsied blastocysts among ranges of maternal age at oocyte retrieval. Figure 2A shows the data clustered according to these variables, and the m-ER with 95% CI always overlap. Linear regressions were then performed among ranges of maternal age and confirmed the absence of a significant association with previous LB (Fig. 2B). Figure 3A displays the m-ER with 95% CI according to the number of previous miscarriages. Again the results overlap. Figure 3B summarises the linear regressions conducted among ranges of maternal age, and the absence of significant associations was confirmed. The m-ER per cohort of biopsied blastocysts was independent also of the number of previous failed IVF cycles (i.e. IVF cycles that did not result in an LB) (Fig. 4A). Again this information was confirmed via linear regressions among ranges of maternal age (Fig. 4B). Similar results were observed also clustering the data according to the number of implantation failures after previous ETs (Fig. 5A and B).
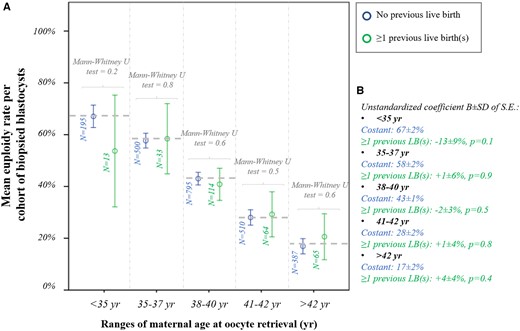
Euploidy rate according to previous live births. (A) Mean euploidy rate per cohort of biopsied blastocysts from each patient according to the number of previous live births (no/≥1) and among different ranges of maternal age at oocyte retrieval. (B) Linear regressions reporting the data in the control (patients with no previous live birth) and the mean difference in patients with ≥1 previous live births.
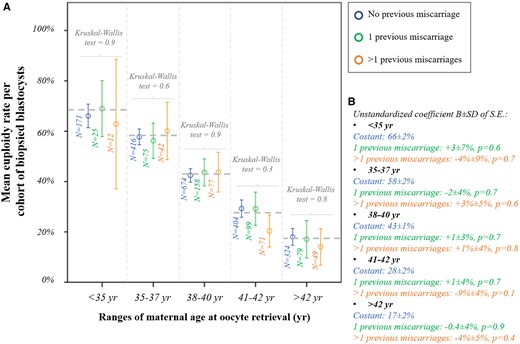
Euploidy rate according to previous miscarriages. (A) Mean euploidy rate per cohort of biopsied blastocysts from each patient according to the number of previous miscarriages experienced (no/1/>1) and among different ranges of maternal age at oocyte retrieval. (B) Linear regressions reporting the data in the control (no previous miscarriage) and the mean difference in patients with 1 or >1 previous miscarriage(s).
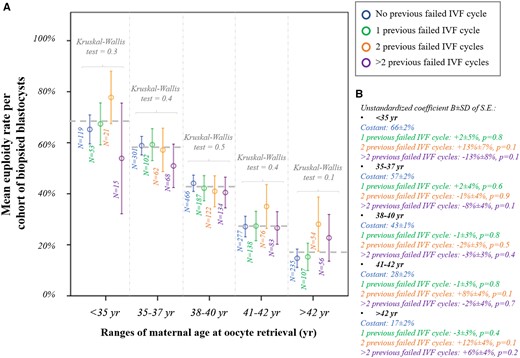
Euploidy rate according to previous failed IVF cycles. (A) Mean euploidy rate per cohort of biopsied blastocysts from each patient according to the number of previous failed IVF cycles (no/1/2/>2) and among different ranges of maternal age at oocyte retrieval. (B) Linear regressions reporting the data in the control (no previous failed IVF cycle) and the mean difference in patients with 1, 2 or >2 previous failed IVF cycle(s).
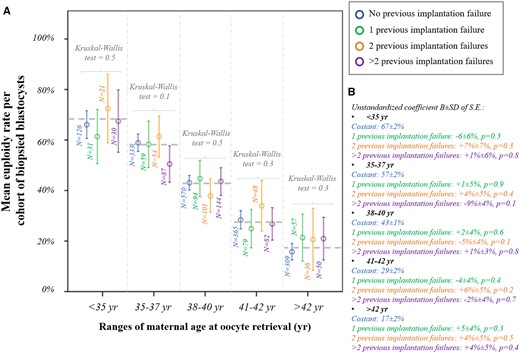
Euploidy rate according to previous implantation failures. (A) Mean euploidy rate per cohort of biopsied blastocysts from each patient according to the number of implantation failures after previous embryo transfers (no/1/2/>2) and among different ranges of maternal age at oocyte retrieval. (B) Linear regressions reporting the data in the control (no previous implantation failure) and the mean difference in patients with 1, 2 or >2 previous implantation failure(s).
For the 440 patients who had already undergone PGT-A before the study period, on average 277 ± 275 days had elapsed between the former treatment and the cycle included in this study. Four ranges were therefore outlined (≤90 days: n = 96/440, 22%; 91–180 days: n =116/440, 26%; 181-360 days: n =133/440, 30%; >360 days: n =95/440, 22%). We then investigated whether having obtained solely aneuploid blastocysts previously would affect the m-ER in the cycle included in this study. Figure 6 displays the absence of significant differences across all ranges of maternal age at oocyte retrieval. A generalised linear model adjusted for maternal age and the days elapsed between consecutive cycles confirmed the absence of an association between the presence or absence of euploid blastocysts in previous PGT-A cycles and the m-ER in the cycle under analysis (partial eta-squared: 0.017, power: 0.34 and P-value = 0.7).
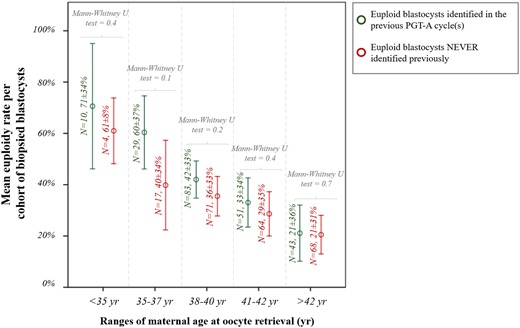
Mean euploidy rate per cohort of biopsied blastocysts from each patient according to the absence (or presence) of euploid embryos in previous PGT-A cycles and among different ranges of maternal age at oocyte retrieval. The analysis was conducted in a subset of 440 patients who had already undergone PGT-A before the study period.
Among the first vitrified-warmed euploid blastocyst SETs, the implantation rate was 51% (N = 802/1580), the miscarriage rate was 14% (N =110/802) and the LBR was 44% (N =692/1580) (Fig. 7A). These results were clustered according to the number of previous miscarriages and of previous implantation failures. No difference was reported in the LBR per SET among patients with 1 (N =100/258, 39%, P = 0.1) or >1 previous miscarriages (N =61/136, 45%; P = 0.99) compared with patients with no previous miscarriage (N =531/1186, 45%) (Fig. 7B). On the contrary, a decreasing trend was shown in the LBR per SET depending on the number of implantation failures after previous ETs, which achieved statistical significance when comparing patients with >2 (N =93/255, 36%; P < 0.01) to patients with no previous implantation failure (N =452/970, 47%). Such a difference was evident already from the implantation rate, whereas the miscarriage rate was similar in the four groups (Fig. 7C). The data in Fig. 7B and C were also confirmed through multivariate logistic regression analyses adjusted for blastocyst morphological quality and day of full blastocyst development (Supplementary Tables SI and SII). In particular the multivariate-OR of a LB per euploid blastocyst SET in patients with >2 previous implantation failures versus patients with no previous implantation failure was 0.64, 95% CI 0.48–0.86, adjusted P-value < 0.01 (Supplementary Table SII).
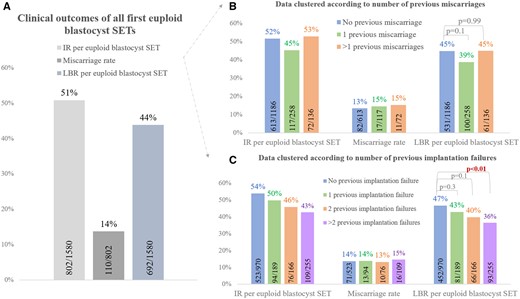
Clinical outcomes of the first euploid blastocyst single embryo transfers (SETs) conducted during the study period. (A) Overall data. (B) Data clustered according to number of previous miscarriages. (C) Data clustered according to number of previous implantation failures. IR, implantation rate; LBR, live birth rate.
Discussion
The experience of adverse events like miscarriage or implantation failure, especially after euploid embryo transfers, further worsens the already frustrating condition of idiopathic infertility, thereby making counselling even more sensitive. Reliable data are hence required to better understanding whether an adverse reproductive history is negatively associated with blastocysts’ euploidy rate, and miscarriage or LBR after euploid blastocyst transfer. This study was aimed at producing some valuable information on this topic, which are still largely missing in the literature. The analysis of 2676 patients with at least one blastocyst biopsied for PGT-A at our centre outlined a scenario where the only relevant parameter on the euploidy rate is maternal age at oocyte retrieval. Apparently, whatever reproductive history characterises the women, the impact of AMA itself is far more relevant. This was shown for previous LBs, for recurrent miscarriages, and for repeated IVF and implantation failures, as well as for the case of only aneuploid blastocysts for patients who had already undergone PGT-A before the study period.
From a clinical perspective, RPL is considered a multifactorial disorder, and several risk factors beyond AMA have been documented, such as antiphospholipid antibodies, uterine malformations, abnormal parental karyotype and immunological issues (Opatrny et al., 2006; Venetis et al., 2014; Arachchillage et al., 2015; Vissenberg et al., 2015; Morin et al., 2017; Alecsandru et al., 2020), whereas others such as stress, environment and smoking have been suggested. Still, in 40–50% of cases, the pathophysiology of RPL remains unknown (Atik et al., 2018). Despite all the recent steps forward achieved in IVF, and the well-known impact of chromosomal abnormalities on both embryo implantation potential and clinical miscarriages, it is still challenging to counsel patients with a RIF or RPL indication and to outline an efficient treatment strategy. Our data further confirm that embryonic chromosomal abnormalities are critical, but they are not the only cause of RIF and RPL (Morin et al., 2017; Colley et al., 2019; Alecsandru et al., 2020; Liu et al., 2020; van Dijk et al., 2020). Dealing with implantation, and later pregnancy, the scenario is in fact more complicated as these processes involve a timely interaction between a competent embryo and a receptive endometrium, two aspects regulated by several complex signalling pathways, cell–cell and cell–matrix interactions (Paria et al., 1993; Ma et al., 2003), which are still poorly understood (Wang and Dey 2006). In our study, the LBR per SET after first vitrified-warmed euploid blastocyst transfers outlined reassuring outcomes in case of RPL, whereas a decreasing trend was still shown based on the number of previous implantation failures, which became significant in women with a previous experience of more than two failed transfers (i.e. RIF). To explain this evidence, the ‘checkpoint hypothesis’ provides an intriguing model: the endometrium at the time of implantation must have a correct balance between receptivity and selectivity to allow a successful implantation (Macklon and Brosens 2014). An extremely receptive but poorly selective endometrium allows more easily the conception of aneuploid blastocysts culminating in early pregnancy loss (i.e. super-fertility); on the contrary, a poorly receptive but extremely selective endometrium could prevent the implantation of even reproductively competent euploid blastocysts. Thus, if transferring an euploid blastocysts might be beneficial in case of RPL (Wang et al., 2019), it is not equally effective in case of RIF. This latter condition could be explained also with the theory of the window of implantation (WOI): apparently the endometrium reaches its maximal receptivity for a limited period of time that in some cases could be premature or more delayed than expected (Bellver and Simon 2018). Altered WOI have been reported among RIF patients, and a personalised embryo transfer strategy, whose timing is defined through a specific molecular signature outlined on an endometrial biopsy might be useful to reduce the risk of a further implantation failure (Ruiz-Alonso et al., 2013; Simón et al., 2020). However, although promising, the evidence to date is still limited and must be expanded in the future (Bellver and Simon, 2018).
Our data should be confirmed in other centres, and with different populations of possibly younger patients. Ideally, specific analyses in patients who experienced RPL or RIF solely after euploid blastocyst transfers would provide an even less biased scenario (Pirtea et al., 2020). In such subsets of women, differences in the euploidy rates might be unveiled, as well as more significant effects on the LBR after euploid blastocyst SET. The clinical relevance of women’s reproductive history should be investigated also in a wider population of patients, including non-PGT cycles and/or cycles where no blastocyst was obtained. This design would, in fact, allow the assessment of putative associations with outcomes other than euploidy and LBRs, and increase the generalisability of this information. In this context, approaches of genomic prediction should not be overlooked in the future since they might unveil the intrinsic predisposition of certain patients to adverse reproductive events, such as total failure of fertilisation or of embryo development, exceedingly low euploidy rates, RIF, RPL, which are unexpected compared to well-established predictive criteria like maternal age (McCoy et al., 2015, 2018; Colley et al., 2019; Buonaiuto et al., 2020; Capalbo et al., 2020b; Kahraman et al., 2020). Finally, causes other than embryological factors deserve future insights: the mechanisms ruling endometrial receptivity, its crosstalk with the embryo, their ongoing interactions and all the inherent immunological implications must be investigated to further improve IVF efficiency especially in poor prognosis patients (Hernandez-Vargas et al., 2020).
Data availability
The data underlying this article are available in the article and in its online supplementary material.
Authors’ roles
D.C. and A.C. designed the study. D.C., D.S., A.G. and E.S. collected the data. D.C. analysed the data. D.C., L.D., L.T. and A.V. drafted the manuscript. All authors contributed to the interpretation and discussion of the results.
Funding
No funding was received.
Conflict of interest
The authors declare no conflict of interest related to this study.



