-
PDF
- Split View
-
Views
-
Cite
Cite
Brenda Eskenazi, Jennifer Ames, Stephen Rauch, Stefano Signorini, Paolo Brambilla, Paolo Mocarelli, Claudia Siracusa, Nina Holland, Marcella Warner, Dioxin exposure associated with fecundability and infertility in mothers and daughters of Seveso, Italy, Human Reproduction, Volume 36, Issue 3, March 2021, Pages 794–807, https://doi.org/10.1093/humrep/deaa324
Close - Share Icon Share
Abstract
Is there an association between 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) exposure and fecundability and infertility among Seveso women and their daughters?
TCDD exposure is associated with a decrease in fecundability and increased risk of infertility in women, as well as their daughters.
In animal studies, maternal exposure to TCDD is associated with decreased fertility in offspring. Effects of TCDD are mediated by activation of the aryl hydrocarbon receptor (AHR) pathway.
The Seveso Women’s Health Study (SWHS) has followed 981 women exposed to TCDD in a 1976 accident since 1996. In 2014, we initiated the Seveso Second Generation Study to follow-up their children.
We obtained information on pregnancy history including time of trying to conceive from SWHS women and their daughters who were 18 years or older. We considered TCDD exposure as initial 1976 serum TCDD concentration and estimated TCDD at pregnancy. We examined relationships of TCDD exposure with time to pregnancy (TTP, the monthly probability of conception within the first 12 months of trying) and infertility (≥12 months of trying to conceive). We also assessed contributions of polymorphisms in the AHR pathway via genetic risk score.
Among SWHS women (n = 446), median TTP was 3 months and 18% reported taking ≥12 months to conceive. Initial 1976 TCDD (log10) was associated with longer TTP (adjusted fecundability odds ratio = 0.82; 95% CI 0.68–0.98) and increased risk of infertility (adjusted relative risk = 1.35; 95% CI 1.01–1.79). TCDD at pregnancy yielded similar associations. Among SWHS daughters (n = 66), median TTP was 2 months and 11% reported taking ≥12 months to conceive. Daughters showed similar, but non-significant, associations with maternal TCDD exposure.
A limitation of this study is time to pregnancy was reported retrospectively, although previous studies have found women are able to recall time to conception with a high degree of accuracy many years after the fact. The number of SWHS daughters who had a live birth was small and we were unable to examine fecundability of SWHS sons.
Consistent with previous findings in animal studies, our study found that TCDD exposure may be associated with decreased fertility in Seveso mothers and potentially in their daughters exposed in utero. There may be susceptible genetic subgroups. The literature has largely considered the genetics of the AHR pathway in the context of male fertility but not female fertility, despite strong biological plausibility. These findings should be replicated in larger populations and of different ancestry. Future studies in Seveso should examine the sons and the grandchildren of exposed mothers given the animal literature suggesting potential heritable epigenetic effects.
This study was supported by grant numbers F06 TW02075-01 from the National Institutes of Health, R01 ES07171 and 2P30-ESO01896-17 from the National Institute of Environmental Health Sciences, R82471 from the U.S. Environmental Protection Agency and #2896 from Regione Lombardia and Fondazione Lombardia Ambiente, Milan, Italy. J.A. was supported by F31ES026488 from the National Institutes of Health. The authors declare they have no actual or potential competing financial interests.
N/A.
Introduction
2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD), a widespread environmental contaminant, is a known human carcinogen (International Agency for Research on Cancer (IARC), 1997) and potent endocrine disrupting compound (Birnbaum, 1994, 1995). TCDD bioaccumulates in the environment, is highly lipophilic, resists metabolism and has a half-life in humans of 7–9 years (Pirkle et al., 1989; Needham et al., 1994). While environmental levels have been declining due to regulatory controls, TCDD and other dioxin-like compounds such as polychlorinated dibenzo-p-dioxins (PCDDs), dibenzofurans (PCDFs) and biphenyls (PCBs), continue to be detected in the lipid stores of humans, especially those living in industrialized areas (Patterson et al., 2008; Consonni et al., 2012).
Although TCDD has the ability to interfere with endocrine function, few epidemiologic studies have examined the relationship of exposure to dioxin-like compounds and women’s fertility. Some studies have suggested an association of serum PCB concentrations (not all dioxin-like) of women and reduced fertility (Law et al., 2005; Axmon et al., 2006; Yang et al., 2008; Buck Louis et al., 2013, 2016; Chevrier et al., 2013). TCDD, due to its ability to cross the placenta, and its accumulation in the uterus (Li et al., 2006), also has the potential to adversely affect a developing fetus. In fact, animal studies suggest greater susceptibility to the effects of TCDD following in utero exposure and that these effects may persist through multiple generations (Gray and Ostby, 1995; Gray et al., 1995; Ikeda et al., 2005; Jin et al., 2008; Kakeyama et al., 2008; Arima et al., 2009; Bruner-Tran and Osteen, 2011; Ding et al., 2011; Manikkam et al., 2012). Three studies have examined maternal exposure to dioxin-like PCBs on daughters’ fertility (Cohn et al., 2011; Gennings et al., 2013; Han et al., 2016) and found longer time to pregnancy (TTP) associated with maternal serum levels. Although no previous studies have examined multigenerational exposure to TCDD in humans, one animal study reported reduced fertility in three generations of offspring of mice exposed to a single dose of TCDD during pregnancy (Bruner-Tran and Osteen, 2011).
Most effects of TCDD are mediated through binding the aryl hydrocarbon receptor (protein: AHR, gene: AHR), a nuclear receptor and transcription factor abundant in human tissues (Okey et al., 1994; Hankinson, 1995). Other dioxin-like compounds (PCDDs, PCDFs and PCBs), also bind to the AHR, but with a lower binding capacity and lower toxicity than TCDD (IARC, 1997). In interaction with TCDD, AHR and its cofactor AHR nuclear translocator (ARNT) induce expression of a pathway of dioxin-responsive genes including Cytochrome P450s (i.e. CYP1A1, CYP1BI) and the Aryl Hydrocarbon Receptor Repressor (AHRR) that metabolize TCDD but also have cross-talk with processes of steroidogenesis and cell growth. This collateral activity could disrupt AHR-mediated regulation of the female reproductive system, including functions of the ovaries, uterus and placenta and reproductive senescence (Hernandez-Ochoa et al., 2009; Cavallini et al., 2016). Differences in AHR ligand binding affinities within and across species and growing epidemiological evidence suggest that genetic variation in AHR may influence susceptibility to TCDD (Harper et al., 2002; Watanabe et al., 2004; Denison et al., 2011; Reitzel et al., 2014), by altering patterns of gene expression, disrupting steroid hormone and growth factor signal transduction pathways, or affecting cell growth and differentiation (Li et al., 2006).
On 10 July 1976, an explosion at a chemical factory in Seveso, Italy exposed nearby residents to the highest human exposure levels to TCDD ever recorded (di Domenico et al., 1980; Mocarelli et al., 1988; Needham et al., 1991). In 1996, we initiated the Seveso Women’s Health Study (SWHS), a retrospective cohort of the women residing in the area and determined exposure to TCDD by analyzing the women’s archived serum samples collected soon after the explosion (Eskenazi et al., 2000). In the only epidemiologic study thus far to investigate the role of exposure to TCDD on fertility, we reported a 25% increase in time to pregnancy and a doubling in odds of infertility with each 10-fold increase in initial TCDD concentration (Eskenazi et al., 2010). Subsequently, we followed up the women twice more and have now captured almost all of the potential pregnancies of the cohort (Eskenazi et al., 2018). This study updates the fecundability and infertility of the SWHS women. In addition, in SWHS we examine whether polymorphisms in the AHR pathway alter susceptibility to TCDD exposure. Lastly, we examine whether there are multigenerational associations, i.e. whether TCDD exposure of SWHS women is related to fertility of their daughters.
Materials and methods
Study population
Seveso Women’s Health Study
Details of the SWHS and the Seveso Second Generation Study have been presented elsewhere (Eskenazi et al., 2000, 2018). Briefly, women eligible for SWHS were newborn to 40 years old and resided in the most highly contaminated areas (Zones A or B) at the time of the explosion, and had a stored blood sample collected around that time in which to measure TCDD exposure. Enrollment and data collection consisted of three waves of follow-up (1996, 2008 and 2014). Of 981 women initially enrolled, 873 reported ever being pregnant and 617 (62.9%) had at least one pregnancy conceived after the explosion. We excluded a total of 23 women who reported a history of infertility pre-explosion (n = 1), a male partner fertility problem (n = 13) or fertility drug use within 12 months of trying to conceive (n = 9), leaving 594 women with eligible pregnancies. We further limited the analysis to women who had complete TTP data and whose pregnancy resulted in a live birth that was not due to contraceptive failure (n = 446). In addition, there were 18 women who reported trying to conceive for at least 12 months but were unsuccessful; these women were included in analyses of infertility only (see Supplementary Fig. S1 for flow diagram of SWHS sample). The sample size was reduced to 442 for gene by TCDD environmental exposure (GxE) analyses, as 4 women did not have genotype data.
Seveso Second Generation Study: the daughters
Children of SWHS participants were eligible to participate in the Seveso Second Generation Study if they were born after the explosion (10 July 1976) and were at least 2 years of age. Questionnaire data were complete for a total of 677 children (341 females, 336 males) born to 438 mothers. Of the 341 female children (75.3% of 453 eligible), 116 were <18 years and, therefore, not asked about pregnancy history, leaving an analysis sample of 225 daughters born to 197 mothers. Of these, 76 daughters reported ever having been pregnant, and 67 reported at least one pregnancy that resulted in a live birth not due to contraceptive failure. We excluded one who reported a male partner fertility problem, leaving 66 daughters born to 62 mothers for the TTP analysis. In addition, there were three daughters who reported trying to conceive for at least 12 months, but were unsuccessful; they were included in the infertility analyses only (N = 69 daughters born to 64 mothers) (see Supplementary Fig. S2 for flow diagram of SWHS daughters). The study was approved by the Institutional Review Boards of the participating institutions. Before data collection, we obtained written informed consent from SWHS women and daughters.
Data collection
Seveso Women’s Health Study
At each follow-up visit, SWHS women underwent a fasting blood draw, anthropometric measurements and personal interview, which included questions about demographic and lifestyle characteristics and medical histories, including reproductive history and characteristics. Interviews were conducted privately in Italian by trained nurse-interviewers who were unaware of participants’ zone of residence or serum TCDD levels. Women were asked basic questions about each pregnancy, including outcome, duration, smoking and maternal and child health. Several additional questions were asked about the first pregnancy conceived after the explosion that resulted in a live birth (i.e. if the first pregnancy resulted in a miscarriage or other non-live birth outcome and the second pregnancy resulted in a live birth, information was collected about the second pregnancy). Detailed information collected about this pregnancy included questions about whether they had been trying to become pregnant, the length of time (in months) the mothers had tried to conceive before becoming pregnant, contraceptive use at the time of conception and the use of fertility drugs in the cycle in which they conceived. Additionally, women (including those without a pregnancy ending in a live birth) were asked whether they had ever tried for 12 months or more to become pregnant.
Seveso Second Generation Study: the daughters
Data collection for the Seveso Second Generation Study was similar to the SWHS, and included a fasting blood draw, anthropometric measurements, personal structured interview and nutritional food frequency questionnaire. Information collected during the interview included sociodemographic and lifestyle characteristics as well as reproductive and medical histories. In addition to questions about each pregnancy, detailed information was collected on the first pregnancy resulting in a live birth, including on time to conception and contraceptive use. Questions on TTP and infertility asked of SWHS daughters mirrored those asked to the SWHS cohort.
TCDD exposure
Seveso Women’s Health Study
We examined TCDD exposure of SWHS women in two ways: (i) initial (1976) serum TCDD concentration and (ii) estimated TCDD concentration at pregnancy. For all SWHS participants, TCDD was measured in archived sera collected soon after the explosion using high-resolution gas chromatography/high-resolution mass spectrometry methods (Patterson et al., 1987); details are presented elsewhere (Eskenazi et al., 2000, 2004). For the subset of SWHS women who reported a live birth between 1996 and 2014 (n = 312), TCDD was also measured in archived serum collected at the 1996 or 2008 follow-up study using high-resolution gas chromatography/isotope-dilution high-resolution mass spectrometry methods (Patterson and Turner, 2005); details are presented elsewhere (Warner et al., 2014; Eskenazi et al., 2018). All concentrations are reported on a lipid-weight basis, as parts-per-trillion (ppt), by dividing TCDD on a whole-weight basis by total serum lipid content, estimated from measurements of triglycerides and total cholesterol (Akins et al., 1989). Non-detectable values were assigned a value of one-half the detection limit (Hornung and Reed, 1990).
As previously described, we estimated TCDD at pregnancy by extrapolation from the TCDD concentration (1976, 1996 and 2008) closest to but preceding the pregnancy using a first-order kinetic model with a half-life varying with initial concentration, age and other covariates (Warner et al., 2014; Eskenazi et al., 2018). TCDD at pregnancy estimates were extrapolated from serum TCDD levels measured in samples collected in 1976 for 319 pregnancies, in 1996 for 108 pregnancies and in 2008 for 19 pregnancies.
Seveso Second Generation Study: the daughters
We utilized the above-described maternal TCDD exposure measures to examine TCDD exposure of SWHS daughters as: (i) maternal initial (1976) serum TCDD concentration and (ii) estimated maternal TCDD at the pregnancy or TCDD in utero. For all 66 daughters, estimated maternal TCDD at the pregnancy (TCDD in utero) was based on extrapolation from maternal serum TCDD levels measured in samples collected in 1976.
SNP selection and genotyping
We used the HapMap browser in the Caucasian population of European descent (CEPH) and the 1000 Genomes Toscani in the Italia population (TSI) to choose single-nucleotide polymorphisms (SNPs) from candidate genes in the AHR pathway that were expected to have minor allele frequencies greater than 5% in our Italian Caucasian sample. In cases where SNPs were in linkage disequilibrium (LD), we prioritized inclusion of tagSNPs and SNPs with known or suspected functional relevance based on genomic location or citations in the literature. Our final genotyping assay comprised 87 SNPs across seven genes: AHR (n = 19), ARNT (n = 7), AHRR (n = 39), CYP1A1 (n = 5), CYP1A2 (n = 3), CYP1B1 (n = 13) and HSP90 (heat shock protein 90) (n = 1) (see descriptions in Supplementary Table SI).
As described previously (Ames et al., 2018), SWHS maternal DNA for genotyping was isolated from archived blood samples using a QIAamp Blood DNA Maxi kit (QIAGEN, Valencia, CA, USA), with modification detailed in Holland et al. (2006). We performed high throughput genotyping of selected SNPs using the multiplex platform iPLEX (Sequenom, San Diego, CA, USA) at the Genomics Center at University of Minnesota. Primary steps involved multiplex PCR, single-base primer extension and then mass spectrometry to determine genotypes. Quality assurance procedures included assessment of duplicates of participant samples and randomly distributed blank samples. We performed additional genotyping to resolve samples with lower success rates and achieved call rates above 90% for all 87 SNPs. All genotype distributions were assessed for accordance with Hardy–Weinberg equilibrium assumptions; SNPs that departed from equilibrium were excluded from further analysis (n = 6).
Statistical analysis
All four TCDD exposure variables (SWHS—initial 1976 TCDD, TCDD at pregnancy; Daughters—maternal initial 1976 TCDD, TCDD in utero) were log10-transformed to reduce the influence of outliers. We evaluated the form of the dose–response curve with generalized additive models using restricted cubic splines with three degrees of freedom; we found no evidence suggesting non-linearity, so we used continuous TCDD exposures in all models.
To examine the association between TCDD exposure and TTP, we estimated fecundability odds ratios (fORs) using Cox proportional hazards models adapted for discrete-time data. The fOR can be viewed as the change in the odds of conceiving in a given cycle associated with a 10-fold increase in TCDD concentration. A fOR <1.0 indicates reduced fecundability or longer TTP. TTP was measured in months and values were censored at 13 months. ‘Infertility’ was defined as 12 or more months to pregnancy. To examine the association between TCDD exposure and infertility, we estimated relative risks (RRs) of infertility with binary models using a log link and Poisson distribution function, and standard errors produced by the Huber–White sandwich estimator.
Seveso Women’s Health Study
Covariates included in adjusted models for SWHS women were based on a directed acyclic graph (DAG) (Supplementary Fig. S3) and included maternal age at start of trying to conceive, oral contraceptive (OC) use in the year before attempt, maternal smoking in the year before conception, parity before the explosion, menstrual cycle irregularity and history of reproductive and endocrine conditions (including pelvic infection, uterine fibroids, endometriosis or thyroid problems). In addition, we adjusted for paternal age at the time of conception due to its relationship with fertility. Additional covariates considered but not included in final models included marital status, frequency of sexual intercourse, whether actively trying or ‘not concerned’ about becoming pregnant, menstrual cycle length, age at menarche, history of Cesarean section, alcohol and coffee consumption, maternal and paternal education, paternal zone of residence at the time of the explosion and paternal smoking around the time of conception. For the 18 women who did not become pregnant, we imputed paternal age at the time of the initial attempt to become pregnant from maternal age and the age they married.
We conducted several sensitivity analyses for TTP as recommended for retrospective studies (Joffe et al., 2005). Models were repeated: (i) limiting the sample to the first post-explosion live birth (n = 371) as done previously (Eskenazi et al., 2010); (ii) including 18 women who had not achieved pregnancy by the time of interview censored at 13 months; (iii) excluding 159 women who reported conceiving in the first cycle of trying; (iv) including 114 women who reported getting pregnant as a result of contraceptive failure; and (v) changing the censoring time to 14, 10 or 7 months. When included in sensitivity analyses, women who reported getting pregnant as a result of contraceptive failure with consistent contraceptive use were assigned a TTP of 0, while women who reported ‘not quite regular’ contraceptive use were assigned a TTP value equal to half the duration of trying. We performed one sensitivity analysis for infertility including as infertile the nine excluded women who reported fertility drug use within 12 months of trying to conceive.
Seveso Second Generation Study: the daughters
Covariates included in adjusted models for SWHS daughters were limited due to the small sample size; final models included daughter’s age at trying to become pregnant, smoking in the year before attempting pregnancy and paternal age. Additional covariates considered one at a time but not included in final models included irregular menstrual cycle, OC use in year before, marital status and history of reproductive or endocrine condition.
Gene × TCDD interaction in SWHS
In SWHS women, we assessed genetic contributions to the TCDD-fertility relationship with measures of initial 1976 TCDD. For genotype analyses, we assumed a dominant penetrance model (i.e. inheriting 0 vs at least 1 minor allele). To evaluate the main effects of each SNP, we constructed models containing terms for TCDD and the SNP. We then considered adjusted models of interaction between genotype and TCDD on fertility by constructing a cross-product term of genotype and TCDD (i.e. SNP × TCDD). We accounted for multiple comparisons by adjusting P-values using the Benjamini–Hochberg correction with an overall false discovery rate of 0.05.
Due to the large number of SNPs, we expected that few if any interactions would remain significant after adjusting for multiple comparisons. To mitigate this issue and to address the possibility of additive polygenic risk, we constructed an overall genetic risk score, a method that has been shown to increase power in genetic studies (Dudbridge, 2013). We selected candidate SNPs from individual models if the interaction P-value was <0.2. In cases where SNPs were in LD (≥0.9), we chose a single representative from the pair or cluster based on known or suspected functional relevance, according to the literature, Ensembl Project’s Variant Effect Predictor tool (Zerbino et al., 2018) or the open-access Regulome SNP database (Boyle et al., 2012). For each SNP, each participant was assigned a score of 0 (no risk alleles) or 1 (one or two risk alleles), and the items were added to create an overall risk score. In addition, we calculated a weighted version of the score, with each SNP’s weight determined by the magnitude of the SNP × TCDD interaction term (using the natural log of the hazard ratio for TTP models and the natural log to the RR for infertility models), standardized by the sum of the weights. For analyses, we divided these weighted scores into tertiles. All statistical analyses were performed using STATA 15.0 (Stata Corporation, College Station, TX, USA), except assessments of LD, for which we used the R package LDheatmap.
Results
Seveso Women’s Health Study
At the time of the explosion, SWHS women averaged 14.4 (SD = 7.9) years and 83% were nulliparous (Table I). Women averaged 27.2 years (SD = 4.5) at the time of their index pregnancy. About a quarter of women (22%) used OCs in the year prior to trying to become pregnant, 26% had smoked cigarettes, 10% had an irregular menstrual cycle and ∼15% had a history of a reproductive or endocrine condition that could affect fertility. The initial 1976 TCDD concentration of SWHS women was high (median = 61.4 ppt). Initial TCDD levels were higher among women who were nulliparous and among those who used OCs in the year before pregnancy. As reported previously, in the full SWHS cohort, initial 1976 TCDD levels were higher among those who were youngest at the time of explosion (Eskenazi et al., 2004). The associations of TCDD levels with parity and OCs likely reflect this association of TCDD with age.
Select characteristics and exposure summaries for participants from the Seveso Women’s Health Study and Seveso Second Generation Study, Seveso, Italy, 1976–2016.
| . | SWHS . | Second generation daughters . | ||||
|---|---|---|---|---|---|---|
| . | N (%) . | Initial 1976 TCDD Median (IQR) . | Estimated TCDD at pregnancy Median (IQR) . | N (%) . | Maternal initial 1976 TCDD Median (IQR) . | Estimated TCDD in utero Median (IQR) . |
| . | ||||||
| Total . | 446 (100) . | 61.4 (29.2–174.0) . | 12.8 (6.2–31.3) . | 66 (100) . | 52.8 (27.0–128.0) . | 35.8 (19.5–85.8) . |
| Age at explosion (years) | ||||||
| 0–10 | 143 (32.1) | 162.0 (55.9–327.0) | 5.7 (3.0–10.5) | |||
| 11–20 | 193 (43.3) | 52.6 (26.7–109.0) | 14.4 (8.3–30.6) | |||
| 21–30 | 102 (22.9) | 41.6 (22.2–90.1) | 31.1 (17.5–69.4) | |||
| 30–40 | 8 (1.8) | 32.4 (25.4–88.4) | 32.9 (20.0–62.9) | |||
| Parity at time of explosion | ||||||
| Nulliparous | 371 (83.2) | 70.0 (31.7–206.0) | 10.9 (5.5–26.4) | |||
| Parous | 75 (16.8) | 38.5 (22.2–86.2) | 30.6 (17.5–69.4) | |||
| Age at pregnancy attempt (years) | ||||||
| <25 | 126 (28.3) | 46.4 (23.2–100.0) | 19.1 (10.3–39.1) | 8 (12.1) | 86.3 (14.8–221.0) | 24.4 (10.8–91.0) |
| 25–29 | 192 (43.1) | 63.2 (28.2–180.4) | 12.4 (6.4–31.4) | 34 (51.5) | 57.8 (35.5–105.0) | 36.1 (21.8–83.7) |
| 30–34 | 98 (22.0) | 92.3 (41.9–220.0) | 8.1 (3.7–18.6) | 22 (33.3) | 37.7 (22.5–126.0) | 34.6 (19.5–85.8) |
| ≥35 | 30 (6.7) | 139.4 (35.8–245.0) | 7.3 (4.9–19.6) | 2 (3.0) | 127.85 (64.7–191) | 127.85 (64.7–191) |
| Marital status | ||||||
| Married/living as married | 380 (85.2) | 40.4 (22.5–140.0) | 15.1 (8.2–37.1) | 61 (92.4) | 54.3 (29.9–128.0) | 35.8 (21.3–85.8) |
| Not married/living as married | 66 (14.8) | 64.0 (31.7–178.0) | 12.0 (6.0–31.1) | 5 (7.6) | 19.5 (18.4–68.6) | 19.5 (18.4–68.6) |
| Education | ||||||
| Less than high school | 103 (23.1) | 62.4 (23.9–212.0) | 26.2 (9.0–49.9) | 13 (19.7) | 37.7 (35.5–128.0) | 35.5 (25.7–70.4) |
| High school or technical | 322 (72.2) | 60.3 (29.9–166.0) | 11.2 (6.0–26.4) | 40 (60.6) | 47.0 (24.5–133.5) | 35.3 (18.6–107.1) |
| Bachelor’s degree or higher | 21 (4.7) | 75.7 (30.2–192.0) | 6.3 (3.4–12.9) | 13 (19.7) | 72.0 (19.5–105.0) | 56.5 (19.5–72.7) |
| Smoked in year before | ||||||
| No | 332 (74.4) | 61.8 (31.4–189.0) | 12.2 (5.7–31.4) | 46 (69.7) | 48.5 (25.1–185.0) | 37.8 (19.5–106.1) |
| Yes | 114 (25.6) | 61.3 (23.3–157.0) | 13.7 (7.2–31.0) | 20 (30.3) | 57.8 (32.9–88.4) | 35.1 (19.7–67.1) |
| OC use in year before | ||||||
| No | 350 (78.5) | 61.1 (28.9–180.0) | 11.9 (5.7–30.4) | 52 (78.8) | 44.0 (24.5–94.7) | 34.6 (18.6–66.7) |
| Yes | 96 (21.5) | 69.6 (37.2–165.0) | 17.3 (7.9–32.5) | 14 (21.2) | 133.5 (61.3–245.0) | 106.9 (35.8–117.0) |
| Menstrual cycle | ||||||
| Regular | 400 (89.7) | 62.6 (29.5–182.5) | 13.5 (6.3–31.7) | 55 (83.3) | 51.3 (23.9–126.0) | 34.8 (18.8–70.4) |
| Irregular | 46 (10.3) | 54.3 (22.0–148.0) | 9.5 (3.9–26.0) | 11 (16.7) | 99.5 (37.5–238.0) | 72.7 (35.5–112.7) |
| History of reproductive or endocrine conditions | ||||||
| No | 381 (85.4) | 60.1 (29.0–163.0) | 14.1 (6.8–32.0) | 59 (89.4) | 51.3 (27.0–135.0) | 35.8 (19.5–88.9) |
| Yes | 65 (14.6) | 98.9 (42.2–264.0) | 7.7 (3.2–15.0) | 7 (10.6) | 54.3 (22.5–126.0) | 35.7 (18.8–85.8) |
| . | SWHS . | Second generation daughters . | ||||
|---|---|---|---|---|---|---|
| . | N (%) . | Initial 1976 TCDD Median (IQR) . | Estimated TCDD at pregnancy Median (IQR) . | N (%) . | Maternal initial 1976 TCDD Median (IQR) . | Estimated TCDD in utero Median (IQR) . |
| . | ||||||
| Total . | 446 (100) . | 61.4 (29.2–174.0) . | 12.8 (6.2–31.3) . | 66 (100) . | 52.8 (27.0–128.0) . | 35.8 (19.5–85.8) . |
| Age at explosion (years) | ||||||
| 0–10 | 143 (32.1) | 162.0 (55.9–327.0) | 5.7 (3.0–10.5) | |||
| 11–20 | 193 (43.3) | 52.6 (26.7–109.0) | 14.4 (8.3–30.6) | |||
| 21–30 | 102 (22.9) | 41.6 (22.2–90.1) | 31.1 (17.5–69.4) | |||
| 30–40 | 8 (1.8) | 32.4 (25.4–88.4) | 32.9 (20.0–62.9) | |||
| Parity at time of explosion | ||||||
| Nulliparous | 371 (83.2) | 70.0 (31.7–206.0) | 10.9 (5.5–26.4) | |||
| Parous | 75 (16.8) | 38.5 (22.2–86.2) | 30.6 (17.5–69.4) | |||
| Age at pregnancy attempt (years) | ||||||
| <25 | 126 (28.3) | 46.4 (23.2–100.0) | 19.1 (10.3–39.1) | 8 (12.1) | 86.3 (14.8–221.0) | 24.4 (10.8–91.0) |
| 25–29 | 192 (43.1) | 63.2 (28.2–180.4) | 12.4 (6.4–31.4) | 34 (51.5) | 57.8 (35.5–105.0) | 36.1 (21.8–83.7) |
| 30–34 | 98 (22.0) | 92.3 (41.9–220.0) | 8.1 (3.7–18.6) | 22 (33.3) | 37.7 (22.5–126.0) | 34.6 (19.5–85.8) |
| ≥35 | 30 (6.7) | 139.4 (35.8–245.0) | 7.3 (4.9–19.6) | 2 (3.0) | 127.85 (64.7–191) | 127.85 (64.7–191) |
| Marital status | ||||||
| Married/living as married | 380 (85.2) | 40.4 (22.5–140.0) | 15.1 (8.2–37.1) | 61 (92.4) | 54.3 (29.9–128.0) | 35.8 (21.3–85.8) |
| Not married/living as married | 66 (14.8) | 64.0 (31.7–178.0) | 12.0 (6.0–31.1) | 5 (7.6) | 19.5 (18.4–68.6) | 19.5 (18.4–68.6) |
| Education | ||||||
| Less than high school | 103 (23.1) | 62.4 (23.9–212.0) | 26.2 (9.0–49.9) | 13 (19.7) | 37.7 (35.5–128.0) | 35.5 (25.7–70.4) |
| High school or technical | 322 (72.2) | 60.3 (29.9–166.0) | 11.2 (6.0–26.4) | 40 (60.6) | 47.0 (24.5–133.5) | 35.3 (18.6–107.1) |
| Bachelor’s degree or higher | 21 (4.7) | 75.7 (30.2–192.0) | 6.3 (3.4–12.9) | 13 (19.7) | 72.0 (19.5–105.0) | 56.5 (19.5–72.7) |
| Smoked in year before | ||||||
| No | 332 (74.4) | 61.8 (31.4–189.0) | 12.2 (5.7–31.4) | 46 (69.7) | 48.5 (25.1–185.0) | 37.8 (19.5–106.1) |
| Yes | 114 (25.6) | 61.3 (23.3–157.0) | 13.7 (7.2–31.0) | 20 (30.3) | 57.8 (32.9–88.4) | 35.1 (19.7–67.1) |
| OC use in year before | ||||||
| No | 350 (78.5) | 61.1 (28.9–180.0) | 11.9 (5.7–30.4) | 52 (78.8) | 44.0 (24.5–94.7) | 34.6 (18.6–66.7) |
| Yes | 96 (21.5) | 69.6 (37.2–165.0) | 17.3 (7.9–32.5) | 14 (21.2) | 133.5 (61.3–245.0) | 106.9 (35.8–117.0) |
| Menstrual cycle | ||||||
| Regular | 400 (89.7) | 62.6 (29.5–182.5) | 13.5 (6.3–31.7) | 55 (83.3) | 51.3 (23.9–126.0) | 34.8 (18.8–70.4) |
| Irregular | 46 (10.3) | 54.3 (22.0–148.0) | 9.5 (3.9–26.0) | 11 (16.7) | 99.5 (37.5–238.0) | 72.7 (35.5–112.7) |
| History of reproductive or endocrine conditions | ||||||
| No | 381 (85.4) | 60.1 (29.0–163.0) | 14.1 (6.8–32.0) | 59 (89.4) | 51.3 (27.0–135.0) | 35.8 (19.5–88.9) |
| Yes | 65 (14.6) | 98.9 (42.2–264.0) | 7.7 (3.2–15.0) | 7 (10.6) | 54.3 (22.5–126.0) | 35.7 (18.8–85.8) |
IQR, interquartile range; OC, oral contraceptive; SWHS, Seveso Women’s Health Study; TCDD, 2,3,7,8-tetrachlorodibenzo-p-dioxin.
Select characteristics and exposure summaries for participants from the Seveso Women’s Health Study and Seveso Second Generation Study, Seveso, Italy, 1976–2016.
| . | SWHS . | Second generation daughters . | ||||
|---|---|---|---|---|---|---|
| . | N (%) . | Initial 1976 TCDD Median (IQR) . | Estimated TCDD at pregnancy Median (IQR) . | N (%) . | Maternal initial 1976 TCDD Median (IQR) . | Estimated TCDD in utero Median (IQR) . |
| . | ||||||
| Total . | 446 (100) . | 61.4 (29.2–174.0) . | 12.8 (6.2–31.3) . | 66 (100) . | 52.8 (27.0–128.0) . | 35.8 (19.5–85.8) . |
| Age at explosion (years) | ||||||
| 0–10 | 143 (32.1) | 162.0 (55.9–327.0) | 5.7 (3.0–10.5) | |||
| 11–20 | 193 (43.3) | 52.6 (26.7–109.0) | 14.4 (8.3–30.6) | |||
| 21–30 | 102 (22.9) | 41.6 (22.2–90.1) | 31.1 (17.5–69.4) | |||
| 30–40 | 8 (1.8) | 32.4 (25.4–88.4) | 32.9 (20.0–62.9) | |||
| Parity at time of explosion | ||||||
| Nulliparous | 371 (83.2) | 70.0 (31.7–206.0) | 10.9 (5.5–26.4) | |||
| Parous | 75 (16.8) | 38.5 (22.2–86.2) | 30.6 (17.5–69.4) | |||
| Age at pregnancy attempt (years) | ||||||
| <25 | 126 (28.3) | 46.4 (23.2–100.0) | 19.1 (10.3–39.1) | 8 (12.1) | 86.3 (14.8–221.0) | 24.4 (10.8–91.0) |
| 25–29 | 192 (43.1) | 63.2 (28.2–180.4) | 12.4 (6.4–31.4) | 34 (51.5) | 57.8 (35.5–105.0) | 36.1 (21.8–83.7) |
| 30–34 | 98 (22.0) | 92.3 (41.9–220.0) | 8.1 (3.7–18.6) | 22 (33.3) | 37.7 (22.5–126.0) | 34.6 (19.5–85.8) |
| ≥35 | 30 (6.7) | 139.4 (35.8–245.0) | 7.3 (4.9–19.6) | 2 (3.0) | 127.85 (64.7–191) | 127.85 (64.7–191) |
| Marital status | ||||||
| Married/living as married | 380 (85.2) | 40.4 (22.5–140.0) | 15.1 (8.2–37.1) | 61 (92.4) | 54.3 (29.9–128.0) | 35.8 (21.3–85.8) |
| Not married/living as married | 66 (14.8) | 64.0 (31.7–178.0) | 12.0 (6.0–31.1) | 5 (7.6) | 19.5 (18.4–68.6) | 19.5 (18.4–68.6) |
| Education | ||||||
| Less than high school | 103 (23.1) | 62.4 (23.9–212.0) | 26.2 (9.0–49.9) | 13 (19.7) | 37.7 (35.5–128.0) | 35.5 (25.7–70.4) |
| High school or technical | 322 (72.2) | 60.3 (29.9–166.0) | 11.2 (6.0–26.4) | 40 (60.6) | 47.0 (24.5–133.5) | 35.3 (18.6–107.1) |
| Bachelor’s degree or higher | 21 (4.7) | 75.7 (30.2–192.0) | 6.3 (3.4–12.9) | 13 (19.7) | 72.0 (19.5–105.0) | 56.5 (19.5–72.7) |
| Smoked in year before | ||||||
| No | 332 (74.4) | 61.8 (31.4–189.0) | 12.2 (5.7–31.4) | 46 (69.7) | 48.5 (25.1–185.0) | 37.8 (19.5–106.1) |
| Yes | 114 (25.6) | 61.3 (23.3–157.0) | 13.7 (7.2–31.0) | 20 (30.3) | 57.8 (32.9–88.4) | 35.1 (19.7–67.1) |
| OC use in year before | ||||||
| No | 350 (78.5) | 61.1 (28.9–180.0) | 11.9 (5.7–30.4) | 52 (78.8) | 44.0 (24.5–94.7) | 34.6 (18.6–66.7) |
| Yes | 96 (21.5) | 69.6 (37.2–165.0) | 17.3 (7.9–32.5) | 14 (21.2) | 133.5 (61.3–245.0) | 106.9 (35.8–117.0) |
| Menstrual cycle | ||||||
| Regular | 400 (89.7) | 62.6 (29.5–182.5) | 13.5 (6.3–31.7) | 55 (83.3) | 51.3 (23.9–126.0) | 34.8 (18.8–70.4) |
| Irregular | 46 (10.3) | 54.3 (22.0–148.0) | 9.5 (3.9–26.0) | 11 (16.7) | 99.5 (37.5–238.0) | 72.7 (35.5–112.7) |
| History of reproductive or endocrine conditions | ||||||
| No | 381 (85.4) | 60.1 (29.0–163.0) | 14.1 (6.8–32.0) | 59 (89.4) | 51.3 (27.0–135.0) | 35.8 (19.5–88.9) |
| Yes | 65 (14.6) | 98.9 (42.2–264.0) | 7.7 (3.2–15.0) | 7 (10.6) | 54.3 (22.5–126.0) | 35.7 (18.8–85.8) |
| . | SWHS . | Second generation daughters . | ||||
|---|---|---|---|---|---|---|
| . | N (%) . | Initial 1976 TCDD Median (IQR) . | Estimated TCDD at pregnancy Median (IQR) . | N (%) . | Maternal initial 1976 TCDD Median (IQR) . | Estimated TCDD in utero Median (IQR) . |
| . | ||||||
| Total . | 446 (100) . | 61.4 (29.2–174.0) . | 12.8 (6.2–31.3) . | 66 (100) . | 52.8 (27.0–128.0) . | 35.8 (19.5–85.8) . |
| Age at explosion (years) | ||||||
| 0–10 | 143 (32.1) | 162.0 (55.9–327.0) | 5.7 (3.0–10.5) | |||
| 11–20 | 193 (43.3) | 52.6 (26.7–109.0) | 14.4 (8.3–30.6) | |||
| 21–30 | 102 (22.9) | 41.6 (22.2–90.1) | 31.1 (17.5–69.4) | |||
| 30–40 | 8 (1.8) | 32.4 (25.4–88.4) | 32.9 (20.0–62.9) | |||
| Parity at time of explosion | ||||||
| Nulliparous | 371 (83.2) | 70.0 (31.7–206.0) | 10.9 (5.5–26.4) | |||
| Parous | 75 (16.8) | 38.5 (22.2–86.2) | 30.6 (17.5–69.4) | |||
| Age at pregnancy attempt (years) | ||||||
| <25 | 126 (28.3) | 46.4 (23.2–100.0) | 19.1 (10.3–39.1) | 8 (12.1) | 86.3 (14.8–221.0) | 24.4 (10.8–91.0) |
| 25–29 | 192 (43.1) | 63.2 (28.2–180.4) | 12.4 (6.4–31.4) | 34 (51.5) | 57.8 (35.5–105.0) | 36.1 (21.8–83.7) |
| 30–34 | 98 (22.0) | 92.3 (41.9–220.0) | 8.1 (3.7–18.6) | 22 (33.3) | 37.7 (22.5–126.0) | 34.6 (19.5–85.8) |
| ≥35 | 30 (6.7) | 139.4 (35.8–245.0) | 7.3 (4.9–19.6) | 2 (3.0) | 127.85 (64.7–191) | 127.85 (64.7–191) |
| Marital status | ||||||
| Married/living as married | 380 (85.2) | 40.4 (22.5–140.0) | 15.1 (8.2–37.1) | 61 (92.4) | 54.3 (29.9–128.0) | 35.8 (21.3–85.8) |
| Not married/living as married | 66 (14.8) | 64.0 (31.7–178.0) | 12.0 (6.0–31.1) | 5 (7.6) | 19.5 (18.4–68.6) | 19.5 (18.4–68.6) |
| Education | ||||||
| Less than high school | 103 (23.1) | 62.4 (23.9–212.0) | 26.2 (9.0–49.9) | 13 (19.7) | 37.7 (35.5–128.0) | 35.5 (25.7–70.4) |
| High school or technical | 322 (72.2) | 60.3 (29.9–166.0) | 11.2 (6.0–26.4) | 40 (60.6) | 47.0 (24.5–133.5) | 35.3 (18.6–107.1) |
| Bachelor’s degree or higher | 21 (4.7) | 75.7 (30.2–192.0) | 6.3 (3.4–12.9) | 13 (19.7) | 72.0 (19.5–105.0) | 56.5 (19.5–72.7) |
| Smoked in year before | ||||||
| No | 332 (74.4) | 61.8 (31.4–189.0) | 12.2 (5.7–31.4) | 46 (69.7) | 48.5 (25.1–185.0) | 37.8 (19.5–106.1) |
| Yes | 114 (25.6) | 61.3 (23.3–157.0) | 13.7 (7.2–31.0) | 20 (30.3) | 57.8 (32.9–88.4) | 35.1 (19.7–67.1) |
| OC use in year before | ||||||
| No | 350 (78.5) | 61.1 (28.9–180.0) | 11.9 (5.7–30.4) | 52 (78.8) | 44.0 (24.5–94.7) | 34.6 (18.6–66.7) |
| Yes | 96 (21.5) | 69.6 (37.2–165.0) | 17.3 (7.9–32.5) | 14 (21.2) | 133.5 (61.3–245.0) | 106.9 (35.8–117.0) |
| Menstrual cycle | ||||||
| Regular | 400 (89.7) | 62.6 (29.5–182.5) | 13.5 (6.3–31.7) | 55 (83.3) | 51.3 (23.9–126.0) | 34.8 (18.8–70.4) |
| Irregular | 46 (10.3) | 54.3 (22.0–148.0) | 9.5 (3.9–26.0) | 11 (16.7) | 99.5 (37.5–238.0) | 72.7 (35.5–112.7) |
| History of reproductive or endocrine conditions | ||||||
| No | 381 (85.4) | 60.1 (29.0–163.0) | 14.1 (6.8–32.0) | 59 (89.4) | 51.3 (27.0–135.0) | 35.8 (19.5–88.9) |
| Yes | 65 (14.6) | 98.9 (42.2–264.0) | 7.7 (3.2–15.0) | 7 (10.6) | 54.3 (22.5–126.0) | 35.7 (18.8–85.8) |
IQR, interquartile range; OC, oral contraceptive; SWHS, Seveso Women’s Health Study; TCDD, 2,3,7,8-tetrachlorodibenzo-p-dioxin.
The median (interquartile range, IQR) TTP of the 446 women was 3 (1–7) months and 80 women (18%) reported taking 12 months or more to conceive. An additional 18 women reported trying for 12 months or more without success. As presented in Table II, a 10-fold increase in initial 1976 TCDD was associated with lower fecundability or longer TTP (adjusted fOR = 0.82; 95% CI 0.68–0.98). Results were similar for TCDD at pregnancy (adjusted fOR = 0.81; 95% CI 0.66–0.99). Results of sensitivity analyses were generally similar to the main models for both TCDD exposure measures (Fig. 1). Adjusted-fORs ranged from 0.77 to 0.86 for initial 1976 TCDD (Fig. 1a) and from 0.78 to 0.85 for TCDD at pregnancy (Fig. 1b).
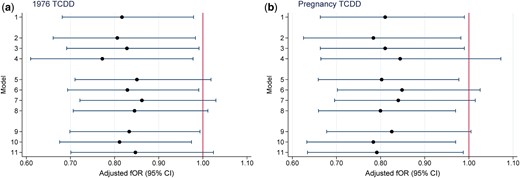
Sensitivity analyses for the relationship between TCDD exposure (log10) and time to pregnancy, Seveso Women’s Health Study, Seveso, Italy, 1976–2016. The fOR can be viewed as the change in the odds of conceiving in a given cycle associated with a 10-fold increase in TCDD concentration. (a) fOR for TCDD in 1976 and (b) fOR for TCDD at pregnancy. Models adjusted for maternal age at trying, smoking in previous year, oral contraceptive use in previous year, pre-explosion parity, irregular menstrual cycle, history of reproductive or endocrine conditions and paternal age. Key to y-axis numbers: primary analysis (Model 1) 1. Live births not due to contraceptive failure (n = 446); alternate subsets (Models 2–4). 2. Exclude women whose first pregnancy did not result in a live birth (n = 371). 3. Include women who attempted for ≥12 months who never conceived (n = 464). 4. Exclude women who conceived in the first cycle (n = 287). Include pregnancies resulting from contraceptive failure (Models 5–8). 5. Add 78 pregnancies after reported regular contraceptive use (TTP = 0) (n = 524). 6. Add 36 pregnancies after reported irregular contraceptive use (TTP = duration of irregular use/2) (n = 482). 7. Add 114 pregnancies after reported regular (TTP = 0) or irregular (TTP = duration of irregular use/2) contraceptive use (n = 560). 8. Add 114 pregnancies after reported regular or irregular contraceptive use (TTP = 0) (n = 560). Change censoring window from 12 months for TTP (Models 9–11). 9. Censor at 14 months (n = 446). 10. Censor at 10 months (n = 446). 11. Censor at 7 months (n = 446). fOR, fecundability odds ratio; TCDD, 2,3,7,8-tetrachlorodibenzo-p-dioxin; TTP, time to pregnancy.
Results for primary models of time to pregnancy and infertility associations with TCDD exposure in the Seveso Women’s Health Study and their daughters born after the explosion, Seveso, Italy, 1976–2016.
| . | Time to pregnancy . | Infertility . |
|---|---|---|
| fOR (95% CI) | RR (95% CI) | |
| SWHS | ||
| N | 446 | 98/464 |
| Initial 1976 TCDD | ||
| Unadjusted | 0.85 (0.72–1.01) | 1.29 (1.00–1.66) |
| Adjusteda | 0.82 (0.68–0.98) | 1.35 (1.01–1.79) |
| Estimated TCDD at pregnancy | ||
| Unadjusted | 0.92 (0.77–1.09) | 1.23 (0.87–1.73) |
| Adjusteda | 0.81 (0.66–0.99) | 1.27 (0.85–1.89) |
| Second generation daughters | ||
| N | 66 | 10 / 69 |
| Maternal initial 1976 TCDD | ||
| Unadjusted | 0.37 (0.25–0.55) | 1.57 (0.57–4.30) |
| Adjustedb | 0.59 (0.31–1.12) | 1.33 (0.66–2.67) |
| Estimated TCDD in utero | ||
| Unadjusted | 0.33 (0.21–0.53) | 1.72 (0.58–5.09) |
| Adjustedb | 0.59 (0.28–1.23) | 1.85 (0.82–4.19) |
| . | Time to pregnancy . | Infertility . |
|---|---|---|
| fOR (95% CI) | RR (95% CI) | |
| SWHS | ||
| N | 446 | 98/464 |
| Initial 1976 TCDD | ||
| Unadjusted | 0.85 (0.72–1.01) | 1.29 (1.00–1.66) |
| Adjusteda | 0.82 (0.68–0.98) | 1.35 (1.01–1.79) |
| Estimated TCDD at pregnancy | ||
| Unadjusted | 0.92 (0.77–1.09) | 1.23 (0.87–1.73) |
| Adjusteda | 0.81 (0.66–0.99) | 1.27 (0.85–1.89) |
| Second generation daughters | ||
| N | 66 | 10 / 69 |
| Maternal initial 1976 TCDD | ||
| Unadjusted | 0.37 (0.25–0.55) | 1.57 (0.57–4.30) |
| Adjustedb | 0.59 (0.31–1.12) | 1.33 (0.66–2.67) |
| Estimated TCDD in utero | ||
| Unadjusted | 0.33 (0.21–0.53) | 1.72 (0.58–5.09) |
| Adjustedb | 0.59 (0.28–1.23) | 1.85 (0.82–4.19) |
fOR, fecundability odds ratio; RR, relative risk.
Models adjusted for maternal age at trying, smoking in previous year, oral contraceptive use in previous year, pre-explosion parity, irregular menstrual cycle, reproductive or endocrine conditions and paternal age.
Models adjusted for maternal and paternal age at trying, and maternal smoking in the previous year.
Results for primary models of time to pregnancy and infertility associations with TCDD exposure in the Seveso Women’s Health Study and their daughters born after the explosion, Seveso, Italy, 1976–2016.
| . | Time to pregnancy . | Infertility . |
|---|---|---|
| fOR (95% CI) | RR (95% CI) | |
| SWHS | ||
| N | 446 | 98/464 |
| Initial 1976 TCDD | ||
| Unadjusted | 0.85 (0.72–1.01) | 1.29 (1.00–1.66) |
| Adjusteda | 0.82 (0.68–0.98) | 1.35 (1.01–1.79) |
| Estimated TCDD at pregnancy | ||
| Unadjusted | 0.92 (0.77–1.09) | 1.23 (0.87–1.73) |
| Adjusteda | 0.81 (0.66–0.99) | 1.27 (0.85–1.89) |
| Second generation daughters | ||
| N | 66 | 10 / 69 |
| Maternal initial 1976 TCDD | ||
| Unadjusted | 0.37 (0.25–0.55) | 1.57 (0.57–4.30) |
| Adjustedb | 0.59 (0.31–1.12) | 1.33 (0.66–2.67) |
| Estimated TCDD in utero | ||
| Unadjusted | 0.33 (0.21–0.53) | 1.72 (0.58–5.09) |
| Adjustedb | 0.59 (0.28–1.23) | 1.85 (0.82–4.19) |
| . | Time to pregnancy . | Infertility . |
|---|---|---|
| fOR (95% CI) | RR (95% CI) | |
| SWHS | ||
| N | 446 | 98/464 |
| Initial 1976 TCDD | ||
| Unadjusted | 0.85 (0.72–1.01) | 1.29 (1.00–1.66) |
| Adjusteda | 0.82 (0.68–0.98) | 1.35 (1.01–1.79) |
| Estimated TCDD at pregnancy | ||
| Unadjusted | 0.92 (0.77–1.09) | 1.23 (0.87–1.73) |
| Adjusteda | 0.81 (0.66–0.99) | 1.27 (0.85–1.89) |
| Second generation daughters | ||
| N | 66 | 10 / 69 |
| Maternal initial 1976 TCDD | ||
| Unadjusted | 0.37 (0.25–0.55) | 1.57 (0.57–4.30) |
| Adjustedb | 0.59 (0.31–1.12) | 1.33 (0.66–2.67) |
| Estimated TCDD in utero | ||
| Unadjusted | 0.33 (0.21–0.53) | 1.72 (0.58–5.09) |
| Adjustedb | 0.59 (0.28–1.23) | 1.85 (0.82–4.19) |
fOR, fecundability odds ratio; RR, relative risk.
Models adjusted for maternal age at trying, smoking in previous year, oral contraceptive use in previous year, pre-explosion parity, irregular menstrual cycle, reproductive or endocrine conditions and paternal age.
Models adjusted for maternal and paternal age at trying, and maternal smoking in the previous year.
In total, 98 of 464 SWHS women (21%) reported trying for 12 months or longer to conceive and included in the infertility analysis. As presented in Table II, a 10-fold increase in initial 1976 TCDD was associated with increased risk of infertility (adjusted RR = 1.35; 95% CI 1.01–1.79). Results were similar, but not significant, for TCDD at pregnancy (adjusted RR = 1.27; 95% CI 0.85–1.89). In the sensitivity analysis, including the women who reported fertility drug use within 12 months of trying to conceive, results for both TCDD exposure measures were dampened (adjusted RR = 1.29; 95% CI 0.98–1.69 for initial 1976 TCDD; adjusted RR = 1.15; 95% CI 0.78–1.69 for TCDD at pregnancy).
The daughters
SWHS daughters (n = 66) averaged 28.4 (SD = 3.7) years at the time of pregnancy. About 21% of daughters used OCs in the year before pregnancy and 30% smoked cigarettes, 17% had an irregular menstrual cycle and 10.6% had a history of a reproductive or endocrine condition that could affect fertility (Table I). The median 1976 TCDD concentration for the mothers of these daughters was 52.8 ppt. Maternal TCDD at pregnancy was lower at 35.8 ppt, reflecting birth years up to 13 years after the explosion (1977–1989). Both measures of maternal TCDD concentrations were higher for the daughters who reported OC use in the last year than in those who did not but did not differ by other covariates.
The median (IQR) TTP of the 66 daughters was 2 (1–5) months and 7 (10.6%) reported taking 12 months or more to conceive. An additional three women reported trying for 12 months or more without success. Among SWHS daughters, maternal initial 1976 TCDD (log10) concentration was associated with lower fecundability, albeit non-significantly (adjusted fOR = 0.59; 95% CI 0.31–1.12; Table II). Results were similar for TCDD concentration in utero (adjusted fOR = 0.59; 95% CI 0.28–1.23). The 10 of 69 daughters (14.5%) who reported trying to conceive for 12 months or longer were included in the infertility analysis. We observed an increased risk of infertility with both measures of TCDD exposure, but the confidence intervals were wide and bracketed unity (maternal initial 1976 TCDD: adjusted RR = 1.33; 95% CI 0.66–2.67; TCDD in utero: adjusted RR = 1.85; 95% CI 0.82–4.19).
Gene × TCDD interaction in SWHS
Six of the 87 SNPs showed a departure from Hardy–Weinberg equilibrium and were excluded from further analyses. Minor allele frequencies of the remaining 81 SNPs ranged from 6.0% (rs6869655) to 49.4% (rs6555205). The allele frequencies in SWHS were similar to those observed in the 1000 Genomes TSI (Auton et al., 2015).
Fecundability
In models including SNPs and TCDD, but no interaction term, six SNPs in AHRR (rs10072668, rs11746079, rs12188164, rs4956935, rs6869655 and rs768479) were independently associated with fecundability, but the inclusion of the SNPs in the model had little effect on the association with TCDD (Supplementary Table SII).
In the examination of the relationship of gene-environment interaction for fecundability, the variant alleles of 10 of the 81 SNPs (AHR: rs2066853, rs6968865; AHRR: rs10044468, rs11746079, rs17562461, rs2015774; CYP1A1: rs4646903; CYP1B1: rs1056836, rs162549, rs163080) exhibited interaction with initial 1976 TCDD on TTP (P < 0.2) (Table III); however, none of the interactions were significant after adjusting for multiple comparisons.
Models of the relationship of initial 1976 TCDD and time to pregnancy stratified by genotype at each locus using dominant model of inheritance, Seveso Women’s Health Study (n = 442), Seveso, Italy, 1976–2016.
| . | . | 0 minor alleles . | 1 or more alleles . | . | . |
|---|---|---|---|---|---|
| Gene | SNP | Adj-fOR (95% CI) | Adj-fOR (95% CI) | Pint | FDR |
| AHR | rs2066853a | 0.77 (0.63–0.94) | 1.04 (0.69–1.58) | 0.19 | 0.81 |
| rs6968865a | 0.64 (0.45–0.92) | 0.91 (0.74–1.11) | 0.09 | 0.81 | |
| AHRR | rs10044468a | 0.88 (0.72–1.08) | 0.61 (0.40–0.92) | 0.12 | 0.81 |
| rs11746079a | 0.85 (0.69–1.04) | 0.53 (0.33–0.85) | 0.08 | 0.81 | |
| rs17562461a | 0.93 (0.73–1.20) | 0.71 (0.54–0.92) | 0.13 | 0.81 | |
| rs2015774a | 0.77 (0.64–0.93) | 1.10 (0.70–1.74) | 0.14 | 0.81 | |
| CYP1A1 | rs4646903a | 0.87 (0.72–1.05) | 0.62 (0.39–0.98) | 0.17 | 0.81 |
| CYP1B1 | rs1056836a | 0.71 (0.52–0.96) | 0.91 (0.72–1.14) | 0.19 | 0.81 |
| rs162549 | 0.75 (0.59–0.94) | 0.96 (0.72–1.27) | 0.18 | 0.81 | |
| rs163080a | 0.74 (0.59–0.93) | 0.98 (0.74–1.30) | 0.13 | 0.81 |
| . | . | 0 minor alleles . | 1 or more alleles . | . | . |
|---|---|---|---|---|---|
| Gene | SNP | Adj-fOR (95% CI) | Adj-fOR (95% CI) | Pint | FDR |
| AHR | rs2066853a | 0.77 (0.63–0.94) | 1.04 (0.69–1.58) | 0.19 | 0.81 |
| rs6968865a | 0.64 (0.45–0.92) | 0.91 (0.74–1.11) | 0.09 | 0.81 | |
| AHRR | rs10044468a | 0.88 (0.72–1.08) | 0.61 (0.40–0.92) | 0.12 | 0.81 |
| rs11746079a | 0.85 (0.69–1.04) | 0.53 (0.33–0.85) | 0.08 | 0.81 | |
| rs17562461a | 0.93 (0.73–1.20) | 0.71 (0.54–0.92) | 0.13 | 0.81 | |
| rs2015774a | 0.77 (0.64–0.93) | 1.10 (0.70–1.74) | 0.14 | 0.81 | |
| CYP1A1 | rs4646903a | 0.87 (0.72–1.05) | 0.62 (0.39–0.98) | 0.17 | 0.81 |
| CYP1B1 | rs1056836a | 0.71 (0.52–0.96) | 0.91 (0.72–1.14) | 0.19 | 0.81 |
| rs162549 | 0.75 (0.59–0.94) | 0.96 (0.72–1.27) | 0.18 | 0.81 | |
| rs163080a | 0.74 (0.59–0.93) | 0.98 (0.74–1.30) | 0.13 | 0.81 |
Models adjusted for maternal age at trying, smoking in previous year, oral contraceptive use in previous year, pre-explosion parity, irregular menstrual cycle, history of reproductive or endocrine conditions and paternal age.
AHR, aryl hydrocarbon receptor; AHRR, aryl hydrocarbon receptor repressor; CYP, cytochrome P450; FDR, false discovery rate; Pint, P-value for interaction; SNP, single-nucleotide polymorphism.
Included in genetic risk allele score calculation.
Models of the relationship of initial 1976 TCDD and time to pregnancy stratified by genotype at each locus using dominant model of inheritance, Seveso Women’s Health Study (n = 442), Seveso, Italy, 1976–2016.
| . | . | 0 minor alleles . | 1 or more alleles . | . | . |
|---|---|---|---|---|---|
| Gene | SNP | Adj-fOR (95% CI) | Adj-fOR (95% CI) | Pint | FDR |
| AHR | rs2066853a | 0.77 (0.63–0.94) | 1.04 (0.69–1.58) | 0.19 | 0.81 |
| rs6968865a | 0.64 (0.45–0.92) | 0.91 (0.74–1.11) | 0.09 | 0.81 | |
| AHRR | rs10044468a | 0.88 (0.72–1.08) | 0.61 (0.40–0.92) | 0.12 | 0.81 |
| rs11746079a | 0.85 (0.69–1.04) | 0.53 (0.33–0.85) | 0.08 | 0.81 | |
| rs17562461a | 0.93 (0.73–1.20) | 0.71 (0.54–0.92) | 0.13 | 0.81 | |
| rs2015774a | 0.77 (0.64–0.93) | 1.10 (0.70–1.74) | 0.14 | 0.81 | |
| CYP1A1 | rs4646903a | 0.87 (0.72–1.05) | 0.62 (0.39–0.98) | 0.17 | 0.81 |
| CYP1B1 | rs1056836a | 0.71 (0.52–0.96) | 0.91 (0.72–1.14) | 0.19 | 0.81 |
| rs162549 | 0.75 (0.59–0.94) | 0.96 (0.72–1.27) | 0.18 | 0.81 | |
| rs163080a | 0.74 (0.59–0.93) | 0.98 (0.74–1.30) | 0.13 | 0.81 |
| . | . | 0 minor alleles . | 1 or more alleles . | . | . |
|---|---|---|---|---|---|
| Gene | SNP | Adj-fOR (95% CI) | Adj-fOR (95% CI) | Pint | FDR |
| AHR | rs2066853a | 0.77 (0.63–0.94) | 1.04 (0.69–1.58) | 0.19 | 0.81 |
| rs6968865a | 0.64 (0.45–0.92) | 0.91 (0.74–1.11) | 0.09 | 0.81 | |
| AHRR | rs10044468a | 0.88 (0.72–1.08) | 0.61 (0.40–0.92) | 0.12 | 0.81 |
| rs11746079a | 0.85 (0.69–1.04) | 0.53 (0.33–0.85) | 0.08 | 0.81 | |
| rs17562461a | 0.93 (0.73–1.20) | 0.71 (0.54–0.92) | 0.13 | 0.81 | |
| rs2015774a | 0.77 (0.64–0.93) | 1.10 (0.70–1.74) | 0.14 | 0.81 | |
| CYP1A1 | rs4646903a | 0.87 (0.72–1.05) | 0.62 (0.39–0.98) | 0.17 | 0.81 |
| CYP1B1 | rs1056836a | 0.71 (0.52–0.96) | 0.91 (0.72–1.14) | 0.19 | 0.81 |
| rs162549 | 0.75 (0.59–0.94) | 0.96 (0.72–1.27) | 0.18 | 0.81 | |
| rs163080a | 0.74 (0.59–0.93) | 0.98 (0.74–1.30) | 0.13 | 0.81 |
Models adjusted for maternal age at trying, smoking in previous year, oral contraceptive use in previous year, pre-explosion parity, irregular menstrual cycle, history of reproductive or endocrine conditions and paternal age.
AHR, aryl hydrocarbon receptor; AHRR, aryl hydrocarbon receptor repressor; CYP, cytochrome P450; FDR, false discovery rate; Pint, P-value for interaction; SNP, single-nucleotide polymorphism.
Included in genetic risk allele score calculation.
We developed a genetic risk allele score to explore joint associations of multiple SNPs in the AHR pathway in interaction with TCDD and fecundability. We considered the 10 SNPs that were interactive with TCDD. One of these SNPs (CYP1B1 rs162549) was excluded from the score due to high LD with another SNP (rs163080) already in the score, and did not contribute appreciably to the association or variance of the model. Thus, the final risk allele score was based on 9 SNPs (AHR rs2066853, rs6968865; AHRR rs10044468, rs11746079, rs17562461, rs2015774; CYP1A1 rs464903; CYP1B1 rs1056836, rs163080) (Table III). Unweighted risk allele scores (range 0–9) were divided into three categories: 0–3 (risk category 1), 4 (risk category 2) and ≥5 (risk category 3). As presented in Fig. 2, we observed no association of initial 1976 TCDD exposure with TTP among women in risk category 1 (fOR = 1.00; 95% CI 0.77–1.30) or risk category 2 (adjusted fOR = 1.18; 95% CI 0.83–1.67). However, among women in risk category 3, a 10-fold increase in initial 1976 TCDD was associated with significantly longer TTP (adjusted fOR 0.54; 95% CI = 0.39–0.73). Similar results were observed for the weighted genetic risk allele score; women in the highest tertile of weighted genetic risk allele score had an ∼50% reduction in the odds of conception during a given cycle for each 10-fold increase in 1976 TCDD (Fig. 2).
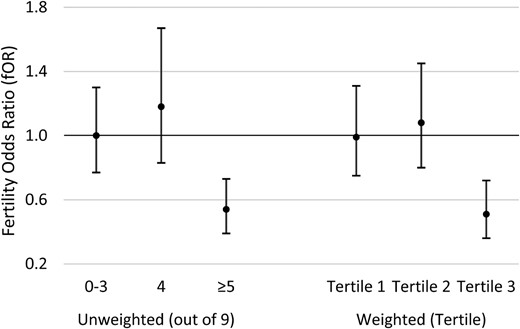
Associations between 1976 TCDD exposure and time to pregnancy based on unweighted and weighted genetic risk allele score category, Seveso Women’s Health Study (n = 442), Seveso, Italy, 1976–2016. Models adjusted for maternal age at trying, smoking in previous year, oral contraceptive use in previous year, pre-explosion parity, irregular menstrual cycle, history of reproductive or endocrine conditions and paternal age.
Infertility
In infertility models including SNPs and TCDD, but no interaction term, four SNPs (AHR rs2158041, rs6960165, rs713150; AHRR rs6869655) were independently associated with infertility, but their inclusion in the model had little effect on the association with TCDD (Supplementary Table SIII). In the examination of the relationship of gene-environment interaction for infertility, the variant alleles of 15 of the 81 SNPs (AHRR rs11746079, rs2015774, rs2672753, rs6869655; ARNT rs11204737, rs11552229, rs1889740, rs2228099; CYP1A1 rs4646421, rs4646903; CYP1B1 rs1056836, rs162549, rs162555, rs162560, rs163080) exhibited interaction with initial 1976 TCDD on infertility (P < 0.2) (Table IV); however, none of the interactions were significant after adjusting for multiple comparisons.
Models of the relationship of initial 1976 TCDD and infertility stratified by genotype at each locus using dominant model of inheritance, Seveso Women’s Health Study (n = 442), Seveso, Italy, 1976–2016.
| . | . | 0 minor alleles . | 1 or more alleles . | . | . |
|---|---|---|---|---|---|
| Gene | SNP | Adj-RR (95% CI) | Adj-RR (95% CI) | Pint | FDR |
| AHRR | rs11746079a | 1.32 (0.98–1.78) | 2.48 (1.21–5.05) | 0.11 | 0.81 |
| rs2015774a | 1.50 (1.09–2.05) | 0.88 (0.42–1.81) | 0.17 | 0.81 | |
| rs2672753a | 1.25 (0.91–1.71) | 2.00 (1.11–3.62) | 0.16 | 0.81 | |
| rs6869655a | 1.32 (0.98–1.77) | 3.46 (1.51–7.88) | 0.03 | 0.81 | |
| ARNT | rs11204737a | 1.83 (1.11–3.01) | 1.23 (0.89–1.70) | 0.17 | 0.81 |
| rs11552229a | 1.00 (0.65–1.54) | 1.60 (1.12–2.27) | 0.09 | 0.81 | |
| rs1889740 | 1.02 (0.64–1.63) | 1.54 (1.10–2.15) | 0.15 | 0.81 | |
| rs2228099 | 1.02 (0.64–1.63) | 1.54 (1.10–2.15) | 0.15 | 0.81 | |
| CYP1A1 | rs4646421 | 1.26 (0.93–1.71) | 2.16 (1.01–4.62) | 0.19 | 0.81 |
| rs4646903a | 1.23 (0.90–1.67) | 2.71 (1.32–5.56) | 0.04 | 0.81 | |
| CYP1B1 | rs1056836a | 1.57 (1.08–2.29) | 1.12 (0.76–1.66) | 0.20 | 0.81 |
| rs162549 | 1.61 (1.18–2.20) | 0.84 (0.49–1.43) | 0.03 | 0.81 | |
| rs162555a | 1.59 (1.16–2.18) | 0.81 (0.49–1.35) | 0.02 | 0.81 | |
| rs162560 | 1.56 (1.14–2.14) | 0.85 (0.49–1.46) | 0.05 | 0.81 | |
| rs163080a | 1.62 (1.19–2.22) | 0.79 (0.46–1.38) | 0.02 | 0.81 |
| . | . | 0 minor alleles . | 1 or more alleles . | . | . |
|---|---|---|---|---|---|
| Gene | SNP | Adj-RR (95% CI) | Adj-RR (95% CI) | Pint | FDR |
| AHRR | rs11746079a | 1.32 (0.98–1.78) | 2.48 (1.21–5.05) | 0.11 | 0.81 |
| rs2015774a | 1.50 (1.09–2.05) | 0.88 (0.42–1.81) | 0.17 | 0.81 | |
| rs2672753a | 1.25 (0.91–1.71) | 2.00 (1.11–3.62) | 0.16 | 0.81 | |
| rs6869655a | 1.32 (0.98–1.77) | 3.46 (1.51–7.88) | 0.03 | 0.81 | |
| ARNT | rs11204737a | 1.83 (1.11–3.01) | 1.23 (0.89–1.70) | 0.17 | 0.81 |
| rs11552229a | 1.00 (0.65–1.54) | 1.60 (1.12–2.27) | 0.09 | 0.81 | |
| rs1889740 | 1.02 (0.64–1.63) | 1.54 (1.10–2.15) | 0.15 | 0.81 | |
| rs2228099 | 1.02 (0.64–1.63) | 1.54 (1.10–2.15) | 0.15 | 0.81 | |
| CYP1A1 | rs4646421 | 1.26 (0.93–1.71) | 2.16 (1.01–4.62) | 0.19 | 0.81 |
| rs4646903a | 1.23 (0.90–1.67) | 2.71 (1.32–5.56) | 0.04 | 0.81 | |
| CYP1B1 | rs1056836a | 1.57 (1.08–2.29) | 1.12 (0.76–1.66) | 0.20 | 0.81 |
| rs162549 | 1.61 (1.18–2.20) | 0.84 (0.49–1.43) | 0.03 | 0.81 | |
| rs162555a | 1.59 (1.16–2.18) | 0.81 (0.49–1.35) | 0.02 | 0.81 | |
| rs162560 | 1.56 (1.14–2.14) | 0.85 (0.49–1.46) | 0.05 | 0.81 | |
| rs163080a | 1.62 (1.19–2.22) | 0.79 (0.46–1.38) | 0.02 | 0.81 |
Models adjusted for maternal age at trying, smoking in previous year, oral contraceptive use in previous year, pre-explosion parity, irregular menstrual cycle, history of reproductive or endocrine conditions and paternal age.
ARNT, aryl hydrocarbon receptor nuclear translocator.
Included in genetic risk allele score calculation.
Models of the relationship of initial 1976 TCDD and infertility stratified by genotype at each locus using dominant model of inheritance, Seveso Women’s Health Study (n = 442), Seveso, Italy, 1976–2016.
| . | . | 0 minor alleles . | 1 or more alleles . | . | . |
|---|---|---|---|---|---|
| Gene | SNP | Adj-RR (95% CI) | Adj-RR (95% CI) | Pint | FDR |
| AHRR | rs11746079a | 1.32 (0.98–1.78) | 2.48 (1.21–5.05) | 0.11 | 0.81 |
| rs2015774a | 1.50 (1.09–2.05) | 0.88 (0.42–1.81) | 0.17 | 0.81 | |
| rs2672753a | 1.25 (0.91–1.71) | 2.00 (1.11–3.62) | 0.16 | 0.81 | |
| rs6869655a | 1.32 (0.98–1.77) | 3.46 (1.51–7.88) | 0.03 | 0.81 | |
| ARNT | rs11204737a | 1.83 (1.11–3.01) | 1.23 (0.89–1.70) | 0.17 | 0.81 |
| rs11552229a | 1.00 (0.65–1.54) | 1.60 (1.12–2.27) | 0.09 | 0.81 | |
| rs1889740 | 1.02 (0.64–1.63) | 1.54 (1.10–2.15) | 0.15 | 0.81 | |
| rs2228099 | 1.02 (0.64–1.63) | 1.54 (1.10–2.15) | 0.15 | 0.81 | |
| CYP1A1 | rs4646421 | 1.26 (0.93–1.71) | 2.16 (1.01–4.62) | 0.19 | 0.81 |
| rs4646903a | 1.23 (0.90–1.67) | 2.71 (1.32–5.56) | 0.04 | 0.81 | |
| CYP1B1 | rs1056836a | 1.57 (1.08–2.29) | 1.12 (0.76–1.66) | 0.20 | 0.81 |
| rs162549 | 1.61 (1.18–2.20) | 0.84 (0.49–1.43) | 0.03 | 0.81 | |
| rs162555a | 1.59 (1.16–2.18) | 0.81 (0.49–1.35) | 0.02 | 0.81 | |
| rs162560 | 1.56 (1.14–2.14) | 0.85 (0.49–1.46) | 0.05 | 0.81 | |
| rs163080a | 1.62 (1.19–2.22) | 0.79 (0.46–1.38) | 0.02 | 0.81 |
| . | . | 0 minor alleles . | 1 or more alleles . | . | . |
|---|---|---|---|---|---|
| Gene | SNP | Adj-RR (95% CI) | Adj-RR (95% CI) | Pint | FDR |
| AHRR | rs11746079a | 1.32 (0.98–1.78) | 2.48 (1.21–5.05) | 0.11 | 0.81 |
| rs2015774a | 1.50 (1.09–2.05) | 0.88 (0.42–1.81) | 0.17 | 0.81 | |
| rs2672753a | 1.25 (0.91–1.71) | 2.00 (1.11–3.62) | 0.16 | 0.81 | |
| rs6869655a | 1.32 (0.98–1.77) | 3.46 (1.51–7.88) | 0.03 | 0.81 | |
| ARNT | rs11204737a | 1.83 (1.11–3.01) | 1.23 (0.89–1.70) | 0.17 | 0.81 |
| rs11552229a | 1.00 (0.65–1.54) | 1.60 (1.12–2.27) | 0.09 | 0.81 | |
| rs1889740 | 1.02 (0.64–1.63) | 1.54 (1.10–2.15) | 0.15 | 0.81 | |
| rs2228099 | 1.02 (0.64–1.63) | 1.54 (1.10–2.15) | 0.15 | 0.81 | |
| CYP1A1 | rs4646421 | 1.26 (0.93–1.71) | 2.16 (1.01–4.62) | 0.19 | 0.81 |
| rs4646903a | 1.23 (0.90–1.67) | 2.71 (1.32–5.56) | 0.04 | 0.81 | |
| CYP1B1 | rs1056836a | 1.57 (1.08–2.29) | 1.12 (0.76–1.66) | 0.20 | 0.81 |
| rs162549 | 1.61 (1.18–2.20) | 0.84 (0.49–1.43) | 0.03 | 0.81 | |
| rs162555a | 1.59 (1.16–2.18) | 0.81 (0.49–1.35) | 0.02 | 0.81 | |
| rs162560 | 1.56 (1.14–2.14) | 0.85 (0.49–1.46) | 0.05 | 0.81 | |
| rs163080a | 1.62 (1.19–2.22) | 0.79 (0.46–1.38) | 0.02 | 0.81 |
Models adjusted for maternal age at trying, smoking in previous year, oral contraceptive use in previous year, pre-explosion parity, irregular menstrual cycle, history of reproductive or endocrine conditions and paternal age.
ARNT, aryl hydrocarbon receptor nuclear translocator.
Included in genetic risk allele score calculation.
We developed a genetic risk allele score to explore joint associations of multiple SNPs in the AHR pathway in interaction with TCDD and infertility. Of the 15 SNPs that were interactive with TCDD, 5 were excluded due to high LD and the final risk allele score was based on 10 SNPs (AHRR rs11746079, rs2015774, rs2672753, rs6869655; ARNT rs11204737, rs11552229; CYP1A1 rs4646903; CYP1B1 rs1056836, rs162555, rs163080) (Table IV). Unweighted risk allele scores (range 0–10) were divided into three categories: 0–3, 4–5 and ≥6. As presented in Fig. 3, a 10-fold increase in initial 1976 TCDD exposure was associated with increased risk of infertility among women in risk category 2 (adj-RR = 1.52; 95% CI 1.03–2.26) and category 3 (adj-RR = 3.01; 95% CI 1.73–5.25). Similar results were observed for the weighted genetic risk allele score (Fig. 3).
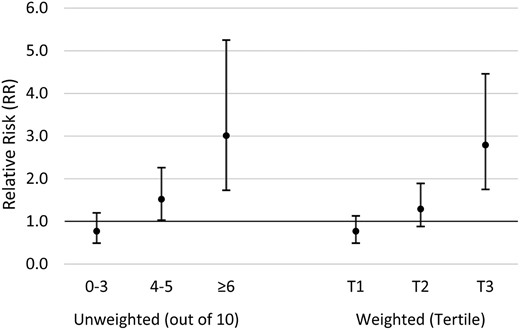
Associations between 1976 TCDD exposure and infertility based on unweighted and weighted genetic risk allele score category, Seveso Women’s Health Study (n = 442), Seveso, Italy, 1976–2016. Models adjusted for maternal age at trying, smoking in previous year, oral contraceptive use in previous year, pre-explosion parity, irregular menstrual cycle, history of reproductive or endocrine conditions and paternal age.
Discussion
In this population of women exposed to high levels of TCDD as a result of an explosion in Seveso, Italy more than 40 years ago, we have now captured almost all of their subsequent live births and have confirmed a previous finding on the earlier births of an ∼20% decrease in fecundability and about a 30% increase in risk of infertility in women per 10-fold increase in their initial serum TCDD measurements and those estimated at the time of the index pregnancy. In addition, we have shown that there may be a susceptible subgroup of women based on their genetic make-up measured by polymorphisms in the AHR pathway. We identified 5 SNPs in AHRR that were directly associated with time to pregnancy and 10 SNPs across AHR, AHRR, CYP1A1 and CYP1B1 that showed evidence of interaction with dioxin exposure. We identified 4 SNPs across AHR and AHRR directly associated with infertility and 15 SNPs across AHRR, ARNT, CYP1A1 and CYP1B1 with interactive associations. In analyses of additive genetic risk, the group with the highest genetic risk score demonstrated nearly half the fecundability and a three-fold increased risk of infertility associated with a 10-fold increase in initial 1976 TCDD. Although the sample size is relatively small and confidence intervals are wide, we found similar reductions in the fecundability and fertility of the daughters of SWHS women associated with their mother’s blood concentrations of TCDD near the time of the explosion or the daughter’s estimated in utero exposure. Unfortunately, sample size prevented an examination of genetic susceptibility in the daughters.
To our knowledge, no other study has examined fertility of women in relation to blood concentrations of TCDD, the most potent endocrine disruptor in the class of dioxin-like compounds including PCBs, furans and dioxins (IARC, 1997). The most similar exposure scenario to the Seveso cohort was that of the Yucheng cohort exposed to dioxin-like compounds (PCBs, PCDFs) in 1978–1979 as a result of ingestion of PCB-contaminated cooking oil in Taiwan (Yang et al., 2008). In 412 women, the authors reported a significantly decreased fOR of 0.90 (95% CI 0.80–1.00) and an increased infertility odds ratio of 2.34 (95% CI 1.23–4.59) for Yucheng women compared with an unexposed reference group.
We had hypothesized that those who were the youngest at the time of the explosion would be most affected because their reproductive systems were developmentally immature and we have shown that the TCDD blood concentrations were highest in those that were youngest at the time of the explosion (Eskenazi et al., 2004). However, the results from the present analyses of 446 pregnancies are similar but less robust than our earlier analysis of 278 pregnancies up to 1996 (Eskenazi et al., 2010), where we observed a 25% increase in time to pregnancy (adjusted fOR = 0.75; 95% CI 0.60–0.95) and an almost doubling in odds of infertility (adjusted OR = 1.9; 95% CI 1.1–3.2, or using the same methods as the present analysis, an adjusted RR = 1.6; 95% CI 1.2–2.3). Those 131 women with new pregnancies since the last analysis were younger at the time of the explosion (mean = 17.3 vs. 7.5 years) and had higher initial 1976 TCDD serum concentrations (GM = 58.2 vs 144.2 ppt), but lower estimated TCDD concentrations at the time of the pregnancy (GM = 22.1 vs 5.5 ppt). It is difficult to disentangle susceptibility based on age at event versus time from event to pregnancy. It is noteworthy, we observed similar results for both measures of TCDD exposure, initial 1976 TCDD and estimated TCDD at pregnancy, which may suggest multiple potential mechanisms.
Evidence from animal studies supports the potential for dioxin to influence fertility and fecundability through several biological mechanisms. In both rodents and primates, TCDD has been shown to affect steroidogenesis and ovulation (Li et al., 1995; Moran et al., 2001). Previously in the Seveso cohort, we found no relationship of TCDD with ovarian function (Warner et al., 2007), but in rats, TCDD exposure has been associated with morphologic changes in the ovary, inhibition of follicular maturation and rupture, altered hormone levels and disruption of the estrous cycle (Li et al., 1995a,b; Roby, 2001; Salisbury and Marcinkiewicz, 2002). In primates, TCDD exposure has been associated with altered hormone levels (Chaffin et al., 1996; Moran et al., 2001). Also in primates, TCDD exposure has been shown to affect early embryo development (Guo et al., 1999), although in SWHS we have consistently found no association between TCDD and clinically recognized spontaneous abortion (Eskenazi et al., 2003, 2018; Wesselink et al., 2014).
Although no other study has examined the impact of maternal exposure to TCDD on the fertility of their daughters, studies have examined the generational impact of exposure to other endocrine disruptors. In particular, studies have demonstrated decreased fertility in daughters of women who used diethylstilbestrol (DES), while pregnant (Palmer et al., 2001; Titus-Ernstoff et al., 2006; Hoover et al., 2011). However, there have been only a few studies that have followed up the daughters of women exposed to endocrine disrupting compounds in the environment. In a study of 151 daughters of Michigan fish anglers, Han et al. (2016) observed reduced fecundability associated with maternal levels of PCBs but not dichlorodiphenyldichloroethylene (DDE); individual PCB congeners were not measured. Cohn et al. (2011) followed up 289 daughters of pregnant women who participated in the Child Health and Development Studies, and found maternal serum concentrations of certain PCB congeners were associated with longer TTP and higher rates of infertility in the daughters. In a follow-up analysis using weighted quartile regression, Gennings et al. (2013) reported the dominant functionality group associated with longer TTP was the dioxin-like, potentially anti-estrogenic PCB group (PCBs 66, 74, 105, 118, 156, 167). Although considerably smaller, our study confirms the observations of Gennings et al. (2013) that maternal exposure to dioxin-like compounds may reduce the fecundability of the daughters.
In a murine model, early life exposure to TCDD resulted in reduced sensitivity of the uterus to progesterone as indicated by lower expression of the progesterone receptors in the adult female offspring (Nayyar et al., 2007). In addition, the rate of infertility observed in the second filial generation (F2) descended from singly-exposed F1 mice was increased similar to their mothers, and likely reflects the impact of TCDD exposure on F1 germ cells. A higher rate of infertility was also observed in F3. These findings are noteworthy since F3 had no direct exposure to TCDD and suggest that heritable epigenetic alterations have been induced by ancestral exposure to this toxicant.
It is biologically plausible that genetic variation in the AHR pathway could impact female reproductive health. This network of genes is highly expressed in female reproductive tissues, including the endometrium and ovaries (Hernandez-Ochoa et al., 2009). Furthermore, CYP1A1 and CYP1B1, regulated by AHR, exhibit crosstalk with estrogen receptors and synthesis of estrogen, a hormone critical to fecundability and fertility (Hernandez-Ochoa et al., 2009). However, previous research has focused primarily on indicators of male fertility. We observed six SNPs in AHRR that may be associated with female fecundability. Previously, rs2292596, a missense variant in AHRR (Pro185Ala) has been linked to male factor infertility (Watanabe et al., 2004; Brokken et al., 2014), but was not significant in the present study. SNPs in AHR that have been found to modulate male fertility and semen quality (rs2066853 and rs2158041) (Gu et al., 2011; Safarinejad et al., 2013; Brokken et al., 2014) were also not associated with longer time to pregnancy in our sample.
Our findings suggest that multiple polymorphisms across the AHR pathway may individually and cumulatively shape risk of infertility in interaction with dioxin exposure. Consistent with the shared biology underlying these two endpoints, there were six SNPs in common across the genetic risk scores for time to pregnancy and infertility (AHRR: rs11746079, rs2015774; CYP1A1: rs4646903; CYP1B1: rs1056836, rs162549, rs163080), several of which have been implicated in pathways of xenobiotic response or estrogenic regulation. Rs11746079, an intron SNP, has been identified as a methylation quantitative trait locus (mQTL) regulating AHRR expression in response to smoking exposure (Gao et al., 2017). Rs4646903, an intergenic variant, has been linked to altered induction of CYP1A1, recurrent pregnancy loss and blood-dioxin concentrations in pregnant Japanese women (Kobayashi et al., 2013; Li et al., 2017). This SNP has also been implicated in endometriosis (Guo, 2006), though inconsistently across populations of differing ancestry (Babu et al., 2005; Juo et al., 2006; Trabert et al., 2011). Rs1056836, a missense variant (Val432Leu) in CYP1B1, is linked to more rapid metabolism of estrogens and modifies infertility in men, possibly via altered sperm motility (Hu et al., 2011). While its role in female reproduction has not been closely studied, this SNP has been associated with several indicators of women’s health including severity of hot flashes in midlife, both independently and in interaction with smoking exposure (Butts et al., 2012; Ziv-Gal et al., 2012), reduced mammographic density (Dumas and Diorio, 2010) and thyroid dysfunction in women with polycystic ovarian syndrome (Zou et al., 2013). Rs162549, a 3' prime UTR variant in CYP1B1, has not been examined with respect to fertility but has been linked to developmental and cancer outcomes, with evidence of interaction with polyaromatic hydrocarbons (Burdon et al., 2010; Wang et al., 2010; Iyer et al., 2014). The remaining two SNPs shared across scores, rs2015774 and rs163080, are located in intronic regions and have no previously known health associations. There were two SNPs in AHR that were significant in fecundability but not infertility models: rs6968865, a regulatory variant, has been implicated in gene-dioxin interactions in a previous study of birthweight in SWHS offspring (Ames et al., 2018) and rs2066853, a missense variant (Arg554Lys), has been linked to infertility in men of Middle Eastern ethnicity (Safarinejad et al., 2013; Mostafa et al., 2017).
One limitation of this study is that the number of SWHS daughters who had a live birth was small and we do not know the TCDD exposure of the daughters at the time of their pregnancies or if their partners were also exposed in utero. The small number of pregnancies of the daughters is likely due to their young age (18–39 years) and the very low fertility rate in Italy (Evans, 1996; Rosina and Caltabiano, 2012). Alternatively, if SWHS women who were most susceptible to dioxin never conceived or had children later (longer TTP), their daughters would not be included in this analysis potentially underestimating the association in daughters. We were unable to examine fecundability of SWHS sons due to the limited number of pregnancies (n = 36), although a previous study in Seveso found decreased sperm concentrations in sons who breastfed from exposed mothers compared to those whose mothers had background levels of TCDD exposure (Mocarelli et al., 2011). In addition, Seveso men exposed in infancy had lower sperm count and motility and serum 17β-estradiol and higher FSH levels compared to a background exposed control group (Mocarelli et al., 2008).
Our study was also limited by the fact that time to pregnancy was reported retrospectively, although Joffe et al. (1995) found that women are able to recall time to conception with a high degree of accuracy many years after the fact. In addition, our fecundability findings are corroborated by the infertility results. As for the genetic analyses, we examined many different SNPs, and the genetic findings may not be generalizable to populations of other ancestry. While these findings warrant replication in other populations, validating the genetic risk score in an independent study sample may be challenging given the present scarcity of studies that have collected a similar combination of exposure, genotype and outcome data. We also lacked sufficient power to examine GxE associations with fertility and reproductive outcomes in the daughters.
Our study has a number of strengths. We had measures of TCDD in the women near the time of the explosion, which is unique in accident populations. Individual TCDD levels were also measured within a half-life of each pregnancy allowing for accurate estimation of in utero exposure. We examined a diverse set of SNPs across exon and introns of genes and considered the genetics of AHR pathway genes. The literature has largely considered the genetics of the AHR pathway in the context of male fertility but not female fertility, despite strong biological plausibility (Cavallini et al., 2016).
Conclusion
In conclusion, our study found that TCDD exposure may be associated with decreased fertility in Seveso mothers and potentially in their daughters exposed in utero. There may be susceptible genetic subgroups. These findings should be replicated in larger populations and of different ancestry. Future studies in Seveso should examine the sons and the grandchildren of exposed mothers given the animal literature suggesting potential heritable epigenetic effects.
Data availability
The data underlying this article cannot be shared publicly due to the privacy of individuals that participated in the study. The data will be shared on reasonable request to the corresponding author.
Acknowledgements
We gratefully acknowledge our collaborators at CDC including Donald G. Patterson, Jr., Wayman Turner and the late Larry L. Needham for their significant contributions to exposure assessment and sample analysis in the Seveso Women’s Health and Second Generation Studies, the field staff at Hospital of Desio and the participants and their families.
Authors’ roles
Study design: B.E. and M.W. Execution, analysis or interpretation of data: all authors. Statistical analysis: B.E., J.A., S.R. and M.W. Drafting of the manuscript: B.E. and M.W. Critical revision of the manuscript for important intellectual content: all authors.
Funding
This study was supported by Grant Numbers F06 TW02075-01 from the National Institutes of Health, R01 ES07171 and 2P30-ESO01896-17 from the National Institute of Environmental Health Sciences, R82471 from the U.S. Environmental Protection Agency, and #2896 from Regione Lombardia and Fondazione Lombardia Ambiente, Milan, Italy. Ms. Ames was supported by F31ES026488 from the National Institutes of Health.
Conflict of interest
The authors declare they have no actual or potential competing financial interests.



