-
PDF
- Split View
-
Views
-
Cite
Cite
Shuang-Zheng Jia, Yang Xiang, Jun-Jun Yang, Jing-hua Shi, Cong-Wei Jia, Jin-Hua Leng, Oncofertility outcomes after fertility-sparing treatment of bilateral serous borderline ovarian tumors: results of a large retrospective study, Human Reproduction, Volume 35, Issue 2, February 2020, Pages 328–339, https://doi.org/10.1093/humrep/dez307
Close - Share Icon Share
Abstract
What are the oncofertility outcomes of young women (≤40 years old) with bilateral serous borderline ovarian tumors (SBOTs) after fertility-sparing surgery?
Fertility preservation with the bilateral ovarian cystectomy procedure is feasible for bilateral SBOTs, with an acceptable oncological outcome and worthwhile pregnancy rates.
Fertility-sparing approaches are becoming the standard management of young patients with unilateral SBOTs and other borderline histological subtypes. However, there is a paucity of evidence to dictate the best management in bilateral SBOTs.
This was a retrospective observational study performed at the Peking Union Medical College Hospital in Beijing, China, between January 1999 and January 2019.
Ninety-four women (≤40 years old) with pathologically confirmed bilateral SBOTs were included. Following preoperative counseling, patients self-selected into one of three treatment modalities: bilateral ovarian cystectomy (n = 48), unilateral adnexectomy plus contralateral cystectomy (UAC; n = 31), and radical surgery (n = 15). Univariate and multivariate analyses were used to determine the clinical and pathological features associated with disease-free survival and reproductive outcomes.
During the median follow-up of 64 months (range, 4–243 months), 61 patients (65%) developed relapse, including 3 (20%) in the radical group, 26 (84%) in the UAC group and 32 (67%) in the bilateral cystectomy group. In the multivariate analyses, preoperative CA-125>300 U/mL, fertility preservation and micropapillary pattern were independently associated with adverse disease-free survival (P = 0.001, 0.03 and 0.026, respectively). Fourteen patients (15%) experienced invasive recurrence, and three (3%) died of progressive disease. The micropapillary pattern was significantly associated with invasive evolution risk (P = 0.006). Of the 49 patients who attempted to conceive, 23 (47%) achieved 27 pregnancies (24 spontaneous and three after IVF-ET), resulting in 19 live births. There was no significant difference in disease-free survival (P = 0.13) or pregnancy rate (41 vs. 50%, P = 0.56) between the UAC and bilateral procedures.
As a retrospective study conducted in a referral center, inherent biases exist. The nonrandom allocation to treatment groups and relatively small number of patients attempt to conceive might limit the statistical power of our findings. Only 41 patients (43.6%) received complete staging during their initial surgeries, so an underestimation bias in terms of the FIGO stage and extraovarian implants might have occurred.
The ultraconservative bilateral ovarian cystectomy procedure should be proposed in bilateral SBOTs when technically feasible. Invasive evolution occurs frequently in these women, and intense follow-up and oncofertility counseling are warranted, especially for those with micropapillary patterns.
No external funding was used for this study. There are no conflicts of interest to declare.
N/A.
Introduction
Serous borderline ovarian tumors (SBOTs) represent the most frequent histological subtype of borderline ovarian tumors(du Bois et al., 2013; Trillsch et al., 2014). Compared to their mucinous counterparts, SBOTs are more commonly associated with certain clinicopathological features, such as the presence of bilateral tumors, a micropapillary pattern, advanced FIGO stage, peritoneal implants and a high recurrence rate (Morice et al., 2012; du Bois et al., 2013; Bendifallah et al., 2014; Uzan et al., 2014a; Vang et al., 2017). Over the last decade, fertility-sparing surgery (FSS) that preserves the uterus and at least part of the ovary has become the gold standard for women with borderline ovarian tumors who desire to maintain their fertility (Daraï et al., 2013; du Bois et al., 2016; Delle Marchette et al., 2019). However, the increased relapse risk exposes patients, especially those who undergo cystectomy, to a potential need for repeated surgery even though no impact on overall survival has been observed (Vasconcelos and de Sousa Mendes, 2015).
Approximately one-third of SBOTs are bilateral, and most of the patients affected are of reproductive age with the desire to bear children (Fauvet et al., 2012). However, there is a paucity of evidence to dictate the best management in this specific subgroup. To date, only one randomized trial, including 32 women with bilateral borderline ovarian tumors (mainly SBOTs), has been published and demonstrated that the use of bilateral ovarian cystectomy (BOC) compared with unilateral adnexectomy plus contralateral cystectomy (UAC) significantly improved reproductive effectiveness without increasing the cumulative recurrence rate (Palomba et al., 2010, 2007), suggesting that BOC should be performed if technically feasible. Nonetheless, patients undergoing BOC had a shorter time to first recurrence and a higher need for radical intervention (Palomba et al., 2010). Unfortunately, in the study by Palomba et al., only women <35 years with early-stage disease were enrolled, and fertility-enhancement programs such as IVF-ET or IUI were initiated 12 months after surgery, restricting the generalization of the study. Bilateral SBOTs are also widely reported to be associated with a higher frequency of surface involvement, the micropapillary pattern and extraovarian implants, all of which adversely impact disease progression (Uzan et al., 2011; Vang et al., 2017). Thus, there is an urgent need to further evaluate the safety of FSS in bilateral SBOTs, especially for patients with advanced-stage disease.
The objective of our study was to establish the feasibility of fertility preservation in young women with bilateral SBOTs, as well as their oncological and reproductive outcomes. To our knowledge, this case series is the most extensive series to date that is specifically dedicated to oncofertility outcomes after conservative treatment of bilateral SBOTs.
Materials & Methods
Patients
Using a computerized database at the Peking Union Medical College Hospital in Beijing, China, consecutive patients with pathologically confirmed bilateral SBOTs who were treated and followed between January 1999 and January 2019 were identified retrospectively. Data on demographics, clinicopathological findings, follow-up information and reproductive outcomes were collected from the patients’ medical records or by telephone interview. The original slides were reviewed by a gynecologic pathologist (C.J.) using the current 2014 WHO Classification of Tumours of the Female Reproductive Organs (Kurman et al., 2014). Patients >40 years and those who were lost to follow-up within 3 months after their initial surgeries were excluded. This study was approved by the Institutional Review Board, and verbal informed consent was obtained during follow-up visits or telephone interviews.
We divided SBOTs into typical SBOTs and SBOTs with micropapillary patterns (SBOT-MPs) according to the criteria established by Burks and Seidman et al. (Burks et al., 1996; Seidman and Kurman 1996). Stromal microinvasion was recorded when foci of the stromal invasion were present but were no more than 10 mm2 (Kurman et al., 2014). Based on the absence or presence of destructive stromal invasion of the underlying tissues, we classified extraovarian peritoneal implants as noninvasive or invasive. The 2014 FIGO classification system was used for staging, based on surgical findings and pathological reports (Pereira et al., 2015).
Treatments and follow-up
FSS was defined as the preservation of the uterus and at least part of one ovary. Following preoperative counseling, patients self-selected into one of three treatment modalities: FSS with BOC; FSS with UAC; and radical surgery, including bilateral adnexectomy with or without a hysterectomy. Peritoneal surgical staging was considered complete when all peritoneal surfaces were carefully explored with cytology, random or oriented multiple biopsies, and omentectomy (Cadron et al., 2007; Colombo et al., 2019). Lymph node dissection was not mandatory, as there is no evidence supporting this procedure in borderline ovarian tumors (Shazly et al., 2016; Trillsch et al., 2015). Adjuvant chemotherapy was offered based on the pathological findings of the surgical specimen.
Patient follow-up consisted of physical and gynecological examinations, CA-125 determination, and an ultrasound scan every 3 months during the first year after surgery, every 6 months for 2 years and then yearly subsequently.
Statistical analyses
The primary outcomes were disease-free survival (DFS) and the pregnancy rate. A recurrence, including ‘borderline’ (in case of a purelyborderline recurrence and/or noninvasive peritoneal implants) or ‘invasive’ (in case of invasive adenocarcinoma or invasive implants) (Uzan et al., 2010, 2014a; Leary et al., 2014), was diagnosed radiographically or clinically and confirmed histologically. We termed pregnancy as visualization of a gestational sac, and the pregnancy rate was determined by dividing the number of patients who succeeded in achieving pregnancy by the number of patients who tried to conceive. Overall survival was defined as the interval from surgery to death or last follow-up.
The distributions of the clinical and demographic features between groups were evaluated using the chi-square test or Fisher’s exact test when appropriate. Survival curves were calculated using the Kaplan–Meier method and compared using the log-rank test. Multivariate analysis was performed with Cox’s proportional hazards model, including prognostic factors that had P values ≤0.10 in the univariable studies and those thought to be clinically significant. The final model was selected based on the forward likelihood ratio method. Furthermore, to determine factors that correlated with a successful pregnancy, the chi-square test or Fisher’s exact test was used when appropriate. All analyses were performed using SPSS software (version 25.0; Chicago, IL, USA), and a P value ≤0.05 in a two-sided test was considered significant.
Results
Patient characteristics
During the study period, 127 patients with bilateral SBOTs were identified, and 33 patients were excluded due to older age (n = 28) or insufficient follow-up data (n = 5), leaving 94 patients for further analysis. Fifteen women underwent radical surgery during their treatment (16%), and 79 patients (84.0%) had a fertility-sparing procedure, with a BOC or UAC process in 48 and 31 patients (51 and 33%), respectively.
Table I summarizes the clinical and tumor characteristics of the study cohort stratified by surgery type. The median age was 29 years (range, 20–40 years), and patients who underwent FSS were younger and more likely to be nulliparous than those in the radical surgery group (P = 0.005 and <0.001, respectively). The median preoperative surgery CA-125 value for all patients was 300.0 U/mL (range, 15.4–6425.0 U/mL), and the values were similar between groups (P = 0.42). However, among the 79 women who received FSS, these demographic features showed no significant difference between those treated with BOC and UAC (Table I).
The characteristics of the 94 young patients with bilateral serous borderline ovarian tumors.
| . | . | Fertility sparing surgery (FSS; n = 79) . | . | . | . | |
|---|---|---|---|---|---|---|
| Characteristics . | All . | Bilateral ovarian cystectomy (BOC; n = 48) . | Unilateral adnexectomy plus contralateral cystectomy (UAC; n = 31) . | Radical surgery (n = 15) . | P value (radical versus FSS) . | P value (BOC versus UAC) . |
| Age years median (range) | 29 (20–40) | 28 (21–40) | 29 (20–40) | 33 (24–40) | 0.005a | 0.95a |
| Referal | 0.14b | 0.77c | ||||
| No | 65 (69.1) | 31 (64.6) | 21 (67.7) | 13 (86.7) | ||
| Yes | 29 (30.9) | 17 (35.4) | 10 (32.3) | 2 (13.3) | ||
| Nulliparous | <0.001b | 0.12c | ||||
| Yes | 67 (71.3) | 41 (85.4) | 22 (71.0) | 4 (26.7) | ||
| No | 27 (28.7) | 7 (14.6) | 9 (29.0) | 11 (73.3) | ||
| CA 125 (n = 78)d | 0.42c | 0.86c | ||||
| ≤300 U/mL | 39 (50.0) | 20 (52.6) | 13 (50.0) | 6 (42.9) | ||
| >300 U/mL | 39 (50.0) | 18 (47.4) | 13 (50.0) | 8 (57.1) | ||
| Surgical approach | 0.016c | 0.011c | ||||
| Laparoscopy | 39 (41.5) | 28 (58.3) | 9 (29.0) | 2 (13.3) | ||
| Laparotomy | 55 (58.5) | 20 (41.7) | 22 (71.0) | 13 (86.7) | ||
| Complete staging | < 0.001c | < 0.001c | ||||
| Yes | 41 (43.6) | 9 (18.8) | 18 (58.1) | 14 (93.3) | ||
| No | 53 (56.4) | 39 (81.2) | 13 (41.9) | 1 (6.7) | ||
| Stage | 0.13c | 0.69c | ||||
| I | 48 (51.1) | 27 (56.3) | 16 (51.6) | 5 (33.3) | ||
| II–III | 46 (48.9) | 21 (43.8) | 15 (48.4) | 10 (66.7) | ||
| Surface involvement (n = 78) | 0.47b | 0.97b | ||||
| No | 9 (11.5) | 4 (10.3) | 3 (12.0) | 2 (14.3) | ||
| Yes | 69 (88.5) | 35 (89.7) | 22 (88.0) | 12 (85.7) | ||
| Micropapillary pattern | 0.37b | 0.15c | ||||
| No | 31 (33.0) | 14 (29.2) | 14 (45.2) | 3 (20.0) | ||
| Yes | 63 (67.0) | 34 (70.8) | 17 (54.8) | 12 (80.0) | ||
| Implant | 0.066b | 0.83b | ||||
| No | 48 (51.1) | 27 (56.3) | 16 (51.6) | 5 (33.3) | ||
| Noninvasive | 36 (38.3) | 17 (35.4) | 13 (41.9) | 6 (40.0) | ||
| Invasive | 10 (10.6) | 4 (8.3) | 2 (6.5) | 4 (26.7) | ||
| Adjuvant chemotherapy | 0.008b | 0.33b | ||||
| No | 76 (80.9) | 5 (10.4) | 6 (19.4) | 8 (53.3) | ||
| Yes | 18 (19.1) | 43 (89.6) | 25 (80.6) | 7 (46.6) | ||
| At least one pregnancy | 0.56c | |||||
| Success | 23/49 (46.9) | 16 (50.0) | 7 (41.2) | |||
| Failure | 26/49 (53.1) | 16 (50.0) | 10 (58.8) | |||
| . | . | Fertility sparing surgery (FSS; n = 79) . | . | . | . | |
|---|---|---|---|---|---|---|
| Characteristics . | All . | Bilateral ovarian cystectomy (BOC; n = 48) . | Unilateral adnexectomy plus contralateral cystectomy (UAC; n = 31) . | Radical surgery (n = 15) . | P value (radical versus FSS) . | P value (BOC versus UAC) . |
| Age years median (range) | 29 (20–40) | 28 (21–40) | 29 (20–40) | 33 (24–40) | 0.005a | 0.95a |
| Referal | 0.14b | 0.77c | ||||
| No | 65 (69.1) | 31 (64.6) | 21 (67.7) | 13 (86.7) | ||
| Yes | 29 (30.9) | 17 (35.4) | 10 (32.3) | 2 (13.3) | ||
| Nulliparous | <0.001b | 0.12c | ||||
| Yes | 67 (71.3) | 41 (85.4) | 22 (71.0) | 4 (26.7) | ||
| No | 27 (28.7) | 7 (14.6) | 9 (29.0) | 11 (73.3) | ||
| CA 125 (n = 78)d | 0.42c | 0.86c | ||||
| ≤300 U/mL | 39 (50.0) | 20 (52.6) | 13 (50.0) | 6 (42.9) | ||
| >300 U/mL | 39 (50.0) | 18 (47.4) | 13 (50.0) | 8 (57.1) | ||
| Surgical approach | 0.016c | 0.011c | ||||
| Laparoscopy | 39 (41.5) | 28 (58.3) | 9 (29.0) | 2 (13.3) | ||
| Laparotomy | 55 (58.5) | 20 (41.7) | 22 (71.0) | 13 (86.7) | ||
| Complete staging | < 0.001c | < 0.001c | ||||
| Yes | 41 (43.6) | 9 (18.8) | 18 (58.1) | 14 (93.3) | ||
| No | 53 (56.4) | 39 (81.2) | 13 (41.9) | 1 (6.7) | ||
| Stage | 0.13c | 0.69c | ||||
| I | 48 (51.1) | 27 (56.3) | 16 (51.6) | 5 (33.3) | ||
| II–III | 46 (48.9) | 21 (43.8) | 15 (48.4) | 10 (66.7) | ||
| Surface involvement (n = 78) | 0.47b | 0.97b | ||||
| No | 9 (11.5) | 4 (10.3) | 3 (12.0) | 2 (14.3) | ||
| Yes | 69 (88.5) | 35 (89.7) | 22 (88.0) | 12 (85.7) | ||
| Micropapillary pattern | 0.37b | 0.15c | ||||
| No | 31 (33.0) | 14 (29.2) | 14 (45.2) | 3 (20.0) | ||
| Yes | 63 (67.0) | 34 (70.8) | 17 (54.8) | 12 (80.0) | ||
| Implant | 0.066b | 0.83b | ||||
| No | 48 (51.1) | 27 (56.3) | 16 (51.6) | 5 (33.3) | ||
| Noninvasive | 36 (38.3) | 17 (35.4) | 13 (41.9) | 6 (40.0) | ||
| Invasive | 10 (10.6) | 4 (8.3) | 2 (6.5) | 4 (26.7) | ||
| Adjuvant chemotherapy | 0.008b | 0.33b | ||||
| No | 76 (80.9) | 5 (10.4) | 6 (19.4) | 8 (53.3) | ||
| Yes | 18 (19.1) | 43 (89.6) | 25 (80.6) | 7 (46.6) | ||
| At least one pregnancy | 0.56c | |||||
| Success | 23/49 (46.9) | 16 (50.0) | 7 (41.2) | |||
| Failure | 26/49 (53.1) | 16 (50.0) | 10 (58.8) | |||
Values are n (%) unless stated otherwise.
aMann–Whitney U test.
bFisher exact test.
cχ2 test.
dBased on the median value.
The characteristics of the 94 young patients with bilateral serous borderline ovarian tumors.
| . | . | Fertility sparing surgery (FSS; n = 79) . | . | . | . | |
|---|---|---|---|---|---|---|
| Characteristics . | All . | Bilateral ovarian cystectomy (BOC; n = 48) . | Unilateral adnexectomy plus contralateral cystectomy (UAC; n = 31) . | Radical surgery (n = 15) . | P value (radical versus FSS) . | P value (BOC versus UAC) . |
| Age years median (range) | 29 (20–40) | 28 (21–40) | 29 (20–40) | 33 (24–40) | 0.005a | 0.95a |
| Referal | 0.14b | 0.77c | ||||
| No | 65 (69.1) | 31 (64.6) | 21 (67.7) | 13 (86.7) | ||
| Yes | 29 (30.9) | 17 (35.4) | 10 (32.3) | 2 (13.3) | ||
| Nulliparous | <0.001b | 0.12c | ||||
| Yes | 67 (71.3) | 41 (85.4) | 22 (71.0) | 4 (26.7) | ||
| No | 27 (28.7) | 7 (14.6) | 9 (29.0) | 11 (73.3) | ||
| CA 125 (n = 78)d | 0.42c | 0.86c | ||||
| ≤300 U/mL | 39 (50.0) | 20 (52.6) | 13 (50.0) | 6 (42.9) | ||
| >300 U/mL | 39 (50.0) | 18 (47.4) | 13 (50.0) | 8 (57.1) | ||
| Surgical approach | 0.016c | 0.011c | ||||
| Laparoscopy | 39 (41.5) | 28 (58.3) | 9 (29.0) | 2 (13.3) | ||
| Laparotomy | 55 (58.5) | 20 (41.7) | 22 (71.0) | 13 (86.7) | ||
| Complete staging | < 0.001c | < 0.001c | ||||
| Yes | 41 (43.6) | 9 (18.8) | 18 (58.1) | 14 (93.3) | ||
| No | 53 (56.4) | 39 (81.2) | 13 (41.9) | 1 (6.7) | ||
| Stage | 0.13c | 0.69c | ||||
| I | 48 (51.1) | 27 (56.3) | 16 (51.6) | 5 (33.3) | ||
| II–III | 46 (48.9) | 21 (43.8) | 15 (48.4) | 10 (66.7) | ||
| Surface involvement (n = 78) | 0.47b | 0.97b | ||||
| No | 9 (11.5) | 4 (10.3) | 3 (12.0) | 2 (14.3) | ||
| Yes | 69 (88.5) | 35 (89.7) | 22 (88.0) | 12 (85.7) | ||
| Micropapillary pattern | 0.37b | 0.15c | ||||
| No | 31 (33.0) | 14 (29.2) | 14 (45.2) | 3 (20.0) | ||
| Yes | 63 (67.0) | 34 (70.8) | 17 (54.8) | 12 (80.0) | ||
| Implant | 0.066b | 0.83b | ||||
| No | 48 (51.1) | 27 (56.3) | 16 (51.6) | 5 (33.3) | ||
| Noninvasive | 36 (38.3) | 17 (35.4) | 13 (41.9) | 6 (40.0) | ||
| Invasive | 10 (10.6) | 4 (8.3) | 2 (6.5) | 4 (26.7) | ||
| Adjuvant chemotherapy | 0.008b | 0.33b | ||||
| No | 76 (80.9) | 5 (10.4) | 6 (19.4) | 8 (53.3) | ||
| Yes | 18 (19.1) | 43 (89.6) | 25 (80.6) | 7 (46.6) | ||
| At least one pregnancy | 0.56c | |||||
| Success | 23/49 (46.9) | 16 (50.0) | 7 (41.2) | |||
| Failure | 26/49 (53.1) | 16 (50.0) | 10 (58.8) | |||
| . | . | Fertility sparing surgery (FSS; n = 79) . | . | . | . | |
|---|---|---|---|---|---|---|
| Characteristics . | All . | Bilateral ovarian cystectomy (BOC; n = 48) . | Unilateral adnexectomy plus contralateral cystectomy (UAC; n = 31) . | Radical surgery (n = 15) . | P value (radical versus FSS) . | P value (BOC versus UAC) . |
| Age years median (range) | 29 (20–40) | 28 (21–40) | 29 (20–40) | 33 (24–40) | 0.005a | 0.95a |
| Referal | 0.14b | 0.77c | ||||
| No | 65 (69.1) | 31 (64.6) | 21 (67.7) | 13 (86.7) | ||
| Yes | 29 (30.9) | 17 (35.4) | 10 (32.3) | 2 (13.3) | ||
| Nulliparous | <0.001b | 0.12c | ||||
| Yes | 67 (71.3) | 41 (85.4) | 22 (71.0) | 4 (26.7) | ||
| No | 27 (28.7) | 7 (14.6) | 9 (29.0) | 11 (73.3) | ||
| CA 125 (n = 78)d | 0.42c | 0.86c | ||||
| ≤300 U/mL | 39 (50.0) | 20 (52.6) | 13 (50.0) | 6 (42.9) | ||
| >300 U/mL | 39 (50.0) | 18 (47.4) | 13 (50.0) | 8 (57.1) | ||
| Surgical approach | 0.016c | 0.011c | ||||
| Laparoscopy | 39 (41.5) | 28 (58.3) | 9 (29.0) | 2 (13.3) | ||
| Laparotomy | 55 (58.5) | 20 (41.7) | 22 (71.0) | 13 (86.7) | ||
| Complete staging | < 0.001c | < 0.001c | ||||
| Yes | 41 (43.6) | 9 (18.8) | 18 (58.1) | 14 (93.3) | ||
| No | 53 (56.4) | 39 (81.2) | 13 (41.9) | 1 (6.7) | ||
| Stage | 0.13c | 0.69c | ||||
| I | 48 (51.1) | 27 (56.3) | 16 (51.6) | 5 (33.3) | ||
| II–III | 46 (48.9) | 21 (43.8) | 15 (48.4) | 10 (66.7) | ||
| Surface involvement (n = 78) | 0.47b | 0.97b | ||||
| No | 9 (11.5) | 4 (10.3) | 3 (12.0) | 2 (14.3) | ||
| Yes | 69 (88.5) | 35 (89.7) | 22 (88.0) | 12 (85.7) | ||
| Micropapillary pattern | 0.37b | 0.15c | ||||
| No | 31 (33.0) | 14 (29.2) | 14 (45.2) | 3 (20.0) | ||
| Yes | 63 (67.0) | 34 (70.8) | 17 (54.8) | 12 (80.0) | ||
| Implant | 0.066b | 0.83b | ||||
| No | 48 (51.1) | 27 (56.3) | 16 (51.6) | 5 (33.3) | ||
| Noninvasive | 36 (38.3) | 17 (35.4) | 13 (41.9) | 6 (40.0) | ||
| Invasive | 10 (10.6) | 4 (8.3) | 2 (6.5) | 4 (26.7) | ||
| Adjuvant chemotherapy | 0.008b | 0.33b | ||||
| No | 76 (80.9) | 5 (10.4) | 6 (19.4) | 8 (53.3) | ||
| Yes | 18 (19.1) | 43 (89.6) | 25 (80.6) | 7 (46.6) | ||
| At least one pregnancy | 0.56c | |||||
| Success | 23/49 (46.9) | 16 (50.0) | 7 (41.2) | |||
| Failure | 26/49 (53.1) | 16 (50.0) | 10 (58.8) | |||
Values are n (%) unless stated otherwise.
aMann–Whitney U test.
bFisher exact test.
cχ2 test.
dBased on the median value.
Regarding management information, approximately two-fifths (n = 39, 42%) of the tumors were resected with the laparoscopic approach during their initial therapy, and this mode was more commonly used in the BOC group than in the UAC group and radical group counterparts (P = 0.011 and 0.002, respectively). Overall less than half of the patients (44%, n = 41/94) had received complete staging surgery but this proportion increased significantly from the BOC to UAC to radical procedures (19 versus 58 versus 93%, respectively, P < 0.001). After surgery, 18 patients (19%) with peritoneal implants, including all but two patients (n = 8/10, 80%) with invasive implants, received adjuvant platinum-based chemotherapy.
Regarding pathological features, 63 (67%) women had micropapillary patterns, and nearly half of the patients (n = 46, 49%) had extraovarian implants, including invasive and noninvasive implants in 10 (11%) and 36 (38%) of affected patients, respectively. No significant differences in these histological characteristics were found between groups (Table I).
Oncological outcomes
During the median follow-up of 64 months (range, 4–243 months), 61 (65%) patients developed primary relapse of borderline or invasive disease (n = 55 and n = 6, respectively) at a median time of 35 months (range, 3–130 months), including 3 (20%) in the radical group, 26 (84%) in the UAC group and 32 (67%) in the BOC group. On Kaplan–Meier analysis, both the UAC and BOC procedures were associated with worse DFS compared to the radical surgery group (P = 0.022 and P = 0.0013, respectively) (Fig. 1a). Univariate analysis demonstrated a significantly poorer prognosis in the patients aged <30 years (P = 0.003), in those with a preoperative CA-125 >300 U/mL (P = 0.012) and in those selecting an FSS procedure (P = 0.035) (Table II). And patients with SBOT-MPs showed marginally significantly poorer prognosis (P = 0.051). Thus, four variables (FSS procedures, age <30 years, CA-125 >300 U/mL and SBOT-MP) were included in our multivariate analysis. Data analysis showed that preoperative CA-125>300 U/mL, fertility preservation and micropapillary pattern were independently associated with adverse DFS (P = 0.001, 0.03 and 0.026, respectively) (Table II).
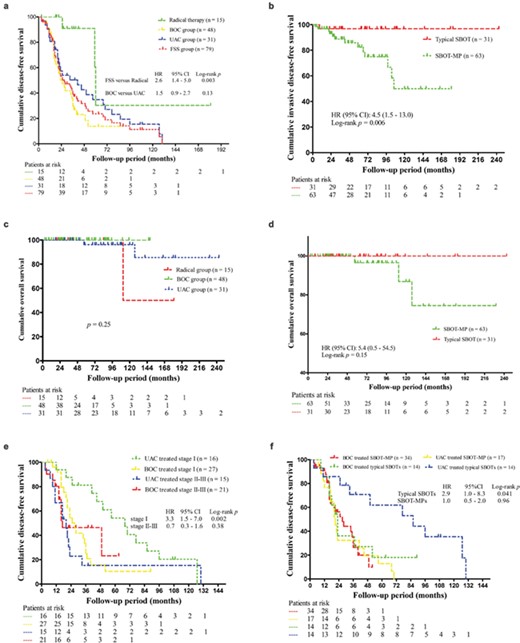
Survival curves in women with bilateral serous borderline ovarian tumors (SBOTs). HR, hazard ratio; CI, confidence interval (a). Disease-free survival (DFS) according to treatment modalities. FSS, fertility sparing surgery; BOC, bilateral ovarian cystectomy; UAC, unilateral adnexectomy plus contralateral cystectomy (b). Invasive DFS according to SBOTs with microcapillary pattern (SBOT-MPs) (c). Overall survival according to treatment modalities (d). Overall survival comparing typical SBOTs to SBOT-MPs (e). DFS after fertility sparing surgery (FSS) stratified by International Federation of Gynecology and Obstetrics (FIGO) stage (f). DFS after FSS stratified by SBOT-MPs.
Prognostic factors for recurrence (borderline and invasive) and invasive recurrence in women with bilateral serous borderline ovarian tumors (SBOT).
| . | N . | Recurrence-free survival . | Invasive recurrence-free survival . | ||||
|---|---|---|---|---|---|---|---|
| Univariate analysis . | Multivariate analysis . | Univariate analysis . | |||||
| 5-year disease-free survival (%) . | P . | Hazard ratio (95% CI) . | P . | 5-year invasive disease-free survival (%) . | P . | ||
| Total | 94 | ||||||
| Fertility sparing surgery | 0.003 | 0.001 | 0.75 | ||||
| No | 15 | 61 | 1 | 66 | |||
| UAC | 31 | 35 | 7.6 (2.1–27.9) | 81 | |||
| BOC | 48 | 14 | 12.8 (3.3–48.9) | 91 | |||
| Age (years) | 0.012 | 0.37 | 0.78 | ||||
| ≤30 | 58 | 39 | 1 | 87 | |||
| >30 | 36 | 19 | 0.8 (0.4–1.4) | 83 | |||
| CA 125 (U/mL) | 0.035 | 0.03 | 0.58 | ||||
| ≤300 | 39 | 42 | 1 | 82 | |||
| >300 | 39 | 15 | 2.1 (1.1–4.1) | 84 | |||
| Surgical approach | 0.90 | 0.97 | |||||
| Laparotomy | 55 | 29 | 84 | ||||
| Laparoscopy | 39 | 21 | 89 | ||||
| Complete staging | 0.27 | 0.54 | |||||
| Yes | 41 | 32 | 84 | ||||
| No | 53 | 22 | 88 | ||||
| FIGO stage | 0.38 | 0.26 | |||||
| I | 48 | 29 | 92 | ||||
| II–III | 46 | 25 | 79 | ||||
| SBOT-MP | 0.051 | 0.026 | 0.006 | ||||
| No | 31 | 42 | 1 | 97 | |||
| Yes | 63 | 15 | 2.0 (1.1–3.7) | 82 | |||
| Implants | 0.68 | 0.60 | |||||
| No | 48 | 29 | 92 | ||||
| Noninvasive | 36 | 28 | 75 | ||||
| Invasive | 10 | 21 | 74 | ||||
| Chemotherapy | 0.33 | 0.15 | |||||
| No | 76 | 30 | 92 | ||||
| Yes | 18 | 11 | 68 | ||||
| . | N . | Recurrence-free survival . | Invasive recurrence-free survival . | ||||
|---|---|---|---|---|---|---|---|
| Univariate analysis . | Multivariate analysis . | Univariate analysis . | |||||
| 5-year disease-free survival (%) . | P . | Hazard ratio (95% CI) . | P . | 5-year invasive disease-free survival (%) . | P . | ||
| Total | 94 | ||||||
| Fertility sparing surgery | 0.003 | 0.001 | 0.75 | ||||
| No | 15 | 61 | 1 | 66 | |||
| UAC | 31 | 35 | 7.6 (2.1–27.9) | 81 | |||
| BOC | 48 | 14 | 12.8 (3.3–48.9) | 91 | |||
| Age (years) | 0.012 | 0.37 | 0.78 | ||||
| ≤30 | 58 | 39 | 1 | 87 | |||
| >30 | 36 | 19 | 0.8 (0.4–1.4) | 83 | |||
| CA 125 (U/mL) | 0.035 | 0.03 | 0.58 | ||||
| ≤300 | 39 | 42 | 1 | 82 | |||
| >300 | 39 | 15 | 2.1 (1.1–4.1) | 84 | |||
| Surgical approach | 0.90 | 0.97 | |||||
| Laparotomy | 55 | 29 | 84 | ||||
| Laparoscopy | 39 | 21 | 89 | ||||
| Complete staging | 0.27 | 0.54 | |||||
| Yes | 41 | 32 | 84 | ||||
| No | 53 | 22 | 88 | ||||
| FIGO stage | 0.38 | 0.26 | |||||
| I | 48 | 29 | 92 | ||||
| II–III | 46 | 25 | 79 | ||||
| SBOT-MP | 0.051 | 0.026 | 0.006 | ||||
| No | 31 | 42 | 1 | 97 | |||
| Yes | 63 | 15 | 2.0 (1.1–3.7) | 82 | |||
| Implants | 0.68 | 0.60 | |||||
| No | 48 | 29 | 92 | ||||
| Noninvasive | 36 | 28 | 75 | ||||
| Invasive | 10 | 21 | 74 | ||||
| Chemotherapy | 0.33 | 0.15 | |||||
| No | 76 | 30 | 92 | ||||
| Yes | 18 | 11 | 68 | ||||
UAC, unilateral adnexectomy plus contralateral cystectomy; BOC, bilateral ovarian cystectomy; FIGO, International Federation of Gynecology and Obstetrics; SBOT-MP, SBOT with microcapillary pattern
Prognostic factors for recurrence (borderline and invasive) and invasive recurrence in women with bilateral serous borderline ovarian tumors (SBOT).
| . | N . | Recurrence-free survival . | Invasive recurrence-free survival . | ||||
|---|---|---|---|---|---|---|---|
| Univariate analysis . | Multivariate analysis . | Univariate analysis . | |||||
| 5-year disease-free survival (%) . | P . | Hazard ratio (95% CI) . | P . | 5-year invasive disease-free survival (%) . | P . | ||
| Total | 94 | ||||||
| Fertility sparing surgery | 0.003 | 0.001 | 0.75 | ||||
| No | 15 | 61 | 1 | 66 | |||
| UAC | 31 | 35 | 7.6 (2.1–27.9) | 81 | |||
| BOC | 48 | 14 | 12.8 (3.3–48.9) | 91 | |||
| Age (years) | 0.012 | 0.37 | 0.78 | ||||
| ≤30 | 58 | 39 | 1 | 87 | |||
| >30 | 36 | 19 | 0.8 (0.4–1.4) | 83 | |||
| CA 125 (U/mL) | 0.035 | 0.03 | 0.58 | ||||
| ≤300 | 39 | 42 | 1 | 82 | |||
| >300 | 39 | 15 | 2.1 (1.1–4.1) | 84 | |||
| Surgical approach | 0.90 | 0.97 | |||||
| Laparotomy | 55 | 29 | 84 | ||||
| Laparoscopy | 39 | 21 | 89 | ||||
| Complete staging | 0.27 | 0.54 | |||||
| Yes | 41 | 32 | 84 | ||||
| No | 53 | 22 | 88 | ||||
| FIGO stage | 0.38 | 0.26 | |||||
| I | 48 | 29 | 92 | ||||
| II–III | 46 | 25 | 79 | ||||
| SBOT-MP | 0.051 | 0.026 | 0.006 | ||||
| No | 31 | 42 | 1 | 97 | |||
| Yes | 63 | 15 | 2.0 (1.1–3.7) | 82 | |||
| Implants | 0.68 | 0.60 | |||||
| No | 48 | 29 | 92 | ||||
| Noninvasive | 36 | 28 | 75 | ||||
| Invasive | 10 | 21 | 74 | ||||
| Chemotherapy | 0.33 | 0.15 | |||||
| No | 76 | 30 | 92 | ||||
| Yes | 18 | 11 | 68 | ||||
| . | N . | Recurrence-free survival . | Invasive recurrence-free survival . | ||||
|---|---|---|---|---|---|---|---|
| Univariate analysis . | Multivariate analysis . | Univariate analysis . | |||||
| 5-year disease-free survival (%) . | P . | Hazard ratio (95% CI) . | P . | 5-year invasive disease-free survival (%) . | P . | ||
| Total | 94 | ||||||
| Fertility sparing surgery | 0.003 | 0.001 | 0.75 | ||||
| No | 15 | 61 | 1 | 66 | |||
| UAC | 31 | 35 | 7.6 (2.1–27.9) | 81 | |||
| BOC | 48 | 14 | 12.8 (3.3–48.9) | 91 | |||
| Age (years) | 0.012 | 0.37 | 0.78 | ||||
| ≤30 | 58 | 39 | 1 | 87 | |||
| >30 | 36 | 19 | 0.8 (0.4–1.4) | 83 | |||
| CA 125 (U/mL) | 0.035 | 0.03 | 0.58 | ||||
| ≤300 | 39 | 42 | 1 | 82 | |||
| >300 | 39 | 15 | 2.1 (1.1–4.1) | 84 | |||
| Surgical approach | 0.90 | 0.97 | |||||
| Laparotomy | 55 | 29 | 84 | ||||
| Laparoscopy | 39 | 21 | 89 | ||||
| Complete staging | 0.27 | 0.54 | |||||
| Yes | 41 | 32 | 84 | ||||
| No | 53 | 22 | 88 | ||||
| FIGO stage | 0.38 | 0.26 | |||||
| I | 48 | 29 | 92 | ||||
| II–III | 46 | 25 | 79 | ||||
| SBOT-MP | 0.051 | 0.026 | 0.006 | ||||
| No | 31 | 42 | 1 | 97 | |||
| Yes | 63 | 15 | 2.0 (1.1–3.7) | 82 | |||
| Implants | 0.68 | 0.60 | |||||
| No | 48 | 29 | 92 | ||||
| Noninvasive | 36 | 28 | 75 | ||||
| Invasive | 10 | 21 | 74 | ||||
| Chemotherapy | 0.33 | 0.15 | |||||
| No | 76 | 30 | 92 | ||||
| Yes | 18 | 11 | 68 | ||||
UAC, unilateral adnexectomy plus contralateral cystectomy; BOC, bilateral ovarian cystectomy; FIGO, International Federation of Gynecology and Obstetrics; SBOT-MP, SBOT with microcapillary pattern
Notably, fourteen patients (15%) developed recurrence with invasive evolution at a median of 29 months (range, 3–106 months), including six (43%) patients whose disease transformed into serous invasive ovarian cancer and eight (57%) patients who had recurrences as invasive peritoneal disease. Detailed information on these 14 patients who had invasive relapse is presented in Table III. The 5-year invasive DFS rate of all studied patients was 88%, and there was no significant difference in invasive DFS among the three treatment groups (P = 0.75). As presented in Table II, only SBOT-MP was significantly associated with invasive malignant evolution of the disease (P = 0.006) (Fig. 1b).
Details of the 14 patients with bilateral serous borderline ovarian tumors (SBOTs) who had invasive recurrences or succumbed to their disease.
| ID . | Age . | Primary surgery . | FIGO stagea . | Implants . | Chemo. . | MPP/min. . | DFS (mo.) . | Initial recurrence . | Time to inv. (mo.) . | F/U (mo.) . | Current status . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Path . | Extent . | Staging . | Sites . | Histology . | Treatment . | ||||||||||
| Patients with invasive recurrence (n = 11) | |||||||||||||||
| #1 | 22 | Open | BOC | No | IIB | Inv. | Yes | Y/N | 3 | Local & spread | LGSC | CRS and chemo. | 3 (ca.) | 43 | AWD |
| #2 | 23 | Open | UAC | No | IIIC | Non-inv. | Yes | Y/N | 22 | Local & spread | Inv. | CRS | 22 (inv.) | 41 | NED |
| #3 | 23 | Open | UAC | Yes | IIIC | Non-inv. | Yes | Y/N | 9 | Local | BOT | UC | 42 (inv.) | 103 | NED |
| #4 | 25 | Open | UAC | Yes | IIIC | Non-inv. | No | Y/N | 7 | Local & spread | Inv. | CRS | 7 (inv.) | 58 | NED |
| #5 | 28 | Lap. | BOC | No | IC | No | No | Y/N | 16 | Local | BOT | Lap. BOC | 66 (ca.) | 85 | NED |
| #6 | 28 | Lap. | UAC | Yes | IIIC | Non-inv. | No | Y/N | 17 | Local & spread | BOT & non-inv. | Lap. UC | 98 (inv.) | 108 | NED |
| #7 | 29 | Open | UAC | Yes | IC | No | No | Y / N | 12 | Local | BOT & min. | UC | 26 (ca.) | 127 | NED |
| #8 | 29 | Lap. | BOC | No | IC | No | No | Y / N | 16 | Local & spread | BOT & non-inv. | CRS | 59 (ca.) | 86 | AWD |
| #9 | 31 | Open | Rad. | Yes | IIIC | Non-inv. | Yes | Y/Y | 25 | Local & spread | LGSC | CRS | 25 (ca.) | 52 | AWD |
| #10 | 33 | Open | UAC | Yes | IC | No | No | Y / N | 57 | Local | BOT | UC | 106 (inv.) | 230 | NED |
| #11 | 40 | Lap. | BOC | No. | IC | No | No | N / N | 9 | Local & spread | BOT & inv. | Lap USO with stating | 9 (inv.) | 85 | NED |
| Patients with invasive recurrence and succumbed to the disease (n = 3) | |||||||||||||||
| #12 | 29 | Open | Rad. | Yes | IIIC | Inv. | Yes | Y / Y | 60 | Spread | Inv. | CRS | 60 (inv.) | 111 | Dead |
| #13 | 37 | Open | UAC | Yes | IC | No | No | Y / N | 20 | Local | BOT | UC | 103 (ca.) | 127 | Dead |
| #14 | 40 | Open | UAC | Yes | IIB | Non-inv. | No | Y / N | 21 | Local & spread | BOT & inv. | CRS | 21 (inv.) | 57 | Dead |
| ID . | Age . | Primary surgery . | FIGO stagea . | Implants . | Chemo. . | MPP/min. . | DFS (mo.) . | Initial recurrence . | Time to inv. (mo.) . | F/U (mo.) . | Current status . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Path . | Extent . | Staging . | Sites . | Histology . | Treatment . | ||||||||||
| Patients with invasive recurrence (n = 11) | |||||||||||||||
| #1 | 22 | Open | BOC | No | IIB | Inv. | Yes | Y/N | 3 | Local & spread | LGSC | CRS and chemo. | 3 (ca.) | 43 | AWD |
| #2 | 23 | Open | UAC | No | IIIC | Non-inv. | Yes | Y/N | 22 | Local & spread | Inv. | CRS | 22 (inv.) | 41 | NED |
| #3 | 23 | Open | UAC | Yes | IIIC | Non-inv. | Yes | Y/N | 9 | Local | BOT | UC | 42 (inv.) | 103 | NED |
| #4 | 25 | Open | UAC | Yes | IIIC | Non-inv. | No | Y/N | 7 | Local & spread | Inv. | CRS | 7 (inv.) | 58 | NED |
| #5 | 28 | Lap. | BOC | No | IC | No | No | Y/N | 16 | Local | BOT | Lap. BOC | 66 (ca.) | 85 | NED |
| #6 | 28 | Lap. | UAC | Yes | IIIC | Non-inv. | No | Y/N | 17 | Local & spread | BOT & non-inv. | Lap. UC | 98 (inv.) | 108 | NED |
| #7 | 29 | Open | UAC | Yes | IC | No | No | Y / N | 12 | Local | BOT & min. | UC | 26 (ca.) | 127 | NED |
| #8 | 29 | Lap. | BOC | No | IC | No | No | Y / N | 16 | Local & spread | BOT & non-inv. | CRS | 59 (ca.) | 86 | AWD |
| #9 | 31 | Open | Rad. | Yes | IIIC | Non-inv. | Yes | Y/Y | 25 | Local & spread | LGSC | CRS | 25 (ca.) | 52 | AWD |
| #10 | 33 | Open | UAC | Yes | IC | No | No | Y / N | 57 | Local | BOT | UC | 106 (inv.) | 230 | NED |
| #11 | 40 | Lap. | BOC | No. | IC | No | No | N / N | 9 | Local & spread | BOT & inv. | Lap USO with stating | 9 (inv.) | 85 | NED |
| Patients with invasive recurrence and succumbed to the disease (n = 3) | |||||||||||||||
| #12 | 29 | Open | Rad. | Yes | IIIC | Inv. | Yes | Y / Y | 60 | Spread | Inv. | CRS | 60 (inv.) | 111 | Dead |
| #13 | 37 | Open | UAC | Yes | IC | No | No | Y / N | 20 | Local | BOT | UC | 103 (ca.) | 127 | Dead |
| #14 | 40 | Open | UAC | Yes | IIB | Non-inv. | No | Y / N | 21 | Local & spread | BOT & inv. | CRS | 21 (inv.) | 57 | Dead |
Chemo. chemotherapy; Min, microinvasion; MPP, micropapillary pattern; DFS, disease-free survival; F/U, follow-up; BOC, bilateral ovarian cystectomy; Inv., invasive; Y, yes; N, no; LGSC, low-grade ovarian carcinoma; CRS, cytoreductive surgery; ca, carcinoma; AWD, alive with disease; UAC, unilateral adnexectomy plus contralateral cystectomy; Non-inv., noninvasive; NED, no evidence of disease; BOT, borderline ovarian tumor; UC, unilateral cystectomy; Lap, laparoscopy; USO, unilateral adnexectomy.
aWhere staging = ‘No’ this is based on surgical findings and pathological reports only.
Details of the 14 patients with bilateral serous borderline ovarian tumors (SBOTs) who had invasive recurrences or succumbed to their disease.
| ID . | Age . | Primary surgery . | FIGO stagea . | Implants . | Chemo. . | MPP/min. . | DFS (mo.) . | Initial recurrence . | Time to inv. (mo.) . | F/U (mo.) . | Current status . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Path . | Extent . | Staging . | Sites . | Histology . | Treatment . | ||||||||||
| Patients with invasive recurrence (n = 11) | |||||||||||||||
| #1 | 22 | Open | BOC | No | IIB | Inv. | Yes | Y/N | 3 | Local & spread | LGSC | CRS and chemo. | 3 (ca.) | 43 | AWD |
| #2 | 23 | Open | UAC | No | IIIC | Non-inv. | Yes | Y/N | 22 | Local & spread | Inv. | CRS | 22 (inv.) | 41 | NED |
| #3 | 23 | Open | UAC | Yes | IIIC | Non-inv. | Yes | Y/N | 9 | Local | BOT | UC | 42 (inv.) | 103 | NED |
| #4 | 25 | Open | UAC | Yes | IIIC | Non-inv. | No | Y/N | 7 | Local & spread | Inv. | CRS | 7 (inv.) | 58 | NED |
| #5 | 28 | Lap. | BOC | No | IC | No | No | Y/N | 16 | Local | BOT | Lap. BOC | 66 (ca.) | 85 | NED |
| #6 | 28 | Lap. | UAC | Yes | IIIC | Non-inv. | No | Y/N | 17 | Local & spread | BOT & non-inv. | Lap. UC | 98 (inv.) | 108 | NED |
| #7 | 29 | Open | UAC | Yes | IC | No | No | Y / N | 12 | Local | BOT & min. | UC | 26 (ca.) | 127 | NED |
| #8 | 29 | Lap. | BOC | No | IC | No | No | Y / N | 16 | Local & spread | BOT & non-inv. | CRS | 59 (ca.) | 86 | AWD |
| #9 | 31 | Open | Rad. | Yes | IIIC | Non-inv. | Yes | Y/Y | 25 | Local & spread | LGSC | CRS | 25 (ca.) | 52 | AWD |
| #10 | 33 | Open | UAC | Yes | IC | No | No | Y / N | 57 | Local | BOT | UC | 106 (inv.) | 230 | NED |
| #11 | 40 | Lap. | BOC | No. | IC | No | No | N / N | 9 | Local & spread | BOT & inv. | Lap USO with stating | 9 (inv.) | 85 | NED |
| Patients with invasive recurrence and succumbed to the disease (n = 3) | |||||||||||||||
| #12 | 29 | Open | Rad. | Yes | IIIC | Inv. | Yes | Y / Y | 60 | Spread | Inv. | CRS | 60 (inv.) | 111 | Dead |
| #13 | 37 | Open | UAC | Yes | IC | No | No | Y / N | 20 | Local | BOT | UC | 103 (ca.) | 127 | Dead |
| #14 | 40 | Open | UAC | Yes | IIB | Non-inv. | No | Y / N | 21 | Local & spread | BOT & inv. | CRS | 21 (inv.) | 57 | Dead |
| ID . | Age . | Primary surgery . | FIGO stagea . | Implants . | Chemo. . | MPP/min. . | DFS (mo.) . | Initial recurrence . | Time to inv. (mo.) . | F/U (mo.) . | Current status . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Path . | Extent . | Staging . | Sites . | Histology . | Treatment . | ||||||||||
| Patients with invasive recurrence (n = 11) | |||||||||||||||
| #1 | 22 | Open | BOC | No | IIB | Inv. | Yes | Y/N | 3 | Local & spread | LGSC | CRS and chemo. | 3 (ca.) | 43 | AWD |
| #2 | 23 | Open | UAC | No | IIIC | Non-inv. | Yes | Y/N | 22 | Local & spread | Inv. | CRS | 22 (inv.) | 41 | NED |
| #3 | 23 | Open | UAC | Yes | IIIC | Non-inv. | Yes | Y/N | 9 | Local | BOT | UC | 42 (inv.) | 103 | NED |
| #4 | 25 | Open | UAC | Yes | IIIC | Non-inv. | No | Y/N | 7 | Local & spread | Inv. | CRS | 7 (inv.) | 58 | NED |
| #5 | 28 | Lap. | BOC | No | IC | No | No | Y/N | 16 | Local | BOT | Lap. BOC | 66 (ca.) | 85 | NED |
| #6 | 28 | Lap. | UAC | Yes | IIIC | Non-inv. | No | Y/N | 17 | Local & spread | BOT & non-inv. | Lap. UC | 98 (inv.) | 108 | NED |
| #7 | 29 | Open | UAC | Yes | IC | No | No | Y / N | 12 | Local | BOT & min. | UC | 26 (ca.) | 127 | NED |
| #8 | 29 | Lap. | BOC | No | IC | No | No | Y / N | 16 | Local & spread | BOT & non-inv. | CRS | 59 (ca.) | 86 | AWD |
| #9 | 31 | Open | Rad. | Yes | IIIC | Non-inv. | Yes | Y/Y | 25 | Local & spread | LGSC | CRS | 25 (ca.) | 52 | AWD |
| #10 | 33 | Open | UAC | Yes | IC | No | No | Y / N | 57 | Local | BOT | UC | 106 (inv.) | 230 | NED |
| #11 | 40 | Lap. | BOC | No. | IC | No | No | N / N | 9 | Local & spread | BOT & inv. | Lap USO with stating | 9 (inv.) | 85 | NED |
| Patients with invasive recurrence and succumbed to the disease (n = 3) | |||||||||||||||
| #12 | 29 | Open | Rad. | Yes | IIIC | Inv. | Yes | Y / Y | 60 | Spread | Inv. | CRS | 60 (inv.) | 111 | Dead |
| #13 | 37 | Open | UAC | Yes | IC | No | No | Y / N | 20 | Local | BOT | UC | 103 (ca.) | 127 | Dead |
| #14 | 40 | Open | UAC | Yes | IIB | Non-inv. | No | Y / N | 21 | Local & spread | BOT & inv. | CRS | 21 (inv.) | 57 | Dead |
Chemo. chemotherapy; Min, microinvasion; MPP, micropapillary pattern; DFS, disease-free survival; F/U, follow-up; BOC, bilateral ovarian cystectomy; Inv., invasive; Y, yes; N, no; LGSC, low-grade ovarian carcinoma; CRS, cytoreductive surgery; ca, carcinoma; AWD, alive with disease; UAC, unilateral adnexectomy plus contralateral cystectomy; Non-inv., noninvasive; NED, no evidence of disease; BOT, borderline ovarian tumor; UC, unilateral cystectomy; Lap, laparoscopy; USO, unilateral adnexectomy.
aWhere staging = ‘No’ this is based on surgical findings and pathological reports only.
As shown in Table III, three patients (3%) eventually succumbed to their disease 57, 111 and 127 months following their primary surgeries, resulting in 5- and 10-year overall survival rates of 94 and 85%, respectively. Two patients had invasive disease at their first peritoneal recurrence, and debulking surgeries were performed. For the third patient, successful pregnancy was achieved after her initial UAC surgery. Twenty months later, local borderline relapse was found during the cesarean section, and unilateral cystectomy was performed on the remaining ovary. Five years after the primary management, she relapsed in the form of borderline disease with noninvasive implants that were treated with radical cytoreductive surgery. She developed a third invasive recurrence three years later and was treated with secondary cytoreductive surgery with adjuvant platinum-based chemotherapy. This patient died 2 years after this last recurrence. As shown in Figure 1c and d, neither the FSS procedures nor SBOT-MP was significantly associated with overall survival (P = 0.25 and P = 0.15, respectively).
Concerning the 79 patients who underwent FSS procedures, 58 (73%) experienced recurrence. The 5-year DFS rate of patients who underwent BOC was 14%, compared with 35% in patients with UAC, but this difference was not significant (P = 0.13) (Fig. 1a). Subsequently, we performed further survival analysis according to different subgroups (Supplementary Table SI). As illustrated in Figure 1e and f, the DFS after BOC was significantly worse than the UAC procedure when confined to patients with FIGO stage I or typical SBOT (P = 0.002 and 0.041, respectively). However, when excluding the 27 referral patients after recurrence, the difference did not retain significance (P = 0.12 and 0.058, respectively).
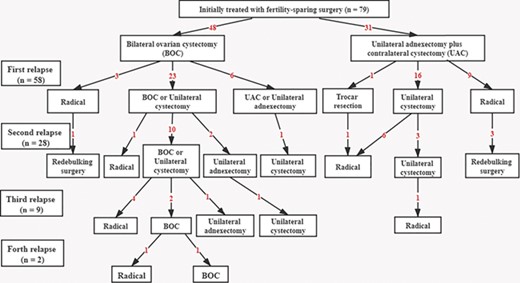
Additionally, as shown in Figure 2, 46 (79%) patients had been treated conservatively for their primary relapses. Twenty-four (52%) patients had a further recurrence after conservative treatment of their first relapse, and nine (38%) of them suffered a third recurrence. During the follow-up period, 26 patients underwent radical surgery after relapse, including simply hysterectomy and removal of remaining ovary/ovaries (n = 9), radical staging surgery (n = 3) and radical cytoreductive surgery (n = 14). The main indications were invasive evolution (n = 11), patient desire (n = 6), recurrence after childbearing (n = 5), relapse with persistent infertility (n = 2) and repeated recurrence more than two times (n = 2). Notably, subjects from the UAC group had a significantly higher need of radical intervention for recurrence than the BOC group (65 versus 28%, P = 0.005).
Fertility results and pregnancy outcomes
Among the 79 women who underwent FSS procedures, 10 patients (13%) were diagnosed as infertile before their ovarian tumors, and 49 patients (62%) attempted to conceive after surgery. During the median follow-up of 85 months (range, 18–243 months), 23 patients (47%) obtained 27 pregnancies (24 spontaneous and 3 after IVF-ET), resulting in 19 live births. As illustrated in Figure 3, among 27 patients who elected to undertake a fertility sparing approach to the management of relapsed disease, only six patients (22%) achieved a pregnancy. Also, among 23 patients who had no fertility desire after the initial surgery, 6 women attempted to conceive after relapse, and 3 patients succeeded in a pregnancy (n = 3/4 in the BOC group and n = 0/2 in the UAC group). Notably, nine patients underwent IVF treatment for 13 cycles, and the pregnancy rate in this subgroup was 33% (n = 3/9). Details regarding the flowchart of reproductive outcomes after fertility preservation in bilateral SBOTs are provided in Figure 3.
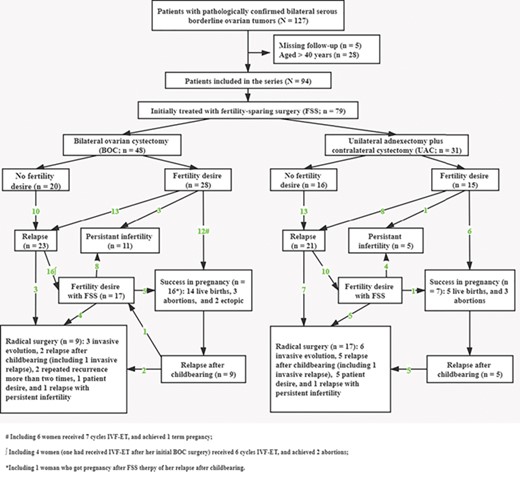
Flowchart of reproductive outcomes after fertility-sparing surgery in young women with bilateral serous borderline ovarian tumors.
To determine factors associated with a successful pregnancy, univariable analysis of our data did not identify tumor-related factors that affected fertility (Table IV). Also, these factors retained no significance after subgroup analysis (Supplementary Table SII).
Factors associated with success in achieving pregnancy in the 49 patients with bilateral serous borderline ovarian tumors.
| Variables . | Pregnancy (n = 23) . | No pregnancy(n = 26) . | P . |
|---|---|---|---|
| Age (years) | 1.00‡ | ||
| ≤30 | 18 | 21 | |
| >30 | 5 | 5 | |
| Infertility | 1.00‡ | ||
| No | 19 | 21 | |
| Yes | 4 | 5 | |
| FSS procedures | 0.56† | ||
| BOC | 16 | 16 | |
| UAC | 7 | 10 | |
| Complete staging | 0.59† | ||
| Yes | 8 | 11 | |
| No | 15 | 15 | |
| Surgical approach | 0.12† | ||
| Open | 9 | 16 | |
| Laparoscopy | 14 | 10 | |
| FIGO stage | 0.28† | ||
| I | 15 | 13 | |
| II–III | 8 | 13 | |
| Implant | 0.53† | ||
| No | 15 | 13 | |
| Noninvasive | 7 | 12 | |
| Invasive | 1 | 1 | |
| Chemotherapy | 1.00‡ | ||
| No | 20 | 22 | |
| Yes | 3 | 4 |
| Variables . | Pregnancy (n = 23) . | No pregnancy(n = 26) . | P . |
|---|---|---|---|
| Age (years) | 1.00‡ | ||
| ≤30 | 18 | 21 | |
| >30 | 5 | 5 | |
| Infertility | 1.00‡ | ||
| No | 19 | 21 | |
| Yes | 4 | 5 | |
| FSS procedures | 0.56† | ||
| BOC | 16 | 16 | |
| UAC | 7 | 10 | |
| Complete staging | 0.59† | ||
| Yes | 8 | 11 | |
| No | 15 | 15 | |
| Surgical approach | 0.12† | ||
| Open | 9 | 16 | |
| Laparoscopy | 14 | 10 | |
| FIGO stage | 0.28† | ||
| I | 15 | 13 | |
| II–III | 8 | 13 | |
| Implant | 0.53† | ||
| No | 15 | 13 | |
| Noninvasive | 7 | 12 | |
| Invasive | 1 | 1 | |
| Chemotherapy | 1.00‡ | ||
| No | 20 | 22 | |
| Yes | 3 | 4 |
†X2 square test.
‡Fisher exact test.
FSS, fertility sparing surgery; UAC, unilateral adnexectomy plus contralateral cystectomy; BOC, bilateral ovarian cystectomy; FIGO, International Federation of Gynecology and Obstetrics
Factors associated with success in achieving pregnancy in the 49 patients with bilateral serous borderline ovarian tumors.
| Variables . | Pregnancy (n = 23) . | No pregnancy(n = 26) . | P . |
|---|---|---|---|
| Age (years) | 1.00‡ | ||
| ≤30 | 18 | 21 | |
| >30 | 5 | 5 | |
| Infertility | 1.00‡ | ||
| No | 19 | 21 | |
| Yes | 4 | 5 | |
| FSS procedures | 0.56† | ||
| BOC | 16 | 16 | |
| UAC | 7 | 10 | |
| Complete staging | 0.59† | ||
| Yes | 8 | 11 | |
| No | 15 | 15 | |
| Surgical approach | 0.12† | ||
| Open | 9 | 16 | |
| Laparoscopy | 14 | 10 | |
| FIGO stage | 0.28† | ||
| I | 15 | 13 | |
| II–III | 8 | 13 | |
| Implant | 0.53† | ||
| No | 15 | 13 | |
| Noninvasive | 7 | 12 | |
| Invasive | 1 | 1 | |
| Chemotherapy | 1.00‡ | ||
| No | 20 | 22 | |
| Yes | 3 | 4 |
| Variables . | Pregnancy (n = 23) . | No pregnancy(n = 26) . | P . |
|---|---|---|---|
| Age (years) | 1.00‡ | ||
| ≤30 | 18 | 21 | |
| >30 | 5 | 5 | |
| Infertility | 1.00‡ | ||
| No | 19 | 21 | |
| Yes | 4 | 5 | |
| FSS procedures | 0.56† | ||
| BOC | 16 | 16 | |
| UAC | 7 | 10 | |
| Complete staging | 0.59† | ||
| Yes | 8 | 11 | |
| No | 15 | 15 | |
| Surgical approach | 0.12† | ||
| Open | 9 | 16 | |
| Laparoscopy | 14 | 10 | |
| FIGO stage | 0.28† | ||
| I | 15 | 13 | |
| II–III | 8 | 13 | |
| Implant | 0.53† | ||
| No | 15 | 13 | |
| Noninvasive | 7 | 12 | |
| Invasive | 1 | 1 | |
| Chemotherapy | 1.00‡ | ||
| No | 20 | 22 | |
| Yes | 3 | 4 |
†X2 square test.
‡Fisher exact test.
FSS, fertility sparing surgery; UAC, unilateral adnexectomy plus contralateral cystectomy; BOC, bilateral ovarian cystectomy; FIGO, International Federation of Gynecology and Obstetrics
Discussion
The natural behavior of bilateral SBOTs remains enigmatic. To our knowledge, the current series represents the most extensive dataset available that precisely describes the oncofertility outcomes of young patients with bilateral SBOTs. Although restricted by its retrospective nature, this study has several meaningful findings and unanswered questions that merit further exploration.
First, our study documented the recurrence rates and noninferior overall survival of fertility-sparing procedures in bilateral SBOTs. Compared with radical therapy, fertility-sparing management significantly worsened DFS (P = 0.003); however, disease-specific overall survival did not differ between the subgroups (P = 0.25). Although the relapse rate is very high, comparable proportions between 48.1 and 68.2% have also been reported in the literature (Palomba et al., 2010; Uzan et al., 2011; Delle Marchette et al., 2019). As stated previously, surgery that preserves fertility potential triples the risk of recurrence compared with radical treatment (du Bois et al., 2013). Several high-risk features for SBOT progression, such as younger age at diagnosis, bilateral involvement, an abnormal CA-125 value, FSS procedures, incomplete staging, tumor residuals, micropapillary patterns and extraovarian implants, have been postulated, though the data are conflicting (Wu et al., 2009; Shih et al., 2011; du Bois et al., 2013; Bendifallah et al., 2014; Trillsch et al., 2014; Uzan et al., 2014b; Vasconcelos et al., 2016; Ouldamer et al., 2017; Delle Marchette et al., 2019). Regrettably, all of these features were highly prevalent in our study population. Moreover, as a referral center, selection bias might exist. Also, when we excluded the 29 referral patients after recurrence, the relapse rate decreased to 49% (n = 32/65). Fortunately, most recurrences could be cured by surgery exclusively without impacting overall survival. Thus, FSS may be considered in these patients after obtaining adequate informed consent.
Regarding the two FSS procedures, comparable DFS and pregnancy rates were observed between the two groups (P = 0.13 and 0.56, respectively), although the nonrandom allocation to treatment groups and relatively small number of patients attempt to conceive might limit the statistical power of our findings. Moreover, these differences retained no significance after subgroup analysis. Similar findings have also been reported in a prospective study by Palomba et al. (2010, 2007), in which comparable cumulative recurrence rates were observed between the UAC and BOC groups (P = 0.14). Furthermore, they suggested that the ultra-conservative BOC approach was more effective than the standard UAC approach in terms of reproductive outcomes. However, this difference was not significant in our patients confined to FIGO stage I (P = 0.41), and a trend towards poorer DFS was found after BOC procedure (P = 0.12). On the other hand, the intention-to-treat analysis revealed an unfavorable risk–benefit ratio in the BOC group, which is to obtain two additional pregnancies at the expense of three additional radical interventions (Palomba et al., 2010, 2007). Nonetheless, BOC is advisable in the case of FIGO stage I bilateral SBOTs before we get more conclusive evidence. However, the patients should be counseled about the fact that undergoing a UAC does not seem to comprise fertility outcomes while achieving a relatively lower recurrence rate.
Interestingly, when confined to patients with ‘high risk’ features, subgroup analysis of our data revealed no statistically significant differences in DFS and pregnancy outcomes in patients with advanced-stage or with SBOT-MP after conservative management (P = 0.38 and 0.96, respectively). Unfortunately, very few studies have specifically addressed this issue to compare the two FSS approaches. As in advanced-stage SBOT, a similar recurrence rate was noticed between the cystectomy-included (including cystectomy, BOC and UAC procedures) and unilateral adnexectomy group (16/28 versus 6/11, P = 1.00) (Uzan et al., 2010). Theoretically, the BOC procedure, when feasible, can retain as much ovarian tissue as possible. Meanwhile, 79% of our patients relapsed at least a second time after conservative therapy, and repeated surgeries indisputably damaged the ovarian reserve, leading to the preservation of part of ovary impossible. Additionally, subjects from the UAC group had a significantly higher proportion of radical intervention (65 versus 28%, P = 0.005). Thus, an ultra-conservative BOC should be favored in advanced-stage bilateral SBOTs, and a fertility evaluation that minimally comprises an AMH measurement and antral follicle count is advised.
The ultimate objective of FSS is to fulfill the childbearing desire of young women. Notably, consistent with previous studies (Daraï et al., 2013; Ouldamer et al., 2016), only 38% (n = 8/21) of our patients with advanced-stage disease successfully achieved pregnancy. Moreover, only 22% (n = 6/27) of our patients became pregnant after repeated surgery. Thus, extensive oncofertility counseling should be proposed before surgery (Mangili et al., 2016). Recently, techniques in assisted reproductive medicine have developed, and encouraging results have been demonstrated in terms of the safety and effectiveness of reproductive medical treatments after fertility preservation for gynecological cancers (Daraï et al., 2013; Zapardiel et al., 2016). Besides, in vitro data have suggested that gonadotropins and high-dose estrogens do not stimulate cultured borderline ovarian cell proliferation (Basille et al., 2006). However, in our current study, among the nine patients who used IVF treatment, the pregnancy rate was only 33% (n = 3/9), which might be due to the delayed initiation of IVF. Thus, to improve fertility outcomes, fertility counseling should become an integral part of bilateral SBOT management, especially for those with advanced-stage disease (Mangili et al., 2016). Also, fertility-enhancement therapy should be encouraged, given its high relapse rate and short recurrence interval (Palomba et al., 2010).
The prognostic value of the micropapillary pattern in SBOTs continues to fuel debate in the literature. In our study, significantly decreased survival in patients with SBOT-MP was observed (P = 0.026). A similar finding has also been observed in Silva’s long-term follow-up series (Silva et al., 2006), and this difference remained meaningful when early (Uzan et al., 2014a) and advanced-stage (Shih et al., 2011, 2010) disease were separately reported. However, the majority of studies have failed to demonstrate the association between SBOT-MP and an unfavorable prognosis, especially when accounting for the concurrent invasive extraovarian implants (Seidman and Kurman 1996; Longacre et al., 2005; Park et al., 2011; Uzan et al., 2011; Fauvet et al., 2012; Morice et al., 2012; du Bois et al., 2013; Hannibal et al., 2014; Trillsch et al., 2014). Restricted by sample size, stratified analysis is impractical in our study, and further studies on this topic are recommended. Nevertheless, intensive surveillance should be mandatory for these young bilateral SBOT patients.
Notably, 15% (n = 14/94) of our patients evolved to carcinoma, and three patients (3%) eventually succumbed to their disease. As observed in the Danish national cohort, our case series confirmed that SBOT-MP was significantly associated with invasive malignant evolution of the disease (21 versus 3%, P = 0.031) (Vang et al., 2017). This rate of progression to invasive carcinoma is comparable to that described in the literature for SBOT-MPs (11–20%) (Morice et al., 2012; Vang et al., 2017) and SBOTs (2–8%) (Zanetta et al., 2001; Morice et al., 2003; Uzan et al., 2014a; Hannibal et al., 2017; Vang et al., 2017). Furthermore, molecular studies have also demonstrated that SBOT-MP is more clonally related and molecularly similar to low-grade serous carcinoma than to typical SBOTs, implying SBOT-MP as a stepwise pattern from SBOT and invasive ovarian cancer (Singer et al., 2002; May et al., 2010). More recently, Seidman et al. suggested another explanation, namely, that occult micrometastases were unsampled in the primary SBOT-MP, and recommended sampling at least two sections/cm of maximum tumor diameter in SBOT-MPs (Seidman et al., 2020). Accordingly, the examination of numerous sections is warranted to rule out occult invasion, and adequate peritoneal sampling should be performed to detect implants in patients with SBOT-MPs. Additionally, for patients who experienced malignant evolution, further molecular investigation of their specimens will help to elucidate the pathogenesis of low-grade ovarian carcinomas.
We are aware that our study has several limitations. First, as a retrospective study conducted in a single-center, inherent biases exist. Because the study utilized data from a period of over 20 years, the referral patterns of our service, as well as the techniques used by our pathologists, changed over the study period. Although we have re-reviewed the slides, sample bias cannot be excluded. Furthermore, selection bias might exist. Patients with ‘lower risk’ or smaller masses might be more inclined to select BOC, although we did not find significant difference in distribution of FIGO stage and SBOT-MP between the two groups. However, the relatively large sample size and the inclusion of all consecutive bilateral SBOT patients are the main strengths of our investigation. Second, nearly one-third of the patients were initially treated outside of our center and then referred to our center after recurrence; thus, selection bias might exist. Most inpatients seeking treatment at our hospital already had unfavorable outcomes, which may have led to the overinterpretation of the results. Last, only 44% of our patients underwent complete staging during their initial surgery, whereas incomplete surgical staging has been proposed as the most prominent prognostic factor for recurrence in a multicenter German series (du Bois et al., 2013; Trillsch et al., 2015). Thus, lower recurrence estimates for advanced stage are probably a consequence of the low staging rate bias (57 versus 73%). Indeed, 12% (n = 2/17) of our patients with the apparently early-stage disease were upstaged (unpublished data) after staging surgery. Thus, an underestimated bias for FIGO and extraovarian implants might occur. Despite these limitations, the current study provides valuable insight into the clinical features and behavior of bilateral SBOTs.
In conclusion, FSS is feasible for young women with BiSBOTs who desire fertility, with an acceptable oncological outcome and meaningful rates of pregnancy. The ultraconservative BOC procedure should be proposed when technically feasible, especially for those with advanced-stage and SBOT-MP. Invasive evolution occurs frequently in these women, and intense follow-up and oncofertility counseling are warranted, especially for those with micropapillary patterns.
Acknowledgements
We thank all of the patients for agreeing to participate in our study.
Authors’ roles
S.J., Y.X. and J.L. contributed substantially to the study concept and design; the acquisition, analysis and interpretation of data; and manuscript writing. J.Y., J.S. and C.J. contributed to the understanding of data and performed critical revisions of relevant intellectual content. All authors approved the publication of the final version.
Funding
No external funding was used for this study.
Conflict of interest
All authors have nothing to declare.



