-
PDF
- Split View
-
Views
-
Cite
Cite
I González-Foruria, I Rodríguez, F Martínez, J Rodríguez-Purata, P Montoya, D Rodríguez, J Nicolau, B Coroleu, P N Barri, N P Polyzos, Clinically significant intra-day variability of serum progesterone levels during the final day of oocyte maturation: a prospective study with repeated measurements, Human Reproduction, Volume 34, Issue 8, August 2019, Pages 1551–1558, https://doi.org/10.1093/humrep/dez091
Close - Share Icon Share
Abstract
Is there significant variability in progesterone levels during the final day of oocyte maturation in women undergoing ovarian stimulation?
Progesterone levels drop from the basal level up to 44% during the final day of oocyte maturation in women undergoing ovarian stimulation.
It has been suggested that elevated progesterone levels on the final day of ovarian stimulation may be related to poorer outcomes in in vitro fertilization fresh cycles due to a negative impact on the endometrium. However, despite conflicting results regarding the actual effect of progesterone on pregnancy rates and the lack of a well-established cut off, currently many IVF patients have their embryo transfer deferred when progesterone values surpass a threshold of 1.5 ng/ml on the day of ovulation triggering.
This was a prospective cohort study conducted in 22 oocyte donors of a university-affiliated fertility centre between November 2017 and January 2018. We calculated the sample size to detect a difference of 15% between the first and last progesterone measurements with a 5% false-positive rate in a two-sided test with 80% statistical power and a 95% confidence interval (CI).
Progesterone circulating levels were evaluated at four different times during the final day of oocyte maturation (08:00, 12:00, 16:00 and 20:00) before ovulation triggering in healthy oocyte donors. A flexible antagonist protocol was used, and ovarian stimulation was achieved with recombinant follicle-stimulating hormone (FSH) in all cases. The pairwise percentage differences in progesterone levels for each patient were calculated. Univariate linear regression analysis was adopted in order to evaluate variables associated with progesterone levels on the first measurement. The intra-day variability of progesterone was analysed using mixed models.
Mean serum progesterone values at 08:00, 12:00, 16:00 and 20:00 were 1.75 ng/ml, 1.40 ng/ml, 1.06 ng/ml and 0.97 ng/ml. The progesterone difference between 08:00 and 20:00 was 0.77 (95% CI, 0.56–0.99), which is equivalent to a 44% decline in the mean progesterone values between the first (08:00) and the last determination (20:00; P < 0.001). Among those patients with basal (08:00) progesterone levels >1.5 ng/ml (n = 10), 70% (n = 7) showed levels reduced to <1.5 ng/ml on the last determination of the day (20:00). A mixed model analysis revealed that the progesterone reduction during the day was significantly associated with time and total recombinant FSH dose administered.
Only young healthy oocyte donors stimulated with an antagonist protocol using recombinant FSH were included. Extrapolation to the general IVF population, with different stimulation protocols and gonadotropins, needs to be confirmed.
This study suggests that a single progesterone determination on the final day of oocyte maturation is not reliable enough to make clinical decisions due to the enormous variation in progesterone during the day. Further studies are needed to better define the impact of the follicular progesterone rise on the endometrium of IVF cycles.
Funding was granted from Fundació Santiago Dexeus Font. N.P.P. received unrestricted grants and/or lectures fees from Roche Diagnostics, MSD, Merck, Ferring Pharmaceuticals, IBSA, Theramex and BESINS International, not associated with the current study. The remaining authors have no competing interests.
Clinicaltrials.gov NCT03366025.
Introduction
Although it is generally accepted that ovarian stimulation leads to an increase in live birth rates following IVF/ICSI (Davis and Rosenwaks, 2001) by increasing the number of available embryos, a potential detrimental effect on endometrial receptivity and implantation rates cannot be excluded (Devroey et al., 2004).
Several pathophysiological mechanisms have been proposed in order to explain the negative effect of ovarian stimulation on embryo implantation rates. One of the most widely discussed appears to be progesterone elevation on the last days of ovarian stimulation, mainly due to accumulating evidence suggesting a profound detrimental effect on pregnancy rates (Al-Azemi et al., 2012). This increase in serum progesterone levels has been directly associated with the use of gonadotropins for ovarian stimulation. Given that GnRH analogues are routinely used to prevent a premature LH surge, the origin of such rise in progesterone is not due to premature luteinization but to granulosa cell production. Granulosa cells from each of the follicles that are being stimulated produce a normal amount of progesterone compatible with the late follicular phase. However, when many follicles are being stimulated at the same time, the total amount of progesterone dramatically increases (Shulman et al., 1996). For this reason, subtle late follicular progesterone elevations, unrelated to a premature LH surge, have been reported in 12–38% of all IVF cycles regardless of artificial pituitary suppression (Silverberg et al., 1991; Ubaldi et al., 1996; Bosch et al., 2003). Although it is unclear how the progesterone rise may negatively affect pregnancy rates, recent evidence supports a detrimental effect on endometrial receptivity rather than oocyte quality (Lawrenz and Fatemi, 2017), by a direct impact on endometrial cells gene expression and displacement of the endometrial window of receptivity (Van Vaerenbergh et al., 2011).
Despite the extensive literature on the effect of progesterone elevation on pregnancy rates, not all published studies are in agreement regarding the actual level of progesterone beyond which pregnancy rates decline or whether in the end a supraphysiological increase in serum progesterone levels on the day of ovulation triggering has any effect at all on final reproductive outcome (Martinez et al., 2004; Venetis et al., 2007; Bosch et al., 2010; Yding Andersen et al., 2011). A major limitation, potentially associated with this discrepancy, may be the huge diversity regarding the exact timing of the measurement and also the assay used in order to determine serum progesterone levels in previous studies. Therefore, taking into account the well-established circadian variability of serum progesterone levels in the spontaneous cycle of normal women (Fujimoto et al., 1990; Bungum et al., 2013), one could seriously question whether indeed a single random measurement of progesterone on the day of final oocyte maturation is adequate to determine a clear cut-off level that could be used in clinical practise. This is even more worrisome if we consider that no data are available on the daily variability of progesterone levels in women undergoing ovarian stimulation, while most of the studies that have evaluated the role of serum progesterone levels in IVF cycle outcomes have used a single measurement on the day of triggering, without specifying at what time the blood sample was collected or if all samples were collected at the same time of the day.
The current study aims through a robust design to provide a simple answer as to whether progesterone levels of the same individual vary significantly depending on the time of blood retrieval before ovulation triggering, by evaluating the daily variability of serum progesterone during the final day of oocyte maturation through multiple blood samplings in 22 healthy women who were undergoing ovarian stimulation for oocyte donation.
Material and Methods
Study population and design
We performed a prospective cohort study with repeated measurements conducted at Dexeus University Hospital, Barcelona (Spain). A total of 22 healthy oocyte donors were recruited between November 2017 and January 2018. Patients were aged between 18 and 35 years and were not under any other medical treatment at the time of the study. Each patient signed an informed consent form before being recruited.
The study protocol was approved by the institutional review board, the local ethics committee and was registered at the ClinicalTrials.gov website (www.clinicaltrials.gov, trial number NCT03366025).
Ovarian stimulation protocol and final oocyte maturation
Patients were treated using a flexible antagonist protocol, and stimulation was achieved with recombinant follicle-stimulating hormone (FSH) administered between 20:00 and 22:00 in all cases. Pituitary down regulation was performed with daily administration of a GnRH antagonist (ganirelix) that was introduced as soon as one follicle reached 14 mm or when estradiol levels exceeded 400 pg/ml.
Cycles were monitored by means of transvaginal ultrasound scans and serum determination of estradiol, LH and progesterone. According to the observed ovarian response, dose adjustments of recombinant FSH were performed. Oocyte maturation was achieved with a GnRH agonist (triptorelin acetate) as soon as 3 follicles of ≥18 mm were observed. Participants proceeded to final oocyte maturation only based on ultrasound criteria, and the clinician was blinded to the progesterone results at the time of planning. Oocyte retrieval was performed by transvaginal aspiration 36 h after triggering.
Progesterone assessment
Progesterone circulating levels were evaluated within each patient at four different times during the final day of oocyte maturation (08:00, 12:00, 16:00 and 20:00) using the Progesterone Elecsys Gen III assay (Cobas 6000®; Roche, Basel, Switzerland) with a measuring range of 0.05–60 ng/ml and a coefficient of variation (CV) of ≤7% for levels >1 ng/ml and ≤ 12% for values ≤1 ng/ml.
Sample size calculation
The sample size calculation was based on a paired t-test. According to this, a sample size of 22 oocyte donors was essential in order to detect a difference of 15% between the first and the last daily progesterone value (08:00 vs. 20:00) with a false-positive rate of 5% (two sided), with a power of 80%, assuming a standard deviation (SD) of change in the outcome of 0.250 (Rosner, 1995).
Statistical analysis
Continuous outcomes were presented as mean (SD) values, whereas categorical outcomes were presented as percentages. Progesterone levels in the first time interval (08:00) were recorded, and univariate linear regression analysis was adopted in order to evaluate variables associated with progesterone levels on the first measurement. The effect of each covariate was measured by r-square.
Progesterone levels between different time intervals were recorded, and pairwise percentage differences for each patient were calculated. The intra-day variability of progesterone (between 08:00, 12:00, 16:00 and 20:00) was analysed using mixed models. A mixed model was adjusted for time plus each covariate [age, estradiol, body mass index (BMI), anti-Müllerian hormone (AMH) levels, last recombinant FSH dose and total FSH dose administered] as fixed effects, with constant and random slope (variance–covariance time dependent) and correlated random effects. Mixed models were adjusted using nlme package (Pinheiro et al., 2018).
Finally, in order to analyse the difference between progesterone levels at 08:00 and 20:00, the 95% confidence interval (CI) for the difference in paired means was calculated.
Statistical analyses were performed with IBM© SPSS© Statistics v 22 and R software (R Core Team, 2018).
Results
Participants baseline characteristics
A total of 22 healthy oocyte donors were included in the study. Participants had a mean age of 26.8 ± 4.9 years and mean BMI of 21.5 ± 2.0 kg/m2. The mean antral follicle count and AMH were 17.4 ± 6.9 follicles and 3.9 ± 2.7 ng/ml, respectively.
Participants stimulation characteristics
The mean recombinant FSH dose used on the first day of ovarian stimulation was 208.0 ± 56.4 IU (range, 150–300 IU). The mean number of days and total recombinant FSH dose needed for stimulation were 10.5 ± 1.3 days and 2263 ± 595.8 IU, respectively. The mean number of oocytes and mature oocytes obtained after retrieval were 15.0 ± 6.1 and 12.0 ± 5.3.
Comparison of the differences in progesterone determinations between 08:00 and 20:00 within patients (n = 22).
| . | 20:00 . | ||||
|---|---|---|---|---|---|
| 08:00 | ≤ 1.5 ng/ml | > 1.5 ng/ml | Total | P | |
| ≤ 1.5 ng/ml | 12 (100%) | 0 (0%) | 12 | 0.016 | |
| > 1.5 ng/ml | 7 (70%) | 3 (30%) | 10 | ||
| 19 | 3 | 22 | |||
| . | 20:00 . | ||||
|---|---|---|---|---|---|
| 08:00 | ≤ 1.5 ng/ml | > 1.5 ng/ml | Total | P | |
| ≤ 1.5 ng/ml | 12 (100%) | 0 (0%) | 12 | 0.016 | |
| > 1.5 ng/ml | 7 (70%) | 3 (30%) | 10 | ||
| 19 | 3 | 22 | |||
Comparison of the differences in progesterone determinations between 08:00 and 20:00 within patients (n = 22).
| . | 20:00 . | ||||
|---|---|---|---|---|---|
| 08:00 | ≤ 1.5 ng/ml | > 1.5 ng/ml | Total | P | |
| ≤ 1.5 ng/ml | 12 (100%) | 0 (0%) | 12 | 0.016 | |
| > 1.5 ng/ml | 7 (70%) | 3 (30%) | 10 | ||
| 19 | 3 | 22 | |||
| . | 20:00 . | ||||
|---|---|---|---|---|---|
| 08:00 | ≤ 1.5 ng/ml | > 1.5 ng/ml | Total | P | |
| ≤ 1.5 ng/ml | 12 (100%) | 0 (0%) | 12 | 0.016 | |
| > 1.5 ng/ml | 7 (70%) | 3 (30%) | 10 | ||
| 19 | 3 | 22 | |||
Determinants of progesterone levels at the first measurement (08:00) on the final day of oocyte maturation
Mean progesterone levels on the final day of oocyte maturation was 1.75 (±0.90) ng/ml at the first measurement (08:00). Overall, 45% of the patients (n = 10) presented with levels >1.5 ng/ml, while 55% (n = 12) showed levels ≤1.5 ng/ml (Table I), representing the high and the low progesterone groups according to the previously established thresholds (Bosch et al., 2010).
According to regression analysis, progesterone levels at the first measurement (08:00) presented a significant association with estradiol levels at that same time (R2 = 0.2049; P = 0.034). No association was found between first progesterone levels and the age of the patient, BMI, AMH levels, last recombinant FSH dose and total FSH dose administered (Fig. 1).
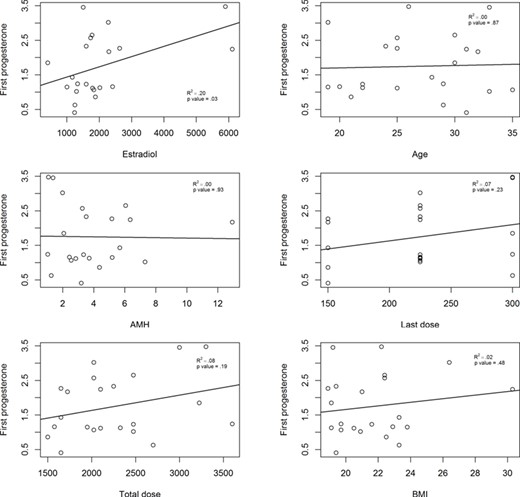
Determinants of serum progesterone levels at the first measurement at 08:00. Last dose, last dose of FSH; Total dose, total dose of FSH.
Intra-day variability of progesterone serum levels
Progesterone levels demonstrated a remarkable progressive decline during the final day of oocyte maturation. The mean progesterone values (±SD) obtained at 8:00, 12:00, 16:00 and 20:00 were 1.75 (±0.90), 1.40 (±0.76), 1.06 (±0.53) and 0.97 (±0.55) ng/ml, respectively.
Overall, the progesterone difference between 08:00 and 20:00 was 0.77 (95% CI, 0.56–0.99), which is equivalent to a 44% decline in the mean progesterone values between the first (08:00) and the last determination (20:00; P < 0.001). In addition, the progesterone difference between 08:00 and 12:00 was 0.35 (95% CI, 0.19–0.50), which is equivalent to a 17% decline in the mean progesterone values between the first (08:00) and the second determination (12:00; P < 0.001). A significant decrease in progesterone values was observed among all the intervals compared, except between 16:00 and 20:00 (P = 0.135) (Fig. 2).
Finally, among those patients with progesterone levels >1.5 ng/ml at 08:00, 70% of them presented values ≤1.5 ng/ml at 20:00 (P = 0.016). None of the patients with progesterone levels ≤1.5 ng/ml at 08:00, presented levels >1.5 ng/ml at 20:00 (Table I and Fig. 2).

Progesterone levels and profiles during the final day of oocyte maturation. (A) Median progesterone levels (ng/ml) over a 12-h interval during the final day of oocyte maturation. (B) Individual serum profiles of progesterone over a 12-h interval during the final day of oocyte maturation.
Determinants of the progesterone decline during the final day of oocyte maturation (mixed models)
The progesterone decline between 08:00 and 20:00 was significantly associated with time and total recombinant FSH dose. Progesterone values presented a fixed mean decline of 0.06 ng/ml (95% CI, 0.04–0.08) for every hour passed between 08:00 and 20:00. In addition, for every 100 IU of recombinant FSH less used in stimulation, progesterone levels decreased 0.3 ng/ml (95% CI, 0.02–0.04). None of the other variables analysed (age, BMI, basal AMH, estradiol and last recombinant FSH dose) presented an association with the progesterone drop during the day (Table II).
Discussion
To our knowledge, this is the first study to evaluate variation in progesterone levels during the final day of oocyte maturation in women undergoing ovarian stimulation. Progesterone levels demonstrated a remarkable clinically and statistically significant decline of up to 44% between 08:00 and 20:00 during the final day of oocyte maturation. Interestingly, among patients considered to have elevated progesterone levels at baseline (08:00), based on the currently accepted threshold of >1.5 ng/ml, the majority (70%) presented levels below this threshold at the last measurement of the day (20:00).
Human ovarian steroidogenesis is regulated by the cyclic variations of gonadotropins occurring during the menstrual cycle. Follicle-stimulating hormone and luteinizing hormone are responsible for the conversion of cholesterol to estradiol, during the follicular phase, and to progesterone, during the luteal phase. According to the two-cell two-gonadotropin concept, LH activates the theca cells to stimulate production of the androgen precursors while FSH activates the granulosa cells to stimulate aromatization of these androgens into estrogens. Both theca and granulosa cells also have the capacity to produce progesterone and to express the enzymes responsible for converting cholesterol to pregnenolone (P450 side-chain cleavage enzyme) and for converting pregnenolone to progesterone (3-b-hydroxysteroid dehydrogenase), and both of these enzymes appear to be up-regulated by FSH and LH (Payne and Hales, 2004; Yu et al., 2005; Oktem et al., 2017). Thus, progesterone is naturally produced by both theca and granulosa cells and is present in circulation during the follicular phase of the human menstrual cycle; however, the precise control of this secretion, especially with ovarian stimulation protocols, is not well understood (Yding Andersen et al., 2011).
Mixed models for association between patient and cycle variables with time (from 08:00 to 20:00) during the final day of oocyte maturation.
| . | Variables . | Estimate . | Std.Error . | P-value . |
|---|---|---|---|---|
| Model time | t | −0.063 | 0.008 | <0.001 |
| Model time + age | t | −0.064 | 0.008 | <0.001 |
| Age | 0.026 | 0.018 | NS | |
| Model time + AMH | t | −0.063 | 0.085 | <0.001 |
| AMH | −0.059 | 0.033 | NS | |
| Model time + BMI | t | −0.063 | 0.008 | <0.001 |
| BMI | −0.005 | 0.035 | NS | |
| Model time + last dose | t | −0.062 | 0.008 | <0.001 |
| Last dose | 0.003 | 0.002 | NS | |
| Model time + total dose | t | −0.063 | 0.008 | <0.001 |
| Total dose | 0.030 | 0.010 | 0.03 | |
| Model time + estradiol | t | −0.061 | 0.001 | <0.001 |
| Estradiol | 0.001 | 0.001 | NS |
| . | Variables . | Estimate . | Std.Error . | P-value . |
|---|---|---|---|---|
| Model time | t | −0.063 | 0.008 | <0.001 |
| Model time + age | t | −0.064 | 0.008 | <0.001 |
| Age | 0.026 | 0.018 | NS | |
| Model time + AMH | t | −0.063 | 0.085 | <0.001 |
| AMH | −0.059 | 0.033 | NS | |
| Model time + BMI | t | −0.063 | 0.008 | <0.001 |
| BMI | −0.005 | 0.035 | NS | |
| Model time + last dose | t | −0.062 | 0.008 | <0.001 |
| Last dose | 0.003 | 0.002 | NS | |
| Model time + total dose | t | −0.063 | 0.008 | <0.001 |
| Total dose | 0.030 | 0.010 | 0.03 | |
| Model time + estradiol | t | −0.061 | 0.001 | <0.001 |
| Estradiol | 0.001 | 0.001 | NS |
Model time is the model with only the hour measurement as covariate.
NS: Not significant
Mixed models for association between patient and cycle variables with time (from 08:00 to 20:00) during the final day of oocyte maturation.
| . | Variables . | Estimate . | Std.Error . | P-value . |
|---|---|---|---|---|
| Model time | t | −0.063 | 0.008 | <0.001 |
| Model time + age | t | −0.064 | 0.008 | <0.001 |
| Age | 0.026 | 0.018 | NS | |
| Model time + AMH | t | −0.063 | 0.085 | <0.001 |
| AMH | −0.059 | 0.033 | NS | |
| Model time + BMI | t | −0.063 | 0.008 | <0.001 |
| BMI | −0.005 | 0.035 | NS | |
| Model time + last dose | t | −0.062 | 0.008 | <0.001 |
| Last dose | 0.003 | 0.002 | NS | |
| Model time + total dose | t | −0.063 | 0.008 | <0.001 |
| Total dose | 0.030 | 0.010 | 0.03 | |
| Model time + estradiol | t | −0.061 | 0.001 | <0.001 |
| Estradiol | 0.001 | 0.001 | NS |
| . | Variables . | Estimate . | Std.Error . | P-value . |
|---|---|---|---|---|
| Model time | t | −0.063 | 0.008 | <0.001 |
| Model time + age | t | −0.064 | 0.008 | <0.001 |
| Age | 0.026 | 0.018 | NS | |
| Model time + AMH | t | −0.063 | 0.085 | <0.001 |
| AMH | −0.059 | 0.033 | NS | |
| Model time + BMI | t | −0.063 | 0.008 | <0.001 |
| BMI | −0.005 | 0.035 | NS | |
| Model time + last dose | t | −0.062 | 0.008 | <0.001 |
| Last dose | 0.003 | 0.002 | NS | |
| Model time + total dose | t | −0.063 | 0.008 | <0.001 |
| Total dose | 0.030 | 0.010 | 0.03 | |
| Model time + estradiol | t | −0.061 | 0.001 | <0.001 |
| Estradiol | 0.001 | 0.001 | NS |
Model time is the model with only the hour measurement as covariate.
NS: Not significant
Although no previous studies have evaluated progesterone variation during the final day of oocyte maturation in women under ovarian stimulation, the intra-day variability of progesterone in unstimulated cycles has previously been reported. It is well established that progesterone is one of the hormones that follow a diurnal pattern during the day (van Kerkhof et al., 2015). In this context, there is evidence demonstrating that serum progesterone levels during early follicular phase are highest in the early morning and show a profound decrease during the day (Fujimoto et al., 1990; Bungum et al., 2013). In our study, only time and total recombinant FSH dose presented a correlation with the progesterone decline along the day. In this regard, it has been previously shown that the amount of progesterone found in the serum of patients during the late follicular phase of gonadotropin-stimulated cycles derives from the granulosa cell production in response to FSH (Fleming and Jenkins, 2010; Fatemi and Van Vaerenbergh, 2015; Lawrenz and Fatemi, 2017; Lawrenz et al., 2018b) without premature luteinization or any involvement of the theca cells. Furthermore, our results show that the only factor associated with progesterone levels on the first determination of the day (08:00) was estradiol. This interesting finding is biologically plausible because on the final day of oocyte maturation both hormones are mainly produced by granulosa cells (Oktem et al., 2017). In this regard, previous studies have demonstrated that the higher the number of follicles stimulated, the higher the levels of progesterone on the day of ovulation triggering (Griesinger et al., 2013). In the same context, there is interesting research showing that the levels of progesterone on the day of ovulation triggering are increased in relation to the total doses of FSH used during ovarian stimulation (Beckers et al., 2000; Andersen and Ezcurra, 2014). However, the results of our study showed that the total dose of exogenous administration of recombinant FSH was not associated with the highest values of progesterone in the early morning (08:00), although it presented a positive correlation with their progesterone decline during the day. These findings may be explained by the number of samples analysed, as 22 samples were used for the univariate analysis (08:00) and 88 for the mixed model evaluating progesterone variation at 4 different times of the day. Besides, it is important to remark that differences in progesterone values may also be explained when using different assays for its measurement. Although our study design was robust, consistently using the same progesterone assay, it has been demonstrated that progesterone results present significant variations depending on the assay used and the range of progesterone level (Patton et al., 2014; Lawrenz et al., 2018c).
A major strength of the current study is its robust design aiming to eliminate confounding bias. Serum progesterone was evaluated in the same laboratory, always under the same conditions, and at the same four specific time intervals during the day of oocyte maturation. In addition, all participants were healthy oocyte donors who were treated with an identical antagonist protocol using recombinant FSH administered daily always at the same time of the day (between 20:00 and 22:00). Finally, a robust sample size calculation was performed in order to detect a difference of 15% between the first and the last progesterone determination during the final day of oocyte maturation.
Nevertheless, we need to highlight that our study refers to young healthy oocyte donors with good ovarian reserve markers, a population that does not reflect the majority of the patients seeking fertility advice, who could be of more advanced age. Still, there is no biological rationale to suggest that serum progesterone levels may follow a different pattern in advanced age patients. In addition, we need to highlight that the CV in progesterone determination by the Progesterone Elecsys Gen III assay has been described to be ≤12%. In this context, we cannot exclude that this variation may have affected progesterone values during different measurements; nevertheless, such variations in progesterone levels among study samples would have randomly occurred in all the samples, and thus, it is unlikely to be related with the clinically and statistical decline of 44% we observed in the progesterone values from 08:00 to 20:00.
The clinical implications of the current study appear to be of paramount importance given the widely accepted detrimental role of serum progesterone levels on the day of ovulation triggering on the final IVF outcomes. Previous studies and meta-analyses have shown that elevated serum progesterone levels in the late follicular phase impair the outcomes of fresh IVF cycles (Lid et al., 2004; Venetis et al., 2007; Bosch et al., 2010; Venetis et al., 2013), a finding that led to changes in the daily clinical practise of many IVF centres worldwide, opening a new indication for the `freeze-all’ approach (Van Vaerenbergh et al., 2011; Esteves et al., 2018; Roque et al., 2019). In addition, further research did not find any association between progesterone elevation on the final day of oocyte maturation and the odds of pregnancy after a subsequent transfer of frozen-thawed embryos, suggesting that the negative influence of such a rise was on endometrial receptivity, rather than on oocyte quality (Labarta et al., 2011; Yang et al., 2015; Healy et al., 2016). Still, other groups failed to reach the same conclusions (Martinez et al., 2004; Martinez et al., 2016) or demonstrated that the negative impact of progesterone elevation occurred mostly in certain subgroups of patients (Griesinger et al., 2013). Furthermore, despite the proposed cut off of 1.5 ng/ml (Bosch et al., 2010) described in the past, there is still no consensus on the exact progesterone cut-off value beyond which fresh IVF transfers have significantly inferior results, especially given the presence of different assays with different CVs. The heterogeneity of the studies performed is enormous, and different threshold values have been proposed depending on patients’ characteristics, the day of embryo transfer and the type of gonadotropin used for stimulation (Papanikolaou et al., 2009; Bosch et al., 2010; Devroey et al., 2012; Huang et al., 2015; Lawrenz et al., 2016). It is now evident that most of the studies do not give information on the time of the last gonadotropin administration and the exact time at which progesterone is measured of the day of triggering. For this reason, although the findings of our study do not question the validity of previous research regarding the negative effect of the progesterone rise during the late follicular phase of stimulated cycles, they seriously question the proposed progesterone thresholds beyond which lower implantation rates in fresh embryo transfer cycles are observed.
Far from being closed, progesterone elevation during the late follicular phase is still an open issue in reproductive medicine (Martinez et al., 2016; Lawrenz et al., 2018a). Further studies are needed to determine whether progesterone, when measured with the same assay and at the same time during the day, is associated with a detrimental effect on pregnancy rates following fresh embryo transfer. Additionally, it is even more important to search for new strategies that can help us to better identify which patients are harmed with fresh IVF transfer and will benefit from a deferred (frozen) embryo transfer policy. If a single progesterone determination is not be valid enough, repeated progesterone assessments during the ovarian stimulation cycle could be used in any way to find an answer to this unresolved hot topic.
In conclusion, our findings clearly show a remarkable decrease in progesterone values during the day of ovulation triggering in oocyte donors who underwent an ovarian stimulation cycle. These results highlight the relevance of standardizing the time of progesterone determination and the importance of future research on the subject.
Authors’ roles
N.P.P. conceived and designed the study. I.G.F., I.R. and D.R. collected the data. I.R. performed the statistical analyses. I.G.F. and N.P.P. wrote the article. F.M., J.R.P., P.M., J.N., B.C. and P.B. contributed with important intellectual content. All the authors read and approved the final version of the article.
Funding
This research was performed under the auspices of the Càtedra d’Investigació en Obsterícia i Ginecologia of the Department of Obstetrics, Gynaecology and Reproductive Medicine, Hospital Universitary Dexeus, Universitat Autònoma de Barcelona. Funding was granted from Fundació Santiago Dexeus Font (grant number: 11102017/01.)
Conflict of interest
N.P.P. received unrestricted grants and/or lectures fees from Roche Diagnostics, MSD, Merck, Ferring Pharmaceuticals, IBSA, Theramex and BESINS International, not associated with the current study. The remaining authors have no conflicts of interest.



