-
PDF
- Split View
-
Views
-
Cite
Cite
J Grosbois, I Demeestere, Dynamics of PI3K and Hippo signaling pathways during in vitro human follicle activation, Human Reproduction, Volume 33, Issue 9, September 2018, Pages 1705–1714, https://doi.org/10.1093/humrep/dey250
Close - Share Icon Share
Abstract
How does biochemical stimulation or inhibition of the PI3K/Akt/mTOR pathway affect the activation and the growth of human primordial follicles in vitro?
The PI3K/Akt activators act synergistically with the Hippo signaling pathway, disrupted by tissue fragmentation, to accelerate primordial follicles recruitment while mTORC1 inhibitor partially prevents the massive spontaneous activation.
Hippo disruption caused by ovarian fragmentation and exposure to PI3K/Akt activators have been successfully used to activate resting follicles prior to grafting in patients with premature ovarian insufficiency. Outside this indication, massive and premature activation is considered deleterious as it leads to the depletion of the follicular pool and developmental defects in vitro.
One hundred and twelve frozen–thawed ovarian cortex fragments from four patients were exposed to dimethylsulfoxide (DMSO—control group), everolimus (inhibitor group) or bpV (HOpic) and 740Y-P (activators group) during the first 24 and 48 h, respectively, and cultured for additional 5 days.
The ovarian fragments (4×2×1 mm3) were analyzed at Days 0, 1, 3 and 5 of culture. Cortex were either directly processed for immunohistological or western blot analysis or subjected to follicular isolation for assessment of gene expression. The follicle number and the developmental stage were evaluated in sections of ovarian fragments to assess the early follicular development. Survival and developmental potential were evaluated using TUNEL labeling, GDF9 and Ki67 immunostainings, Kit ligand mRNA relative expression and measurement of 17β-estradiol (E2) levels in the culture medium. qPCR and western blotting were used to explore the expression of PI3K/Akt and Hippo pathways (TSC1, mTOR, LATS1, BIRC1 and CCN2 genes and PTEN/p-PTEN, Akt/p-Akt and rpS6/p-rps6 proteins), and localization of YAP in human ovarian tissue was performed by immunofluorescence.
Around 80% of the follicles spontaneously activated during the culture period, triggered by the activation of the PI3K/Akt/mTOR signaling. Moreover, ovarian fragmentation immediately promoted translocation of the Hippo effector YAP into the nucleus of granulosa cells, leading to upregulation of BIRC1 and CCN2 downstream targets at Day 0 and an increase of follicular growth near the cutting site. Short term exposure of thawed human ovarian fragments to PI3K/Akt activators had significant (P < 0.05 at D1 and D3; P < 0.001 at D6) but limited and potentially negative effects on follicular activation compared to spontaneous follicular growth as suggested by impaired E2 secretion. In contrast, transient incubation with an mTORC1 inhibitor partially prevented spontaneous activation by limiting growing follicle counts (P < 0.01 at D6), granulosa cell proliferation, and Kit ligand expression while ensuring appropriate steroidogenesis.
N/A.
Impact of in vitro culture might cover up the potential benefit of the everolimus on growing follicles morphology after 6 days.
Our results demonstrate the synergistic effects of Hippo and PI3K/Akt/mTOR signaling, contributing to the acceleration of early primordial follicle recruitment in thawed human ovarian fragments, and suggest a beneficial effect of the mTORC1 inhibitor.
This work was supported by a grant from the Fond National de la Recherche Scientifique de Belgique (Grant Télevie Nos. 7.6516.16 F and 7.4578.14 F) and the Fonds Erasme. No competing interests declared.
Introduction
Primordial follicles are oocytes surrounded by a single layer of flattened granulosa cells that can remain quiescent in the ovary for years before being recruited into the growing pool. The recruitment of primordial follicles is regulated by highly controlled mechanisms that ensure a sustainable balance between growing and quiescent follicular pools, providing a selected mature healthy oocyte each month while preventing premature exhaustion of the ovarian reserve. Among these regulators, the critical role of the PI3K/Akt/mTOR signaling pathway is well established (Hsueh et al., 2015). This pathway is negatively regulated by phosphatase and tensin homolog deleted on chromosome 10 (PTEN) and tuberous sclerosis complex (TSC1/2). PTEN or TSC1/2 deletion in mice induces overexpression of Akt and/or mTORC, and consequently, the phosphorylation of downstream ribosomal protein S6 (rpS6) in the oocyte, resulting in a massive and precocious activation of primordial follicles (Reddy et al., 2008; Adhikari et al., 2010; Tanaka et al., 2012). Removal of ovarian tissue from its in vivo environment can also dysregulate this pathway and induce massive, spontaneous and accelerated growth of primordial follicles (Hovatta et al., 1999; Telfer et al., 2008).
Recent experiments suggested that the Hippo signaling also plays a crucial role in follicular activation in non-physiological condition (Kawamura et al., 2013). The Hippo pathway has been identified as the major suppressor of tissue overgrowth (Varelas, 2014). It involves a serine/threonine protein kinase cascade that includes the large tumor suppressor kinase (LATS1/2), mammalian Ste-20 like kinase (MST1/2), the adaptor Salvador (Sav) and Mps one binder (Mob) proteins which impede nuclear access of the key effectors Yes-associated protein (YAP) and transcriptional coactivators PDZ-binding motif (TAZ) by phosphorylation and cytoplasmic retention mechanisms. When ovarian tissue is fragmented, the Hippo signaling pathway is disrupted, leading to the translocation of non-phosphorylated YAP into the nucleus to interact with partners such as TEAD1-4 transcription factors that target downstream CCN growth factors and baculoviral IAP repeat containing (BIRC) apoptosis inhibitor genes (Pan, 2007).
In humans, the incubation of ovarian tissue in the presence of PTEN inhibitor or PI3K activator generates massive primordial follicle growth initiation (Li et al., 2010; Kawamura et al., 2013; Lerer-Serfaty et al., 2013; McLaughlin et al., 2014; Novella-Maestre et al., 2015). Taking advantage of the effects of both the PI3K/Akt/mTOR and Hippo pathways on follicular recruitment and growth, pregnancies have been obtained after grafting of small ovarian fragments exposed to PI3K/Akt activators into patients diagnosed with premature ovarian insufficiency (POI) (Kawamura et al., 2013; Suzuki et al., 2015). While functional links have been described between the PI3K/Akt/mTOR and the Hippo pathways in other organs, direct crosstalk remains to be established in the ovary. Moreover, the real benefit of such accelerated follicle activation is controversial, as it may lead to a rapid depletion of the follicle stock and have detrimental effects on the acquisition of oocyte competence (Lerer-Serfaty et al., 2013; McLaughlin et al., 2014). We hypothesized that pharmacological inhibition of the PI3K/Akt/mTOR pathway could limit spontaneous follicular activation, thus promoting a more physiological developmental time frame of the follicles within frozen–thawed human ovarian fragments. Among mTORC1 inhibitors, everolimus (EVE), an analog of rapamycin, is commonly used in human transplantation to prevent organ rejection (Gabardi and Baroletti, 2010). In the ovary, Goldman and colleagues recently showed that EVE co-treatment with chemotherapy preserved ovarian reserve and fertility in mice (Goldman et al., 2017).
The aim of this study was to compare the effect of an inhibition versus an activation of the PI3K/Akt/mTOR pathway on the activation and the subsequent development of human primordial follicles, and to clarify the interaction between the Hippo and the PI3K/Akt/mTOR signaling during early in vitro folliculogenesis.
Materials and Methods
Ovarian tissue collection
Ovarian cortex was obtained from four patients aged 19–29 years (mean 25.25 ± 4.5 years) who underwent ovarian tissue cryopreservation.
Ethical approval
The fertility preservation procedure and the research project were both approved by the local Ethical Committee. All patients gave written informed consent to donate their unused frozen ovarian tissue to the research program at the end of the storage period.
Freezing and thawing procedures
Ovarian fragments measuring around 5 × 5 × 2 mm3 were cryopreserved using a controlled slow-freezing protocol, stored in liquid nitrogen (−196°C) and rapidly thawed as previously described (Demeestere et al., 2003, 2006). After thawing, fragments were rinsed in 10 ml of Leibovitz medium (Gibco, Life Technologies, Belgium) supplemented with 2 mM sodium pyruvate (Sigma, Belgium), 2 mM glutamine (Sigma), 3 mg/ml human serum albumin (HSA) (CAF DCF, Belgium), 30 μg/ml penicillin G (Sigma) and 50 μg/ml streptomycin (Sigma).
In vitro culture
Frozen–thawed ovarian tissue was cut into fragments of 4 × 2 × 1 mm3. One to three fragments were selected from each biopsy as Day 0 (D0) controls and fixed for histological evaluation. The remaining fragments were randomly divided into three groups: dimethylsulphoxide (DMSO—control group), mTORC1 inhibitor (Everolimus [EVE] group) or PI3K/AKT activator (740Y-P) and PTEN inhibitor (bpV) (activator group). Fragments were placed individually into 4-well cell culture plates (ThermoFisher, Belgium) containing 300 μl of culture medium [McCoy’s 5a medium with bicarbonate supplemented with 25 mM HEPES (Gibco, Life Technologies), 0.1% HSA, 3 mM glutamine, 30 μg/ml penicillin G, 50 μg/ml streptomycin, 2.5 μg/ml transferrin (Fisher Scientific), 4 ng/ml selenium (Sigma) and 50 μg/ml ascorbic acid (Sigma)] supplemented with either 30 μM bpV (HOpic) (Merck Chemicals, Belgium) + 150 μg/ml 740Y-P (Bio-Techne, UK) for the first 24 h and 48 h, respectively, or 100 nM EVE (Selleck Chemicals, Germany) or DMSO 1% (Sigma) for the first 24 h, at 37°C in humidified air with 5% CO2. At Day 1, media were replaced with fresh culture medium supplemented with 10 ng/ml insulin (Sigma) and 1 ng/ml hFSH (Gonal F, Merck Serono, UK) and tissues were cultured for an additional 5 days with half of the medium being replaced every other day. After 0, 1, 3 and 6 days of culture, a total of 56 cortical strips were fixed in 4% paraformaldehyde for 24 h at 4°C for histological and immunohistological analysis. Overall, 21 fragments, cultured for 0, 1 or 6 days, were used for immunoblotting analysis, and follicles isolated from 35 fragments were used for gene expression analysis by qPCR.
Histological analysis
Fixed control (D0) and cultured fragments (D1, D3, D6) were dehydrated in increasing concentrations of ethanol (70–100%), embedded in paraffin and serially sectioned at 5 μm thickness. Every fifth section was stained with hematoxylin and eosin to perform morphological evaluation and follicular counts; the remaining sections were used for immunohistological analysis.
Follicles were counted after 0, 1, 3 or 6 days of culture and classified according to their developmental stage as primordial follicles (oocyte surrounded by a few flattened granulosa cells), transitory follicles (oocyte surrounded by flattened and at least one cuboidal granulosa cell) or growing follicles (oocyte surrounded by one or more complete layer(s) of cuboidal granulosa cells). Only follicles that contained an oocyte nucleus were counted to prevent double counting.
Apoptosis assay
DNA fragmentation was assessed using the In Situ Cell Death Detection Kit (Roche, Germany). Paraffin sections were deparaffinized, rehydrated and permeabilized with 20 μg/ml proteinase K in 10 mM Tris pH7.4 (Qiagen, Netherlands). After washing, the slides were incubated with TdT-mediated dUTP-biotin nick-end labeling (TUNEL) reagents according to the manufacturer’s instructions and counterstained with Hoechst (1 μg/ml). Sections were treated with 50 units/ml DNase I (Invitrogen, USA) as a positive control or without the enzyme as a negative control.
Immunohistochemistry
Paraffin sections were deparaffinized and rehydrated in order to investigate the expression of follicular proliferation (Ki67) and oocyte developmental (GDF9) markers as well as the main Hippo effector, YAP. Antigen retrieval by heat was performed in citrate buffer pH 6.0, and endogenous peroxidase activity was quenched using 3% hydrogen peroxide (Merck Millipore, Belgium). Non-specific sites were blocked by normal goat serum and the slides were incubated with primary antibodies (Supplementary Table S1) overnight at 4°C. For Ki67 and GDF9, sections were incubated in biotinylated secondary antibody (Vector Laboratories, UK) and processed using an ABC kit according to the manufacturer’s instructions (Vectastain Elite ABC systems, Vector). The reaction was revealed using 3,3′-diaminobenzidine (DAB) peroxidase substrate kit (Vector) followed by counterstaining with hematoxylin. For YAP, the slides were incubated with a biotinylated goat anti-rabbit secondary antibody, then with avidin-fluorescein isothiocyanate (avidin-FITC, 1:200, Vector) and counterstained with Hoechst. GDF9 and Ki67 were examined using a Leica DM 2000 fluorescence microscope, and YAP was localized using a Zeiss LSM 710 laser scanning confocal microscope.
Immunoblotting analysis
Tissue samples were homogenized in a MagNA Lyser instrument (Roche) for two cycles (6500 rpm, 50 s) in Laemmli buffer containing a cocktail of proteases (ThermoFisher) and phosphatases (Roche) inhibitors. Protein lysates were separated by sodium dodecyl sulfate—polyacrylamide gel electrophoresis (SDS-PAGE) (4–12%) and transferred to nitrocellulose membranes. The membranes were incubated with primary antibodies (Supplementary Table S1) overnight at 4°C, washed and incubated with secondary antibody for 1 h at room temperature. Protein bands were revealed using a SuperSignal West Femto Chemiluminescent Substrate (Thermo Scientific, Belgium) and visualized with a Bio-Rad gel imager using Image Lab software (Bio-Rad, France).
Follicles isolation
Follicles cultured were isolated from tissue using a modified method described previously (Xu et al., 2009). Briefly, ovarian cortical strips were cut into 0.5 mm thickness using a Tissue Slicer (Thomas Scientific, USA) and fragmented with a tissue sectioner (McIlwain Tissue Chopper, The Mickle Laboratory, UK) to obtain uniform-size cubes of 0.5 mm3. The tissue was then enzymatically digested by incubation in culture medium supplemented with 0.2% collagenase IV (Life Technologies) and 0.02% DNase (Sigma) for 1.5 h at 37°C in humidified air with 5% CO2. The ovarian digest was shaken every 10 min with a pipette to mechanically disrupt the tissue. Digestion was stopped by adding an equal volume of culture medium at 4°C supplemented with 10% fetal bovine serum (Sigma). Follicles at all stages were collected directly or after mechanical isolation from the cortex using 29-gauge needles and rinsed two times with phosphate buffered saline (PBS) before RNA extraction.
Real-time PCR analysis
Total RNA from 20 isolated follicles was extracted using RNAqueous-Micro kit (Life Technologies) according to the manufacturer’s instructions. cDNA was reverse-transcribed using MultiScribe Reverse Transcriptase with random primers (Applied Biosystems, USA). RT-qPCR was performed in 96-well plates using Power SYBR Green Master Mix on ABI 7500 (Applied Biosystems), and the relative abundance of the target genes (TSC1, mTOR, LATS1, BIRC1, CCN2 and Kit Ligand) was normalized based on HPRT and YWHAZ levels (Supplementary Table S2). Gene amplification was relatively quantified using the comparative cycle threshold (Ct) method (ΔΔCt).
Hormonal assays
17β-estradiol (E2) was measured in the culture media collected at D1, D3, D5 and D6. Media from the cortex of the same patient were pooled for each day and each experimental condition and stored at −80°C. E2 was measured using a competitive immunoassay with an electrochemiluminescence detection on a Roche analytical platform (Estradiol III, e602, Roche). The inter-assay coefficient of variation was between 2.5 and 5.7%.
Statistical analysis
Results are presented as mean ± SD for follicle counting and mean ± SEM for qPCR analysis, western blots and hormonal concentrations, and were analyzed with SPSS 24 software (IBM, Belgium). Statistical significance was determined either using one-way ANOVA followed by the Fisher test (difference in follicle stage rate and gene/protein expression) or by the Kruskall Wallis test followed by the Mann Whitney test (hormonal assay). P-values <0.05 were considered significant.
Results
Effect of PI3K activators and mTORC1 inhibitor on follicular recruitment
We used an established culture system (Telfer et al., 2008) to compare the effect of transient PI3K/Akt activation or mTORC1 inhibition on primordial follicle activation. After thawing, 89.2% of the follicles were at the primordial or transitory stage of development (Fig. 1A and B). Follicular activation was observed in all groups during culture. Exposure to PI3K/Akt activators triggered a significantly more profound drop in the percentage of primordial follicles compared to control from the first day of culture that correlated with an increase in the transitory population. Nevertheless, the numbers of growing follicles were similar in both groups at Day 6 (Fig. 1C). In contrast, cortex treated with the mTORC1 inhibitor (EVE) exhibited a higher proportion of primordial and transitory follicles at Day 6, as well as a lower number of growing follicles throughout the culture period.
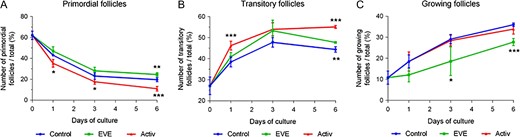
PI3K/Akt activators promote primordial follicle activation whereas everolimus partially limits follicle recruitment. Distribution of follicular stages: (A) primordial follicles, (B) transitory follicles and (C) growing follicles after short term treatment with mTORC1 inhibitor (EVE) or PI3K activators (Activ). Results are expressed as the percentage of follicles at different stages per fragment (mean ± SD); N = 56 fragments (4 × 2 × 1mm3) of ovarian cortex from four patients (control n = 18; EVE n = 18; Activ n = 20; total of 11 403 follicles counted). Statistical analyses were performed in comparison with the control group. *P < 0.05; **P < 0.01; ***P < 0.001 using one-way ANOVA analysis.
Importantly, follicular survival was similar in all groups during culture. No differences were found in the average follicle number per fragment, regardless of the group or the day of culture (Table I). After 6 days of culture, when present, apoptosis was most often detected in the stromal cells in all groups while follicles remained alive (Fig. 2A). Follicular growth was confirmed by GDF9- and Ki67-positive staining in oocyte and granulosa cells, respectively (Fig. 2B). However, histological analysis revealed some morphological abnormalities in the growing follicles in all groups, including vacuoles within the oocyte or irregular layers of granulosa cells after in vitro culture (Fig. 2C).
Follicle number per fragment of ovarian cortex of 4×2×1 mm3 (mean± SEM). N = 56 fragments of ovarian cortex from four patients (control n = 18; EVE n = 18; activ n = 20), everolimus (EVE), activator (activ).
| Day of culture . | Thawing . | Control . | EVE . | Activ . |
|---|---|---|---|---|
| 0 | 197.8 ± 119.3 | – | – | – |
| 1 | – | 184.3 ± 60.4 | 148.8 ± 60.8 | 187.8 ± 75.5 |
| 6 | – | 171.5 ± 90.6 | 177.6 ± 62.2 | 162.1 ± 59.4 |
| Day of culture . | Thawing . | Control . | EVE . | Activ . |
|---|---|---|---|---|
| 0 | 197.8 ± 119.3 | – | – | – |
| 1 | – | 184.3 ± 60.4 | 148.8 ± 60.8 | 187.8 ± 75.5 |
| 6 | – | 171.5 ± 90.6 | 177.6 ± 62.2 | 162.1 ± 59.4 |
Follicle number per fragment of ovarian cortex of 4×2×1 mm3 (mean± SEM). N = 56 fragments of ovarian cortex from four patients (control n = 18; EVE n = 18; activ n = 20), everolimus (EVE), activator (activ).
| Day of culture . | Thawing . | Control . | EVE . | Activ . |
|---|---|---|---|---|
| 0 | 197.8 ± 119.3 | – | – | – |
| 1 | – | 184.3 ± 60.4 | 148.8 ± 60.8 | 187.8 ± 75.5 |
| 6 | – | 171.5 ± 90.6 | 177.6 ± 62.2 | 162.1 ± 59.4 |
| Day of culture . | Thawing . | Control . | EVE . | Activ . |
|---|---|---|---|---|
| 0 | 197.8 ± 119.3 | – | – | – |
| 1 | – | 184.3 ± 60.4 | 148.8 ± 60.8 | 187.8 ± 75.5 |
| 6 | – | 171.5 ± 90.6 | 177.6 ± 62.2 | 162.1 ± 59.4 |
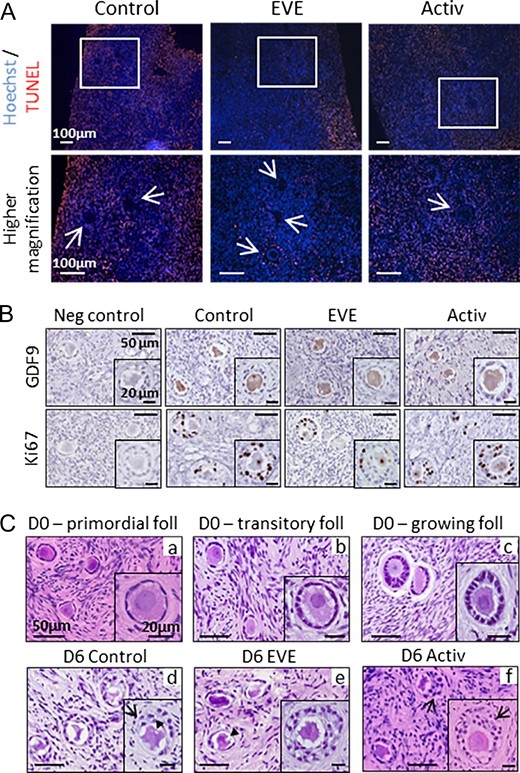
Follicular survival and morphology after treatment exposure and in vitro growth. (A) After 6 days of culture, apoptosis was observed in some stromal cells in all groups but only rarely in in vitro grown follicles. Apoptotic cells were stained by TUNEL (red). Arrows indicate follicles. (B) Oocytes of in vitro grown follicles expressed GDF9, and Ki67 was detected in the granulosa cells of follicles after 6 days of culture. In accordance with the lower rate of growing follicles observed in the EVE group, Ki67 staining was lower in this group compared to control and activator groups. Negative controls (neg control) were unexposed to primary antibodies. (C) The follicular shapes after thawing were well preserved in primordial (a), transitory (b) and growing (c) follicles but secondary follicles displayed morphological abnormalities and presented over-growth patterns after 6 days of culture (d–f), including vacuoles within the oocyte (arrowhead) or anarchic proliferation of the granulosa cells (arrow). Foll = follicle; D0, D6 = Days 0 and 6 of culture, respectively.
Role of the PI3K/Akt/mTOR pathway in primordial follicle activation
The efficiency of both suppression of PTEN and overexpression of PI3K (activator group) or inhibition of mTORC1 (EVE group) to disrupt the PI3K/Akt/mTOR pathway was evaluated by western blotting of ovarian fragments and RT-qPCR analysis of isolated follicles after 0, 1 or 6 days of culture. No differences in mTOR transcript levels were observed, regardless of the treatment used or the day of culture (Fig. 3A). However, the relative expression of TSC1, a critical negative regulator of mTORC1, was significantly downregulated after one day of culture in all groups, suggesting a spontaneous removal of PI3K/Akt/mTOR inhibition at initiation of culture which was reestablished on D6 (Fig. 3B). As a consequence, the downstream effector phospho-rpS6 was increased in both control (P = 0.016) and activator (P = 0.030) groups at D1 (Fig. 3C). This spontaneous activation of the mTORC1 pathway was associated with a slight increase in p-Akt expression at D1 in the control group (P < 0.001), whereas no change in p-PTEN/PTEN ratio was demonstrated. In contrast, the presence of mTORC1 inhibitor dramatically inhibited rpS6 phosphorylation and prevented changes in the p-Akt/Akt ratio at D1. As expected, short term exposure to bpV, which acts as an inhibitor of the protein phosphotyrosine phosphatases with selectivity for PTEN, increased the p-PTEN/PTEN ratio but the difference did not reach significance (Fig. 3D). Moreover, the presence of activators was associated with a significant increase in the phospho-Akt/Akt ratio through decreasing interactions with phosphatidylinositol phosphate (PIP) and direct promotion of PI3K activation (740Y-P). As a consequence, downstream p-rpS6 protein levels were enhanced at D1, although differences compared to control did not reach significance (Fig. 3C and E), confirming that the additional effect of PI3K/Akt activators was limited compared to spontaneous activation.
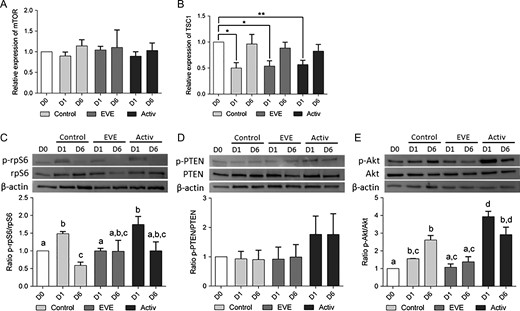
PI3K activators and mTORC1 inhibitor act on primordial follicle activation through the PI3K/Akt/mTORC1 pathway. RT-qPCR and western blotting analysis were performed on isolated follicles and ovarian fragments, respectively, after 0, 1 or 6 days of culture (D0, D1 and D6). (A, B) mTOR transcript levels within the follicles were constant throughout the culture period (A) while TSC1 mRNA relative expression was downregulated after 1 day of culture (B). The relative amount of each transcript was normalized based on HPRT and YWHAZ expression levels. *P < 0.05; **P < 0.01. (C–E) Short term exposure with activators increased phospho-PTEN levels, upregulated the phosphorylation of S473 Akt and downstream rpS6 proteins at D1 compared to control, without affecting total PTEN, Akt and rpS6 content. Treatment with EVE dramatically reduced phospho-rpS6 at D1 compared to control, without affecting either p-PTEN/PTEN or p-Akt/Akt ratios. β-actin was used as loading control. Different letters indicate P < 0.05.
Effect of PI3K/Akt activators and mTORC1 inhibitor on follicular development
The Kit ligand is a pluripotent growth factor involved in preantral follicle growth and oocyte development (Hutt, 2006). Here, Kit ligand expression was significantly increased between D1 and D6 in both control (P = 0.006) and activator (P = 0.001) groups. However, this difference was not observed in the EVE group (P = 0.167) (Fig. 4A), in accordance with the reduced growing follicular rate. Surprisingly, cumulative estradiol (E2) secretion, produced by granulosa cells at the secondary follicle stage onwards, tended to be lower in the activator group than in the control (Fig. 4B). Given that both the activator and control groups displayed a similar rate of growing follicles, these results suggest that short term exposure to activators may impair granulosa cell function. In contrast, E2 levels were comparable between the control and the EVE groups despite reduced follicular recruitment, suggesting that EVE delayed the development of in vitro cultured follicles without impacting steroidogenesis.

Follicular functionality after treatment exposure and in vitro development. (A) Kit ligand (KL) mRNA relative expression in isolated follicles was increased after 6 days of culture (D6) compared to Day 1 (D1), except in the EVE group. (B) Cumulative estradiol (E2) production levels in the culture media tended to be lower in the activator group (Activ) compared to control and mTORC1 inhibitor (EVE) groups despite the higher rate of growing follicles observed. Each point indicates individual measurement and lines indicate evolution of the mean (± SEM) at Days 1, 3, 5 and 6 of culture. *P < 0.05; **P < 0.01; ***P < 0.001.
Interactions with the Hippo pathway
In order to limit potential differences in follicular activation related to the variability in Hippo signaling disruption during fragmentation, we standardized the size of all the cortical fragments (4 × 2 × 1 mm3). As shown in Fig. 5A, YAP was specifically observed in granulosa cells and to a lesser extent in oocytes from primordial to growing follicles, as previously described in a mouse model (Kawamura et al., 2013). After thawing, YAP was transiently located both in the nucleus and in the cytoplasm of granulosa cells from the primordial follicles, whereas it was restricted to the cytoplasm in growing follicles. Moreover, we observed a significant downregulation of the expression of downstream targets of Hippo, BIRC1 and CCN2 at D1 compared to D0 (Fig. 5B and C). Altogether, these data suggest that Hippo disturbance takes place during the tissue preparation and cryopreservation process and then decreases during the first 24 h of culture.
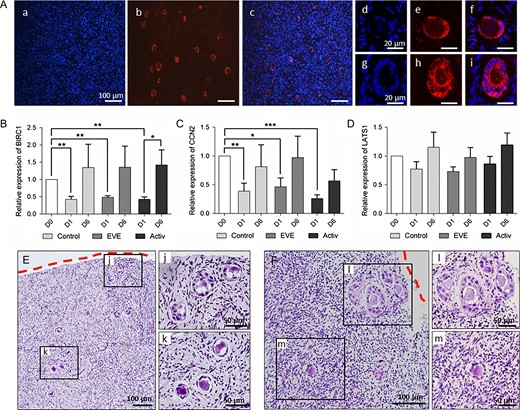
Hippo signaling disruption following ovarian fragmentation promotes follicular activation. (A) Localization of YAP protein within human ovarian cortex after thawing. YAP was specifically expressed by the follicles (a–c). YAP was located in the nucleus of the flattened granulosa cells of the primordial follicles (d–f) and translocated into the cytoplasm of the cuboidal cells of the activated follicles (g–i). a, d, g: Hoechst staining; b, e, h: YAP staining; c, f, i: merge. (B, C) BIRC1 and CCN2 transcript levels were upregulated immediately after thawing, and tended to increase again at Day 6. (D) No significant difference was observed in LATS1 mRNA relative expression in isolated follicles. The relative amount of each transcript was normalized based on HPRT and YWHAZ expression levels. *P < 0.05; **P < 0.01; ***P < 0.001. (E, F) Localization of in vitro grown follicles within the cortex after 3 (E) and 6 (F) days of culture in control medium. Follicles located close to the section site were more developed (j, l) than those localized deeper in the cortical tissue (k, m). Dotted red lines = section area.
Regardless of treatment exposure, the relative expression of LATS1 followed an expression profile similar to that of TSC1 at D1 compared to D0 (Fig. 5D), without reaching significance. As TSC1 and LATS1 are negative regulators of PI3K/Akt/mTOR and Hippo signaling, respectively, this suggests that these pathways act synergistically to promote spontaneous follicular activation. Surprisingly, BIRC1 and CCN2 expression increased again after 6 days of culture in all groups, suggesting a second wave of Hippo disturbance during in vitro follicular growth. Similarly, culturing of mouse ovaries for 8 days resulted in decreased expression levels of negative regulators of the Hippo pathway, MST1 and LATS2, and a reduction in p-YAP/YAP ratio (Xiang et al., 2015), reflecting the removal of physiological Hippo inhibition during early follicular development. The role of the Hippo pathway was further confirmed by the presence of a higher proportion of growing follicles at the periphery of the fragments (Fig. 5E and F).
Discussion
Findings across multiple organ systems and model organisms have implicated Hippo signaling in the control of organ size, tissue homeostasis and regeneration (Lu et al., 2010; Heallen et al., 2011; Xin et al., 2013). Its involvement in reproduction appears to be crucial but several mechanistic features remain elusive. LATS1-null female mice exhibit a POI phenotype due to massive follicular activation (John et al., 1999). Likewise, deletion of CCN2 in ovarian granulosa cells leads to subfertility and impaired follicle development (Nagashima et al., 2011). Our results confirm that Hippo signaling is disturbed after ovarian cortex fragmentation and is involved in the subsequent massive follicular activation in vitro. First, granulosa cells of the primordial follicles specifically expressed the YAP protein, with intensified and transient nuclear localization after thawing. Second, relative expression levels of downstream BIRC1 and CCN2 mRNA were higher in follicles immediately after thawing compared to Day 1. This is corroborated by similar observations in mice, in which increased expression of CCN growth factors and BIRC apoptosis inhibitors within a few hours following ovary excision (Kawamura et al., 2013) or treatment with actin polymerization-enhancing drugs have been reported (Cheng et al., 2015). However, CCN2 expression may also be regulated by other factors as FOXO1/3 (Liu et al., 2013) or GDF9 (Harlow et al., 2002) in granulosa cells. Nevertheless, GDF9 acts probably later as it is mainly expressed by the growing oocyte and FOXO1/3 activation is associated with an increase in P-Akt activity, which was not observed at D0. Third, follicles at the most advanced developmental stage were observed at the periphery of the tissue, close to the section site. Taken together, these data suggest a central role of Hippo during fragmentation-induced follicular activation and early development in non-physiological condition. However, there is no evidence of a physiologic role of Hippo during activation of primordial follicles. Although it has been hypothesized that ovulation, associated with structural changes in actin polymerization disrupting the Hippo signaling, may trigger activation of neighboring quiescent follicles, or that large follicles may activate dormant follicles via inter-follicle communications involving Hippo pathway factors (Hsueh et al., 2015), YAP was undetectable in the nucleus of granulosa cells throughout postnatal follicular growth in mice (Abbassi et al., 2016).
The PI3K/Akt/mTOR pathway is also a master regulator of cell growth and proliferation. Recent studies have identified functional links between Hippo and PI3K/Akt/mTOR pathways in different cell types (Fan et al., 2013; Hansen et al., 2015; Tumaneng et al., 2012). However, direct crosstalk between these two pathways during early follicular development has yet to be identified. Here, we observed a parallel transient drop of TSC1 and LATS1 mRNA expression levels after 1 day of culture, which could trigger the spontaneous follicular activation observed in vitro through synchronized mechanisms. Moreover, we report a second wave of Hippo disturbance at the end of culture, leading to increased BIRC1 and CCN2 expression, concomitant with high expression levels of KL and Akt in the control and the activator groups, suggesting a coordinated action of both pathways in the ovary during folliculogenesis.
Hippo signaling is significantly enriched from human primordial to primary follicles, occurring concomitantly with high expression of SMAD2 transcripts (Ernst et al., 2017). SMADs are downstream intracellular signaling molecules of the transforming growth factor beta (TGFβ) superfamily members, including GDF and BMP ligands. Several lines of evidence indicate that YAP and TAZ interact with this pathway by forming complexes with SMAD2/3, regulating their nuclear shuttling. Once phosphorylated, SMAD2/3 binds to SMAD4 and forms a complex with YAP/TAZ, enabling nuclear translocation and targeting transcription of genes such as CCN2 (Varelas et al., 2008; Grannas et al., 2015). During follicular growth, oocyte-specific growth factors GDF9 or BMP15 activate the canonical TGF-β signaling cascade in the granulosa cells of growing follicles where CCN2 mRNA is abundantly expressed (Harlow and Hillier, 2002). In vivo, this stimulatory effect could be negatively regulated by the Hippo pathway, promoting YAP/TAZ phosphorylation, degradation and cytoplasmic sequestration. By disrupting the Hippo pathway in vitro, TAZ/YAP-TEAD and SMAD may synergize to promote follicular growth, leading to an accelerated developmental pattern that differs from physiological conditions (Fig. 6).
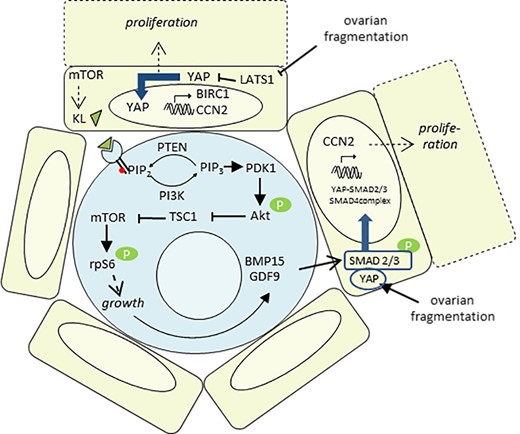
Proposed model for human primordial follicle activation. (i) Ovarian fragmentation disrupts the Hippo pathway within the granulosa cells (GC), resulting in YAP translocation into the nucleus and the transcription of CCN2 and BIRC1, involved in GC proliferation. (ii) mTOR promotes Kit ligand (KL) secretion by the GC, which binds to its oocyte c-Kit receptor. In response, the PI3K/Akt/mTORC1 pathway within the oocyte is activated, leading to its growth and the production of BMP15 and GDF9. (iii) BMP15 and GDF9 secretion by the oocyte, together with YAP activation following ovarian fragmentation, may cooperate to activate SMAD2/3 and form the YAP-SMAD2/3/4 complex. After nuclear translocation, this complex would be able to promote the transcription of growth factors. Altogether, these two pathways allow a coordinated development of both the oocyte and its neighboring GC.
The initiation of growth is characterized by a morphological transition in the granulosa cells from flattened to cuboidal, an enlargement of the oocyte and an increase in mitotic activity of the granulosa cells, requiring close dialog between cells (Hirshfield, 1991). Focusing on morphological shape, we observed chaotic granulosa cell proliferation in growing follicles from all groups after 6 days of culture, inconsistent with physiological growth patterns. Similarly, morphological alterations have been reported in global reawakening mutants such as Kit, PTEN and FOXO3 (Castrillon, 2003; Reddy et al., 2008; Saatcioglu et al., 2016). Excessive induction of the PI3K/Akt/mTOR pathway was associated with enlarged oocytes despite a lack of granulosa cell growth and maturation. Morphological defects in follicles and atresia were also reported after spontaneous activation in vitro (Wang et al., 2016) or in human follicles activated with bpV (HOpic) (Lerer-Serfaty et al., 2013; McLaughlin et al., 2014). These damages could reflect a desynchronization between granulosa cell proliferation and the oocyte resulting from a dysregulation of key growth signaling pathways. However, direct effects due to culture conditions or technical artefacts cannot be excluded.
Our results confirm that transient exposure of human ovarian cortex to the PTEN inhibitor bpV (HOpic) and the PI3K activator 740Y-P accelerated the initiation of primordial follicle development through a marked increase in phospho-PTEN levels and Akt and rpS6 phosphorylation. However, this acceleration of follicular recruitment was not associated with an increase in E2 secretion by the growing follicles, bringing into question the efficiency of PI3K/Akt activators to promote further follicular development and to generate follicles of good quality. Alternatively, short term exposure to mTORC1 inhibitor led to a reduction of growing follicle rate and Kit ligand relative expression. It was, therefore, effective in partially preserving the ovarian reserve. However, this efficacy might be partly offset by Hippo disruption following ovarian fragmentation or by feedback mechanisms such as mTORC2-mediated phosphorylation of Akt. In addition, inhibition of mTORC1 in cancer cell lines also induces activation of the Akt kinase by relieving feedback inhibition of insulin-like growth factor 1 (IGF-I) upstream signaling (O’Reilly et al., 2006). Although mTORC1 inhibition-induced Akt phosphorylation has been observed in human liver or ovarian cancer cell lines (Mabuchi et al., 2011; Masola et al., 2015), in the current study, treatment with EVE did not result in activation of Akt in human ovarian cortex, as reported by recent studies on mouse ovaries (Goldman et al., 2017).
By producing competent and fertilizable oocytes from primordial follicles, the development of suitable in vitro systems for the culture of ovarian cortex could revolutionize assisted reproduction, especially fertility preservation procedures. Our findings bring into question the advantage and the safety of using PI3K/Akt activators to trigger global activation of quiescent follicles within ovarian cortex from patients without ovarian insufficiency. In contrast, this study reveals the significant benefit of using mTORC1 inhibitors to partially safeguard the ovarian reserve. As the role of the Hippo pathway appears to be crucial as early as when the tissue is thawed, a pharmacological-based strategy that combines a slow-down of follicular recruitment with ovarian tissue processing might prevent a precocious burn out of the ovarian reserve.
Acknowledgements
We gratefully acknowledge the IVF Unit of Erasme Hospital for providing the human ovarian cortex samples; Fleur Wolff and the Medical Chemistry Lab of Erasme Hospital for assistance with hormonal measurements; Clément Chevalier and the Center for Microscopy and Molecular Imaging platform of ULB for confocal image acquisition; Géraldine Van Den Steen and Julie Dechène for technical and logistical support; and Sandy Field for English language editing.
Authors’ roles
J.G. and I.D. designed experiments; J.G. performed experiments; J.G. and I.D. analyzed data; J.G. and I.D. wrote the article.
Funding
Grant from the Fonds National de la Recherche Scientifique de Belgique (Grant Télevie Nos. 7.6516.16F and 7.4578.14F) and the Fonds Erasme.
Conflict of interest
None declared.
References
- signal transduction
- cell nucleus
- granulosa cells
- hair follicle
- ovarian follicle
- bovine papillomavirus
- rna, messenger
- stem cell factor
- transcriptional activation
- everolimus
- pten gene
- proto-oncogene proteins c-akt
- phosphoinositide 3-kinase
- primordial follicles
- mtor serine-threonine kinases
- ovarian tissue
- hippo signaling pathway
- mechanistic target of rapamycin complex 1



