-
PDF
- Split View
-
Views
-
Cite
Cite
Marta Devesa, Rosa Tur, Ignacio Rodríguez, Buenaventura Coroleu, Francisca Martínez, Nikolaos P Polyzos, Cumulative live birth rates and number of oocytes retrieved in women of advanced age. A single centre analysis including 4500 women ≥38 years old, Human Reproduction, Volume 33, Issue 11, November 2018, Pages 2010–2017, https://doi.org/10.1093/humrep/dey295
Close - Share Icon Share
Abstract
Is there any relationship between the number of oocytes retrieved and cumulative live birth rates (CLBRs) in women of advanced age?
CLBRs increase with the number of oocytes retrieved in women of advanced reproductive age up to 41 years old, the added value is minimal in women more than 41 years and futile in women 44 years or older.
CLBR is actually the most relevant outcome of IVF from patients’ perspective. There are several studies that have analysed CLBR’s but some of them have included several stimulation cycles, others have not included the frozen embryo transfers (FETs) in their analysis and none has focused on women of advanced reproductive age. We aimed to assess CLBR in women ≥38 years after a single stimulation cycle plus the subsequent frozen embryo transfers.
This is a retrospective analysis carried out in a University-affiliated tertiary centre between January 2000 and December 2013. Overall, 4570 infertile women aged ≥38 years who underwent their first cycle in our centre were included.
Patients were categorized in four age-groups: 38–39 years (G1 = 1875 cycles), 40–41 years (G2 = 1380 cycles), 42–43 years (G3 = 833 cycles) and ≥44 years (G4 = 482 cycles). CLBR’s were evaluated by adding the pregnancies and live births achieved in the FET’s to the ones obtained in the fresh cycle. In order to find out the actual effect of the number of oocytes retrieved in these patients, a predictive model of CLBR according to age and oocyte yield was built.
CLBRs significantly decrease with increasing age among women ≥38 years of age, with the most prominent and clinically relevant decline observed at 42–43 years old, and clear evidence for futility in women aged ≥44 years (25.9% at 38–39 years, 16.4% at 40–41 years, 7% at 42–43 years and 1.2% from 44 years onwards). The higher the number of oocytes retrieved, the higher the CLBR; however, this is more evident up to 41 years old and no clear benefit is observed from 44 years and beyond.
Limitations are related to the retrospective nature of the study; however, no significant differences were observed in the treatment protocols used. Other potential limitations could be the fact that embryo cryopreservation was carried out with slow freezing in 80% of cases and that a small proportion of patients still have frozen embryos; nevertheless, we do not expect a relevant impact of these issues as slow freezing showed excellent results that did not differ significantly compared to vitrification and, on the other hand, the extra benefit coming from the FETs was very limited.
The number of oocytes retrieved is significantly associated with CLBR also in women of advanced reproductive age. However, the added benefit appears to be restricted mainly in women up to 41 years old. Women over 43 do not experience any benefit in CLBR irrespective of the number of oocytes retrieved, and thus should be discouraged from doing an IVF cycle with their own oocytes; for the other age-groups, recommendations should be given considering the age and the expected ovarian response.
None.
NA
Introduction
Defining success in assisted reproduction technologies (ART) has always been controversial, given that most of the published studies have been powered to assess surrogate outcomes such as implantation rates, clinical or ongoing pregnancy rates. Although unanimously most of the clinicians agree that live birth rate is the most important outcome for defining treatment success, cumulative live birth rate (CLBR), including fresh and subsequent frozen embryo transfers (FETs), may actually be the most meaningful outcome from the patients’ perspective (McLernon et al., 2016; Vaughan et al., 2017).
Several single centre studies and large registry analysis utilized CLBR as their primary outcome measure. Nevertheless, definitions largely differed, given that some of these studies have taken into account more than one stimulation cycle (Klipstein et al., 2005; van Disseldorp et al., 2007; Malizia et al., 2009; Stern et al., 2010; Cetinkaya et al., 2013; De Brucker et al., 2013; Smith et al., 2015; McLernon et al., 2016), while others did not at all include the contribution of FETs in their analysis (Klipstein et al., 2005; van Disseldorp et al., 2007; Cetinkaya et al., 2013; De Brucker et al., 2013). Furthermore, although four studies have defined CLBR as the first live birth of the first baby after one ovarian stimulation, including fresh and subsequent FETs, none of them included or specifically analysed outcomes among women of advanced reproductive age. One of the studies pertained only to very young women <35 years old (Ji et al., 2013), the other two to patients not exceeding 40 years of age (Drakopoulos et al., 2016; Toftager et al., 2017), while the last one included women up to 46 years but did not specifically analyse the outcome in advanced aged women (Vaughan et al., 2017).
Despite that women in their mid 30s are the majority of the patients undergoing ART procedures, the reproductive behaviour is shifting worldwide. The availability of contraceptive methods, professional and financial issues, together with the lack of a partner, contribute to childbearing delay. Thus, owing to this continuous postponement of motherhood, ART demand has substantially increased among women of advanced reproductive age, with one out of five IVF cycles in Spain in 2015 involving women ≥40 years old (Sociedad Española de Fertilidad, SEF). This is even more prominent if we actually consider the turning point at which pregnancy rates significantly decline, which is considered by many the age of 38 years old (Faddy et al., 1992), given that the number of IVF cycles performed worldwide in women ≥38 years old is steadily increasing and comprises more than half of our IVF programme.
Taking into account all the above-mentioned evidence, we set out to evaluate for the first time CLBR’s after a single stimulation cycle in women of advanced reproductive age in order to provide to patients and clinicians a tool that can allow them to build realistic expectations and provide accurate individual counselling.
Materials and Methods
This is a retrospective study of 4570 women undergoing their first IVF/ICSI cycle in our centre. The study was carried out in a university-affiliated private fertility clinic between January 2000 and December 2013 following Institutional Review Board (IRB) approval.
Patients’ inclusion criteria
All women ≥38 years at the time of treatment who underwent their first ovarian stimulation cycle for IVF/ICSI in our centre between 2000 and 2013 were included in the analysis. Cycles with oocyte donation and preimplantational genetic screening (PGS) were excluded.
Patients were categorized in four age-groups:
Group 1 (G1=1875 cycles): women aged 38–39 years
Group 2 (G2=1380 cycles): women aged 40–41 years
Group 3 (G3=833 cycles): women aged 42–43 years
Group 4 (G4=482 cycles): women aged ≥44 years.
Demographic and basal characteristics per group are displayed in Table I.
| . | G1 (38–39 yrs) . | G2 (40–41 yrs) . | G3 (42–43 yrs) . | G4 (≥44 yrs) . | P . |
|---|---|---|---|---|---|
| No. of patients/cycles (%) | 1875 (41) | 1380 (30) | 833 (18) | 482 (11) | |
| Age (mean ± SD) | 38.5 ± 0.5 | 40.4 ± 0.5 | 42.4 ± 0.5 | 45 ± 1.3 | <0.001 |
| D3 FSH (IU/ml) (mean ± SD) | 8.9 ± 7.8 | 9.4 ± 7 | 11.6 ± 7.4 | 10.3 ± 5.9 | <0.001 |
| AFC (mean ± SD)a | 9.2 ± 4.8 | 8.2 ± 4.6 | 7.1 ± 4 | 5.8 ± 3.4 | <0.001 |
| Infertility factor (%) | <0.001 | ||||
| Female | 36.4 | 40.4 | 41.9 | 53.7 | |
| Male | 29.5 | 25.7 | 21.2 | 13.7 | |
| Mixed | 11 | 10.3 | 11.2 | 10.8 | |
| Unexplained | 23.1 | 23.6 | 25.7 | 21.8 |
| . | G1 (38–39 yrs) . | G2 (40–41 yrs) . | G3 (42–43 yrs) . | G4 (≥44 yrs) . | P . |
|---|---|---|---|---|---|
| No. of patients/cycles (%) | 1875 (41) | 1380 (30) | 833 (18) | 482 (11) | |
| Age (mean ± SD) | 38.5 ± 0.5 | 40.4 ± 0.5 | 42.4 ± 0.5 | 45 ± 1.3 | <0.001 |
| D3 FSH (IU/ml) (mean ± SD) | 8.9 ± 7.8 | 9.4 ± 7 | 11.6 ± 7.4 | 10.3 ± 5.9 | <0.001 |
| AFC (mean ± SD)a | 9.2 ± 4.8 | 8.2 ± 4.6 | 7.1 ± 4 | 5.8 ± 3.4 | <0.001 |
| Infertility factor (%) | <0.001 | ||||
| Female | 36.4 | 40.4 | 41.9 | 53.7 | |
| Male | 29.5 | 25.7 | 21.2 | 13.7 | |
| Mixed | 11 | 10.3 | 11.2 | 10.8 | |
| Unexplained | 23.1 | 23.6 | 25.7 | 21.8 |
AFC, antral follicle count.
| . | G1 (38–39 yrs) . | G2 (40–41 yrs) . | G3 (42–43 yrs) . | G4 (≥44 yrs) . | P . |
|---|---|---|---|---|---|
| No. of patients/cycles (%) | 1875 (41) | 1380 (30) | 833 (18) | 482 (11) | |
| Age (mean ± SD) | 38.5 ± 0.5 | 40.4 ± 0.5 | 42.4 ± 0.5 | 45 ± 1.3 | <0.001 |
| D3 FSH (IU/ml) (mean ± SD) | 8.9 ± 7.8 | 9.4 ± 7 | 11.6 ± 7.4 | 10.3 ± 5.9 | <0.001 |
| AFC (mean ± SD)a | 9.2 ± 4.8 | 8.2 ± 4.6 | 7.1 ± 4 | 5.8 ± 3.4 | <0.001 |
| Infertility factor (%) | <0.001 | ||||
| Female | 36.4 | 40.4 | 41.9 | 53.7 | |
| Male | 29.5 | 25.7 | 21.2 | 13.7 | |
| Mixed | 11 | 10.3 | 11.2 | 10.8 | |
| Unexplained | 23.1 | 23.6 | 25.7 | 21.8 |
| . | G1 (38–39 yrs) . | G2 (40–41 yrs) . | G3 (42–43 yrs) . | G4 (≥44 yrs) . | P . |
|---|---|---|---|---|---|
| No. of patients/cycles (%) | 1875 (41) | 1380 (30) | 833 (18) | 482 (11) | |
| Age (mean ± SD) | 38.5 ± 0.5 | 40.4 ± 0.5 | 42.4 ± 0.5 | 45 ± 1.3 | <0.001 |
| D3 FSH (IU/ml) (mean ± SD) | 8.9 ± 7.8 | 9.4 ± 7 | 11.6 ± 7.4 | 10.3 ± 5.9 | <0.001 |
| AFC (mean ± SD)a | 9.2 ± 4.8 | 8.2 ± 4.6 | 7.1 ± 4 | 5.8 ± 3.4 | <0.001 |
| Infertility factor (%) | <0.001 | ||||
| Female | 36.4 | 40.4 | 41.9 | 53.7 | |
| Male | 29.5 | 25.7 | 21.2 | 13.7 | |
| Mixed | 11 | 10.3 | 11.2 | 10.8 | |
| Unexplained | 23.1 | 23.6 | 25.7 | 21.8 |
AFC, antral follicle count.
Treatment protocol
Patients were treated by recombinant and/or urinary gonadotrophins (rFSH/hMG) in a long GnRH agonist or a flexible GnRH antagonist protocol followed by IVF or ICSI. The stimulation protocol and gonadotrophin dose were chosen depending on age, BMI and ovarian reserve tests. The decision to use GnRH agonist or antagonist depended more on the hospital policy at the specific time, with a shift towards GnRH antagonist protocol starting in 2009. Criteria for cancellation were: absence of follicular growth after 10 days of intense ovarian stimulation or less than four growing follicles.
Ovulation was triggered with recombinant hCG (r-hCG) in all cases, when at least three follicles >18 mm in diameter were observed. For luteal phase support, vaginal progesterone (200 mg every 8 h) was started the day after the oocyte retrieval and maintained until a negative beta-hCG or the sixth week of pregnancy. Embryo quality was assessed according to number and regularity of blastomeres, percentage of fragmentation and multinucleation; according to our embryo scoring system (Clua et al., 2010), embryos graded with a score ≥8 on a scale of 1 to 10 were considered good-quality embryos.
Embryo transfer was carried out under ultrasound guidance two or three days after oocyte retrieval (Coroleu et al., 2000). Supernumerary embryos were cryopreserved on Day 3 post oocyte retrieval following slow-freezing or vitrification standard protocols.
For FET cycles, hormonal replacement therapy (oral oestrogens and micronized progesterone) was administered after pituitary suppression with Triptorelin Depot 3.75 mg, according to the protocol described elsewhere (Martínez et al., 2011). Once thawed and after 24 h of culture, embryos available for transfer were replaced.
Pregnancy was diagnosed with plasma beta-hCG determination and subsequently confirmed by foetal heartbeat on vaginal ultrasound at Week 6 of gestation.
Statistical analysis
For each age group, we analysed pregnancy, miscarriage and live birth rates both in the fresh and in the frozen cycles. Cumulative pregnancy rates (CPRs) and CLBRs were evaluated by adding the pregnancies and live births achieved in the FETs to the ones obtained in the fresh cycle. With the aim of finding out the real benefit of cryopreservation in women of advanced reproductive age, we analysed separately CLBRs depending on whether cryopreservation had been possible or not, in each age group.
The association between categorical variables and age variable was analysed using the Chi-Square test. Continuous variables were compared with the ANOVA test.
Taking into account the large study period of time and possible clinical practice changes that may act as confounding factors, a sensitivity logistic regression analysis was performed to assess the Odds Ratio (OR) of CLBR for the variable ‘age’ and ‘number of oocytes retrieved’, adjusting for year of treatment, stimulation protocol and cryopreservation technique.
Additionally, a predictive model of CLBR according to age and oocyte yield was built. Smoothing logistic regression model GAM (generalized additive model) was adjusted with the R software to relate the cumulative live birth rate depending on the number of oocytes retrieved and patient age group (Hastie and Tibshirani, 1990; R Development Core Team, 2008). The results of this analysis are displayed graphically. All tests were bilateral with a P value of 5%.
Results
Fresh cycle outcomes
As shown in Table II, although fertilization rates remained stable across all groups, advancing age resulted in a decrease in the number of oocytes retrieved and the number of available and transferred embryos (P < 0.001). Similarly, implantation rates significantly decreased with increasing age, dropping from 13.5% in G1 to 2.2% in G4, leading to a significant decrease in pregnancy rates per cycle and live birth rates, ranging from 22.2% (G1) to 1.2% (G4).
| . | G1 (38–39 yrs) . | G2 (40–41 yrs) . | G3 (42–43 yrs) . | G4 (≥44 yrs) . | P . |
|---|---|---|---|---|---|
| (1875 cycles) . | (1380 cycles) . | (833 cycles) . | (482 cycles) . | ||
| Treatment protocol (%) | NS | ||||
| GnRH agonist | 68.4 | 67.6 | 65.6 | 64.9 | |
| GnRH antagonist | 28.8 | 29.9 | 31.2 | 30.1 | |
| Other | 2.8 | 2.5 | 3.2 | 5 | |
| Total gonadotrophin dose | 3288 ± 1352 | 3614 ± 1143 | 3714 ± 658 | 3817 ± 355 | <0.001 |
| Cancellation rate (%) | 12.9 | 13.5 | 19.9 | 24.7 | <0.001 |
| No. of oocytes retrieved (mean ± SD) | 8 ± 6.7 | 7.2 ± 6.2 | 5.5 ± 5.4 | 4 ± 4.4 | <0.001 |
| Method of fertilization (%) | <0.001 | ||||
| IVF | 25.9 | 30.7 | 37.8 | 44.7 | |
| ICSI | 65.5 | 62.7 | 56.1 | 50 | |
| Combined | 8.6 | 6.6 | 6.1 | 5.3 | |
| Fertilization rate (%) (95% CI) | 70.1 (69–71.3) | 70.6 (69.2–71.9) | 67.9 (66–69.9) | 68.2 (65.1–71.2) | NS |
| No. of cleaved embryos (%) | 3.9 ± 2.6 | 3.7 ± 2.6 | 3.2 ± 2 | 2.6 ± 1.8 | <0.001 |
| Embryo transfer rate (%) | 79.8 | 78.3 | 66.5 | 62.7 | <0.001 |
| No. of embryos transferred (mean ± SD) | 2.1 ± 0.7 | 2.2 ± 0.7 | 2.2 ± 0.8 | 2 ± 0.8 | 0.003 |
| Implantation rate (%) (95% CI) | 13.5 (12.1–14.8) | 9.5 (8.2–10.8) | 5.6 (4.2–6.9) | 2.2 (1.1–3.4) | <0.001 |
| Embryo quality (mean ± SD) | 6.3 ± 2.3 | 6.2 ± 2.3 | 6.1 ± 2.2 | 5.9 ± 2.3 | 0.018 |
| Pregnancy rate (%) | |||||
| Per cycle | 29.7 | 21.9 | 12.6 | 4.1 | <0.001 |
| Per ovum pick-up | 34 | 25.3 | 15.7 | 5.5 | <0.001 |
| Per embryo transfer | 37.2 | 28 | 19 | 6.6 | <0.001 |
| Miscarriage rate (%) | 23.2 | 35.8 | 48.6 | 70 | <0.001 |
| LBR (%) | 22.2 | 13.5 | 6.4 | 1.2 | <0.001 |
| . | G1 (38–39 yrs) . | G2 (40–41 yrs) . | G3 (42–43 yrs) . | G4 (≥44 yrs) . | P . |
|---|---|---|---|---|---|
| (1875 cycles) . | (1380 cycles) . | (833 cycles) . | (482 cycles) . | ||
| Treatment protocol (%) | NS | ||||
| GnRH agonist | 68.4 | 67.6 | 65.6 | 64.9 | |
| GnRH antagonist | 28.8 | 29.9 | 31.2 | 30.1 | |
| Other | 2.8 | 2.5 | 3.2 | 5 | |
| Total gonadotrophin dose | 3288 ± 1352 | 3614 ± 1143 | 3714 ± 658 | 3817 ± 355 | <0.001 |
| Cancellation rate (%) | 12.9 | 13.5 | 19.9 | 24.7 | <0.001 |
| No. of oocytes retrieved (mean ± SD) | 8 ± 6.7 | 7.2 ± 6.2 | 5.5 ± 5.4 | 4 ± 4.4 | <0.001 |
| Method of fertilization (%) | <0.001 | ||||
| IVF | 25.9 | 30.7 | 37.8 | 44.7 | |
| ICSI | 65.5 | 62.7 | 56.1 | 50 | |
| Combined | 8.6 | 6.6 | 6.1 | 5.3 | |
| Fertilization rate (%) (95% CI) | 70.1 (69–71.3) | 70.6 (69.2–71.9) | 67.9 (66–69.9) | 68.2 (65.1–71.2) | NS |
| No. of cleaved embryos (%) | 3.9 ± 2.6 | 3.7 ± 2.6 | 3.2 ± 2 | 2.6 ± 1.8 | <0.001 |
| Embryo transfer rate (%) | 79.8 | 78.3 | 66.5 | 62.7 | <0.001 |
| No. of embryos transferred (mean ± SD) | 2.1 ± 0.7 | 2.2 ± 0.7 | 2.2 ± 0.8 | 2 ± 0.8 | 0.003 |
| Implantation rate (%) (95% CI) | 13.5 (12.1–14.8) | 9.5 (8.2–10.8) | 5.6 (4.2–6.9) | 2.2 (1.1–3.4) | <0.001 |
| Embryo quality (mean ± SD) | 6.3 ± 2.3 | 6.2 ± 2.3 | 6.1 ± 2.2 | 5.9 ± 2.3 | 0.018 |
| Pregnancy rate (%) | |||||
| Per cycle | 29.7 | 21.9 | 12.6 | 4.1 | <0.001 |
| Per ovum pick-up | 34 | 25.3 | 15.7 | 5.5 | <0.001 |
| Per embryo transfer | 37.2 | 28 | 19 | 6.6 | <0.001 |
| Miscarriage rate (%) | 23.2 | 35.8 | 48.6 | 70 | <0.001 |
| LBR (%) | 22.2 | 13.5 | 6.4 | 1.2 | <0.001 |
Note: NS, not significant; LBR, live birth rate.
| . | G1 (38–39 yrs) . | G2 (40–41 yrs) . | G3 (42–43 yrs) . | G4 (≥44 yrs) . | P . |
|---|---|---|---|---|---|
| (1875 cycles) . | (1380 cycles) . | (833 cycles) . | (482 cycles) . | ||
| Treatment protocol (%) | NS | ||||
| GnRH agonist | 68.4 | 67.6 | 65.6 | 64.9 | |
| GnRH antagonist | 28.8 | 29.9 | 31.2 | 30.1 | |
| Other | 2.8 | 2.5 | 3.2 | 5 | |
| Total gonadotrophin dose | 3288 ± 1352 | 3614 ± 1143 | 3714 ± 658 | 3817 ± 355 | <0.001 |
| Cancellation rate (%) | 12.9 | 13.5 | 19.9 | 24.7 | <0.001 |
| No. of oocytes retrieved (mean ± SD) | 8 ± 6.7 | 7.2 ± 6.2 | 5.5 ± 5.4 | 4 ± 4.4 | <0.001 |
| Method of fertilization (%) | <0.001 | ||||
| IVF | 25.9 | 30.7 | 37.8 | 44.7 | |
| ICSI | 65.5 | 62.7 | 56.1 | 50 | |
| Combined | 8.6 | 6.6 | 6.1 | 5.3 | |
| Fertilization rate (%) (95% CI) | 70.1 (69–71.3) | 70.6 (69.2–71.9) | 67.9 (66–69.9) | 68.2 (65.1–71.2) | NS |
| No. of cleaved embryos (%) | 3.9 ± 2.6 | 3.7 ± 2.6 | 3.2 ± 2 | 2.6 ± 1.8 | <0.001 |
| Embryo transfer rate (%) | 79.8 | 78.3 | 66.5 | 62.7 | <0.001 |
| No. of embryos transferred (mean ± SD) | 2.1 ± 0.7 | 2.2 ± 0.7 | 2.2 ± 0.8 | 2 ± 0.8 | 0.003 |
| Implantation rate (%) (95% CI) | 13.5 (12.1–14.8) | 9.5 (8.2–10.8) | 5.6 (4.2–6.9) | 2.2 (1.1–3.4) | <0.001 |
| Embryo quality (mean ± SD) | 6.3 ± 2.3 | 6.2 ± 2.3 | 6.1 ± 2.2 | 5.9 ± 2.3 | 0.018 |
| Pregnancy rate (%) | |||||
| Per cycle | 29.7 | 21.9 | 12.6 | 4.1 | <0.001 |
| Per ovum pick-up | 34 | 25.3 | 15.7 | 5.5 | <0.001 |
| Per embryo transfer | 37.2 | 28 | 19 | 6.6 | <0.001 |
| Miscarriage rate (%) | 23.2 | 35.8 | 48.6 | 70 | <0.001 |
| LBR (%) | 22.2 | 13.5 | 6.4 | 1.2 | <0.001 |
| . | G1 (38–39 yrs) . | G2 (40–41 yrs) . | G3 (42–43 yrs) . | G4 (≥44 yrs) . | P . |
|---|---|---|---|---|---|
| (1875 cycles) . | (1380 cycles) . | (833 cycles) . | (482 cycles) . | ||
| Treatment protocol (%) | NS | ||||
| GnRH agonist | 68.4 | 67.6 | 65.6 | 64.9 | |
| GnRH antagonist | 28.8 | 29.9 | 31.2 | 30.1 | |
| Other | 2.8 | 2.5 | 3.2 | 5 | |
| Total gonadotrophin dose | 3288 ± 1352 | 3614 ± 1143 | 3714 ± 658 | 3817 ± 355 | <0.001 |
| Cancellation rate (%) | 12.9 | 13.5 | 19.9 | 24.7 | <0.001 |
| No. of oocytes retrieved (mean ± SD) | 8 ± 6.7 | 7.2 ± 6.2 | 5.5 ± 5.4 | 4 ± 4.4 | <0.001 |
| Method of fertilization (%) | <0.001 | ||||
| IVF | 25.9 | 30.7 | 37.8 | 44.7 | |
| ICSI | 65.5 | 62.7 | 56.1 | 50 | |
| Combined | 8.6 | 6.6 | 6.1 | 5.3 | |
| Fertilization rate (%) (95% CI) | 70.1 (69–71.3) | 70.6 (69.2–71.9) | 67.9 (66–69.9) | 68.2 (65.1–71.2) | NS |
| No. of cleaved embryos (%) | 3.9 ± 2.6 | 3.7 ± 2.6 | 3.2 ± 2 | 2.6 ± 1.8 | <0.001 |
| Embryo transfer rate (%) | 79.8 | 78.3 | 66.5 | 62.7 | <0.001 |
| No. of embryos transferred (mean ± SD) | 2.1 ± 0.7 | 2.2 ± 0.7 | 2.2 ± 0.8 | 2 ± 0.8 | 0.003 |
| Implantation rate (%) (95% CI) | 13.5 (12.1–14.8) | 9.5 (8.2–10.8) | 5.6 (4.2–6.9) | 2.2 (1.1–3.4) | <0.001 |
| Embryo quality (mean ± SD) | 6.3 ± 2.3 | 6.2 ± 2.3 | 6.1 ± 2.2 | 5.9 ± 2.3 | 0.018 |
| Pregnancy rate (%) | |||||
| Per cycle | 29.7 | 21.9 | 12.6 | 4.1 | <0.001 |
| Per ovum pick-up | 34 | 25.3 | 15.7 | 5.5 | <0.001 |
| Per embryo transfer | 37.2 | 28 | 19 | 6.6 | <0.001 |
| Miscarriage rate (%) | 23.2 | 35.8 | 48.6 | 70 | <0.001 |
| LBR (%) | 22.2 | 13.5 | 6.4 | 1.2 | <0.001 |
Note: NS, not significant; LBR, live birth rate.
Similarly, increasing age significantly reduced the probability of cryopreservation and the number of supernumerary embryos frozen (P < 0.001).
Frozen cycle outcomes
Overall, 30.9% of cycles had surplus embryos for cryopreservation (Table III). A total of 1413 FET’s were analysed, of which 1163 (82.3%) had been carried out with slow freezing and 250 (17.7%) with vitrification. Cryopreservation was carried out with the slow freezing protocol until 2011. From 2012 onwards, vitrification was the cryopreservation technique of choice.
| . | G1 (38–39 yrs) . | G2 (40–41 yrs) . | G3 (42–43 yrs) . | G4 (≥44 yrs) . | P . |
|---|---|---|---|---|---|
| Cryopreservation rate (%) | 38.9 | 32 | 21.4 | 13.1 | <0.001 |
| No. of thawing cycles | 730 | 442 | 178 | 63 | |
| No. of FET with transfer | 594 | 395 | 167 | 57 | |
| No. of transferred embryos (mean ± SD) | 1.9 ± 0.7 | 2 ± 0.7 | 1.8 ± 0.7 | 1.9 ± 0.7 | NS |
| Implantation rate (%) (95% CI) | 14.2 (12–16.3) | 12.9 (10.3–15.4) | 5 (2.3–7.7) | 3.1 (0–6.7) | |
| Pregnancy rate/cycle (%) | 23.7 | 21.3 | 7.8 | 5.3 | <0.001 |
| Miscarriage rate (%) | 34.8 | 47.6 | 61.5 | 100 | 0.016 |
| LBR/cycle (%) | 14.1 | 10.4 | 3 | 0 | <0.001 |
| . | G1 (38–39 yrs) . | G2 (40–41 yrs) . | G3 (42–43 yrs) . | G4 (≥44 yrs) . | P . |
|---|---|---|---|---|---|
| Cryopreservation rate (%) | 38.9 | 32 | 21.4 | 13.1 | <0.001 |
| No. of thawing cycles | 730 | 442 | 178 | 63 | |
| No. of FET with transfer | 594 | 395 | 167 | 57 | |
| No. of transferred embryos (mean ± SD) | 1.9 ± 0.7 | 2 ± 0.7 | 1.8 ± 0.7 | 1.9 ± 0.7 | NS |
| Implantation rate (%) (95% CI) | 14.2 (12–16.3) | 12.9 (10.3–15.4) | 5 (2.3–7.7) | 3.1 (0–6.7) | |
| Pregnancy rate/cycle (%) | 23.7 | 21.3 | 7.8 | 5.3 | <0.001 |
| Miscarriage rate (%) | 34.8 | 47.6 | 61.5 | 100 | 0.016 |
| LBR/cycle (%) | 14.1 | 10.4 | 3 | 0 | <0.001 |
Note: NS, not significant; FET, frozen embryo transfer; LBR, live birth rate.
| . | G1 (38–39 yrs) . | G2 (40–41 yrs) . | G3 (42–43 yrs) . | G4 (≥44 yrs) . | P . |
|---|---|---|---|---|---|
| Cryopreservation rate (%) | 38.9 | 32 | 21.4 | 13.1 | <0.001 |
| No. of thawing cycles | 730 | 442 | 178 | 63 | |
| No. of FET with transfer | 594 | 395 | 167 | 57 | |
| No. of transferred embryos (mean ± SD) | 1.9 ± 0.7 | 2 ± 0.7 | 1.8 ± 0.7 | 1.9 ± 0.7 | NS |
| Implantation rate (%) (95% CI) | 14.2 (12–16.3) | 12.9 (10.3–15.4) | 5 (2.3–7.7) | 3.1 (0–6.7) | |
| Pregnancy rate/cycle (%) | 23.7 | 21.3 | 7.8 | 5.3 | <0.001 |
| Miscarriage rate (%) | 34.8 | 47.6 | 61.5 | 100 | 0.016 |
| LBR/cycle (%) | 14.1 | 10.4 | 3 | 0 | <0.001 |
| . | G1 (38–39 yrs) . | G2 (40–41 yrs) . | G3 (42–43 yrs) . | G4 (≥44 yrs) . | P . |
|---|---|---|---|---|---|
| Cryopreservation rate (%) | 38.9 | 32 | 21.4 | 13.1 | <0.001 |
| No. of thawing cycles | 730 | 442 | 178 | 63 | |
| No. of FET with transfer | 594 | 395 | 167 | 57 | |
| No. of transferred embryos (mean ± SD) | 1.9 ± 0.7 | 2 ± 0.7 | 1.8 ± 0.7 | 1.9 ± 0.7 | NS |
| Implantation rate (%) (95% CI) | 14.2 (12–16.3) | 12.9 (10.3–15.4) | 5 (2.3–7.7) | 3.1 (0–6.7) | |
| Pregnancy rate/cycle (%) | 23.7 | 21.3 | 7.8 | 5.3 | <0.001 |
| Miscarriage rate (%) | 34.8 | 47.6 | 61.5 | 100 | 0.016 |
| LBR/cycle (%) | 14.1 | 10.4 | 3 | 0 | <0.001 |
Note: NS, not significant; FET, frozen embryo transfer; LBR, live birth rate.
Globally, post thawing/warming survival rate was 75%, without statistically significant difference between slow freezing and vitrification: 74.9% (95% CI 72.9–76.8) and 75.6% (95% CI 71.8–79.4), respectively. The mean number of transferred embryos in the frozen cycles did not differ significantly among the groups. Nonetheless, in accordance with fresh cycles, a similar age-related decline in live birth rates was also observed in FET cycles (P < 0.001) (Table III). Overall, live birth rates in FET cycles did not differ significantly between both methods of cryopreservation (10% for slow freezing, 13.2% for vitrification).
Cumulative live birth rates
Cumulative outcome, taking into account fresh and frozen cycles, significantly decreased with increasing age (P < 0.001), with the most prominent and thus clinically relevant decline observed at 42–43 years old, from G2 to G3 (CLBR of 16.4% in G2 and of 7% in G3) and clear evidence for futility in women aged 44 years or older with a CLBR of 1.2% (Fig. 1a).
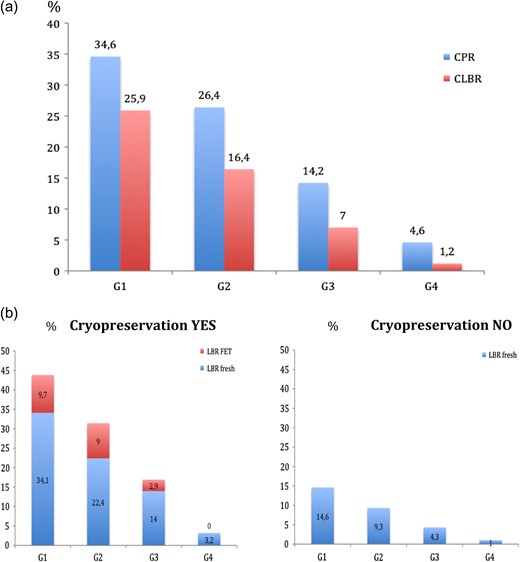
(a) Overall Cumulative pregnancy rates (CPRs) and cumulative live birth rates (CLBRs) for each age group (G1:38–39 years, G2:40–41 years, G3:42–43 years, G4: ≥44 years). (b) CLBRs for each age group (G1:38–39 years, G2:40–41 years, G3:42–43 years, G4: ≥44 years) according to whether cryopreservation was performed (graph in the left hand side) or not (graph in the right hand side). Graph in the left hand side shows percentage of livebirths coming from the fresh cycle (blue part of the bar) and percentage of livebirths coming from FETs (red part of the bar).
Analysis focusing on the additional effect from supernumerary frozen embryos has shown that CLBRs were statistically higher in the cycles in which there were frozen embryos than in the cycles in which there were no supernumerary embryos to be frozen (G1:43.8% vs. 14.6%; G2:31.4% vs. 9.3%; G3:16.9% vs. 4.3%; G4:3.2% vs. 1%). However, this difference was mainly attributed to the differences observed in the live births rates from the fresh cycle between cycles with embryo cryopreservation and those without, rather than from an additional benefit from the frozen cycle itself. Cryopreserved embryos substantially contributed to CLBR in the first two age categories, leading to additional live births of 9.7% in women 38–39 years old, 9% in women 40–41 years, with however only 2.9% in women 42–43 years and none above 44 years having a liveborn from the additional FETs (Fig. 1b).
To control for possible confounding variables that might have changed over the study period of time, we performed a sensitivity logistic regression analysis to estimate the OR of CLBRs for the variable ‘age’ and ‘number of oocytes retrieved’, adjusting for year of stimulation, stimulation protocol and cryopreservation technique. The results show that none of the adjustment variables was significant. The adjusted OR with the 95% confidence interval (CI) are shown in Table IV.
| N = 3846 (patients that reached oocyte retrieval) . | OR adjusted . | 95% CI . |
|---|---|---|
| G1 (38–39 yrs) | 19.27 | 8.50–43.67 |
| G2 (40–41 yrs) | 10.94 | 4.80–24.93 |
| G3 (42–43 yrs) | 4.91 | 2.09–11.55 |
| G4 (≥44 yrs) | 1 | |
| Oocytes | 1.07 | 1.08–1.10 |
| N = 3846 (patients that reached oocyte retrieval) . | OR adjusted . | 95% CI . |
|---|---|---|
| G1 (38–39 yrs) | 19.27 | 8.50–43.67 |
| G2 (40–41 yrs) | 10.94 | 4.80–24.93 |
| G3 (42–43 yrs) | 4.91 | 2.09–11.55 |
| G4 (≥44 yrs) | 1 | |
| Oocytes | 1.07 | 1.08–1.10 |
Adjusted for year of stimulation, stimulation protocol and cryopreservation technique.
| N = 3846 (patients that reached oocyte retrieval) . | OR adjusted . | 95% CI . |
|---|---|---|
| G1 (38–39 yrs) | 19.27 | 8.50–43.67 |
| G2 (40–41 yrs) | 10.94 | 4.80–24.93 |
| G3 (42–43 yrs) | 4.91 | 2.09–11.55 |
| G4 (≥44 yrs) | 1 | |
| Oocytes | 1.07 | 1.08–1.10 |
| N = 3846 (patients that reached oocyte retrieval) . | OR adjusted . | 95% CI . |
|---|---|---|
| G1 (38–39 yrs) | 19.27 | 8.50–43.67 |
| G2 (40–41 yrs) | 10.94 | 4.80–24.93 |
| G3 (42–43 yrs) | 4.91 | 2.09–11.55 |
| G4 (≥44 yrs) | 1 | |
| Oocytes | 1.07 | 1.08–1.10 |
Adjusted for year of stimulation, stimulation protocol and cryopreservation technique.
CLBR probability was evaluated according to age and number of oocytes retrieved in a predictive model. According to our results, the higher the number of oocytes retrieved, the higher the probability of a live born after the use of fresh and frozen embryos. However, as shown in Fig. 2, although this is more evident in women up to 41 years old, women aged 42–43 experience a less profound benefit associated with the number of oocytes retrieved, and no clear benefit is observed in patients aged 44 years old or more.
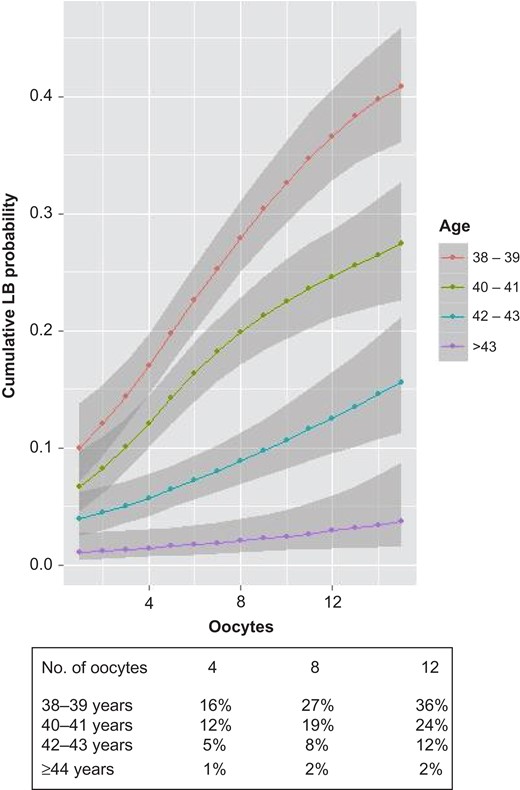
Predictive model of CLBRs according to age and number of oocytes retrieved: G1:38–39 years, G2:40–41 years, G3:42–43 years, G4: ≥44 years. As an example, the table below the graph shows CLBRs, for each age group, if 4, 8 or 12 oocytes are retrieved.
Differences in CLBR between age groups for a given number of oocytes retrieved were statistically significant and are graphically depicted in Fig. 2 (the non-overlapped shadowed area between two groups indicates significant differences). As shown in Fig. 2, for an oocyte yield of 12, the CLBR is 36% for a woman aged 38–39 years, 24% for patients at 40–41, 12% at 42–43 years and only 2% CLBR for a woman of 44 and over (Fig. 2). Of interest, in women 44 years or older a CLBR of 3% would never be reached irrespective of the number of oocytes retrieved (Fig. 2).
Discussion
To our knowledge, this is the first study that focuses on creating a prediction model for CLBRs after a single stimulation cycle in women of advanced reproductive age. According to our results, although CLBR reached reasonable figures of 25.9% in women aged 38–39 years and 16.4% for women of 40–41 years old, a clinically significant decline of almost 60% was identified with progressing age to 42–43 years, while treatment appears futile in women aged above 44 years old or more (The Ethics Committee of the American Society for Reproductive Medicine, 2012). Furthermore, the added value of an increase in the number of oocytes retrieved and of the transfer of supernumerary cryopreserved embryos on CLBR was mostly evident in women up to 41 years old, with older patients experiencing little or no benefit.
Our study is in complete agreement with other studies (Klipstein et al., 2005), demonstrating that an increase in the number of oocytes retrieved and generation of surplus cryopreserved embryos can increase CLBRs. However, according to our results this effect is mainly due to an improvement of the fresh cycle performance (probably due to a better embryo selection when more embryos are available). A considerable additional benefit is also seen from FETs; still this is only evident in women up to 41 years old.
In an attempt to provide a reasonable explanation for our findings and the apparent inefficiency of IVF cycles performed in women of advanced reproductive age, one could assume that this is associated with the age-related increase in embryo aneuploidy rates (Franasiak et al., 2014). For this reason, several groups are strongly in favour of preimplantation aneuploidy screening as a valid approach for increasing live birth rates in aged women. However, one of the main challenges for aged women is getting enough available embryos for biopsy and more oocytes are needed to have a chance of finding euploid embryos. Recently, Ubaldi et al. have reported in selected women aged ≥44 years that preimplantation genetic diagnosis for aneuploidy screening may lead to a delivery rate per cycle of 8% (Ubaldi et al., 2017); still results should be interpreted with caution given that this may only be applicable in women with good ovarian reserve and the potential to produce a good number of oocytes following ovarian stimulation for IVF/ICSI. Accumulation of oocytes or embryos in several cycles has also been proposed, as an attempt to allow PGS and identification of euploid embryos (Moragianni and Penzias, 2010). Nevertheless, available data is rather weak to allow proper recommendations.
On the other hand, the number of oocytes retrieved was a very important variable independently associated with CLBR. This is in agreement with other studies in younger patients showing that oocyte yield is probably one of the most important contributing factors when considering success rates in IVF, with recent evidence that CLBR significantly increases with the number of oocytes retrieved (Drakopoulos et al., 2016; Briggs et al., 2017). However, the added value of our study in the current literature is that in women of advanced age the benefit seems to be anticipated only for women aged up to 42 years old. Thus, although for example many believe that 4–5 oocytes are required to obtain minimally acceptable results (Çiray et al., 2006; Spandorfer et al., 2007; Tsafriret al., 2007), our predictive model revealed that four oocytes can only result in a 16% CLBR in women aged 38–39 years old, 12% for women of 40–41 years, 5% for 42–43 and 1% from 44 and over. Furthermore, in women aged more than 44 years old and over, a CLBR of 3% would never be reached irrespective of the number of oocytes retrieved, clearly suggesting futility of treatment in this population.
A major strength of the current study is that, contrary to previously published studies including several stimulation cycles (Klipstein et al., 2005; van Disseldorp et al., 2007; Malizia et al., 2009; Stern et al., 2010; Cetinkaya et al., 2013; De Brucker et al., 2013; Smith et al., 2015; McLernon et al., 2016), we have considered only results coming from a single stimulation cycle (one fresh embryo transfer plus subsequent FETs), given that this may be more meaningful from the patients’ perspective before starting an IVF cycle. CLBR after several stimulation cycles is an important outcome and may reach 17% in women aged 41–43 and of 9.1% in women >43 (van Disseldorp et al., 2007; Cetinkaya et al., 2013); nevertheless, these figures may not reflect everyday clinical practice, given that only half of the patients end up repeating a second ovarian stimulation for IVF/ICSI (van Disseldorp et al., 2007; Stern et al., 2010; Cetinkaya et al., 2013). Cycle discontinuation rates after the first IVF cycle has been shown to increase with women’s age, irrespective of financial constraints, suggesting that other issues such as success rates are being taken into account (Soullier et al., 2011).
Another strength of our analysis is the construction of a predictive model of CLBR in women of advanced age in relation to age and number of oocytes retrieved, after considering the added value of the transfer of frozen–thawed embryos. To our knowledge this is the first study aiming to create such a model in advanced reproductive age women given that others, either described a prediction model according to age and the number of oocytes retrieved without considering the added value of frozen cycles (Sunkara et al., 2011), or provided data on LBRs and CLBRs according to the number of oocytes retrieved, focusing only on women <40 years old (Smith et al., 2015). Addition of ovarian reserve markers in the future in such a model and evaluation on whether they can predict CLBR could be very interesting given that this may allow clinicians to predict with a relative certainly CLBR prior to ovarian stimulation.
However, apart from the above-mentioned strengths, several limitations are associated with our study and need to be mentioned, most of which are associated with the retrospective study design and large study period. Owing to this, compared age groups may have differed in several baseline characteristics which is apparently associated with patients’ age. On the other hand, despite the fact the study was carried out in a private setting in which ovarian reserve markers are not strictly considered as inclusion criteria for undergoing IVF instead of oocyte reception, a selection bias cannot be excluded. Thus, it may be the case that better prognosis patients have opted in for treatment with their own eggs. Still, such an effect will be minimal given that many of the patients treated in the current study experienced a poor ovarian response.
We need to highlight that embryo cryopreservation was carried out with the slow freezing protocol in 80% of cases, as vitrification was not established as a routine in our laboratory during the total length of the study period. This could indeed have resulted in a potential underestimation of the actual live birth rates attributed to supernumerary cryopreserved embryos, especially if we consider the results of the literature when comparing slow freezing with embryo vitrification, in favour of the latter (Rienzi et al., 2017). For this reason, results need to be interpreted with caution. Nevertheless, slow freezing outcomes were excellent in our setting given that in the current analysis the overall live birth rates were comparable between both methods of cryopreservation.
Although the vast majority of patients have used all their supernumerary embryos, a small percentage of them still had surplus embryos frozen which have not been transferred. This might have actually lead to a slight underestimation the CLBRs; however, we do not expect a relevant impact on the final outcome, given that the extra benefit of the frozen embryo transfers was indeed extremely limited. In fact, the higher CLBRs observed in women with surplus embryos were mainly due to an improvement in the live birth rates following the fresh embryo transfer, reflecting probably a better embryo selection in the first embryo transfer attempt.
Finally, changes over the study period concerning the stimulation protocol and cryopreservation technique might have acted as confounding factors and affected our outcomes. However, they should have affected all groups in a similar way and, in any case, it is unlikely that the probability of CLBR depends on the protocol used given on robust evidence from large randomized trials (Toftager et al., 2017). Furthermore, our logistic regression analysis for CLBRs showed that only age and the number of oocytes retrieved were significant, whereas the year of treatment, the stimulation protocol used and the cryopreservation technique were not significant, suggesting that any change in these variables over the study period did not have a significant impact on CLBR’s.
Allowing for the limitations mentioned above, the current study validates previous findings that the number of oocytes retrieved is significantly associated with CLBR not only in younger patients, but also in women of advanced reproductive age. However, the added benefit appears to be restricted mainly in women up to 41 years old. Women over 43 do not experience any benefit in CLBR irrespective of the number of oocytes retrieved, and thus should be discouraged from doing an IVF cycle with their own oocytes; for the other age-groups, recommendations should be given considering the age and the expected ovarian response.
Acknowledgements
This work was performed under the auspices of ‘Catedra d’Investigació en Obstetricia i Ginecología’ of Department of Obstetrics, Gynaecology and Reproductive Medicine; Hospital Universitario Quirón-Dexeus, Universitat Autònoma de Barcelona.
Authors’ roles
R.T., M.D. and N.P. designed the study. M.D. and N.P. reviewed the literature and wrote the article. I.R. conducted the statistical analysis. All authors approved the final version of the manuscript.
Funding
No funding was required or obtained.
Conflict of interest
There is no conflict of interest.



