-
PDF
- Split View
-
Views
-
Cite
Cite
Danilo Cimadomo, Antonio Capalbo, Paolo Emanuele Levi-Setti, Daria Soscia, Giovanna Orlando, Elena Albani, Valentina Parini, Marta Stoppa, Lisa Dovere, Luisa Tacconi, Elena Ievoli, Roberta Maggiulli, Filippo Maria Ubaldi, Laura Rienzi, Associations of blastocyst features, trophectoderm biopsy and other laboratory practice with post-warming behavior and implantation, Human Reproduction, Volume 33, Issue 11, November 2018, Pages 1992–2001, https://doi.org/10.1093/humrep/dey291
Close - Share Icon Share
Abstract
Are trophectoderm biopsy or other pre-vitrification features or laboratory practices associated with differences in blastocyst post-warming behavior (degeneration, re-expansion and live birth after single embryo transfer (SET))?
Blastocyst morphology, day of full development and artificial shrinkage (either laser-assisted or biopsy-induced) are the pre-vitrification parameters/practices most strongly associated with post-warming behavior and implantation potential while there was no association with trophectoderm biopsy.
Since the introduction of vitrification, the adoption of cycle segmentation, freeze-all and SET policies, as well of trophectoderm biopsy-based aneuploidy testing (i.e. pre-implantation genetic testing for aneuploidies (PGT-A)), the number of blastocysts vitrified worldwide has increased greatly. Previous studies already defined generally high blastocyst cryo-survival rates after vitrification-warming (>95%), along with a positive correlation between blastocyst re-expansion and morphology with implantation. Additionally, artificial shrinkage has been outlined as a potentially beneficial procedure, while the association between embryo cryo-survival and trophectoderm biopsy is still unclear.
Cohort study conducted at two IVF centers (1 and 2). A total of 2129 consecutive SETs using vitrified-warmed blastocysts in either non-PGT or PGT-A cycles between June 2016 and August 2017 were included. A freeze-all strategy was in place and three main pre-vitrification practices were used: (i) no biopsy and no artificial shrinkage (Clinic 1); (ii) trophectoderm biopsy and vitrification of collapsed blastocyst within 30 min (Clinics 1 and 2); and (iii) no biopsy but laser-assisted artificial shrinkage (Clinic 2). The primary outcome was the blastocyst degeneration rate. Overall, 2108 surviving blastocysts were graded at 1.5 h after warming for degeneration (absent or partial) and re-expansion (full, partial or absent) grades and post-warming morphological quality. Logistic regression analyses were conducted to assess the association of any pre-vitrification feature with blastocyst post-warming behavior. The logistic regressions conducted upon live birth after either untested or euploid SET also included the post-warming characteristics.
Center 1 is a private IVF facility, while center 2 is the IVF facility of a University hospital. In non-PGT cycles, ICSI with blastocyst culture up to full-expansion and vitrification were performed. At center 1 the untested blastocysts were vitrified when still expanded, while at center 2 they underwent laser-assisted artificial shrinkage. In PGT-A cycles, in both clinics, trophectoderm biopsy (which involves laser-assisted shrinkage) was done without previous zona-opening on Day 3, and vitrification was performed within 30 min whilst the blastocyst remained collapsed. A qPCR-based chromosome analysis was conducted. Only SETs were performed (euploid-SET in case of PGT-A). Any cycle-, laboratory- and embryo-based feature which could impact blastocyst post-warming behavior was included in the analyses as putative confounder.
The overall degeneration rate was 1% (N = 21/2129). The results were consistent among different vitrification/warming operators or kits used, as well as any other IVF laboratory-related parameter. Blastocyst artificial shrinkage (either laser-assisted or biopsy-induced) involved a lower risk of degeneration after warming (odds ratio (OR) [95% CI] = 0.26 [0.09–0.79]). Conversely, both poor morphological quality pre-vitrification and taking 7 days to reach full blastulation resulted in a significantly higher risk (OR [95% CI] = 11.67 [3.42–39.83] and 4.43 [1.10–20.55], respectively). Importantly, trophectoderm biopsy did not show any association with blastocyst cryo-survival/degeneration. Overall 2.5% (N = 53/2108) blastocysts failed to re-expand post-warming. The only parameters significantly associated with no blastocyst re-expansion post-warming were average (OR [95% CI] = 4.96 [2.20–11.21]) or poor (OR [95% CI] = 19.54 [8.39–45.50]) morphological quality and taking 7 days to reach full blastulation (OR [95% CI] = 3.19 [1.23–8.29]), as well as prevention of spontaneous hatching pre-vitrification (OR [95% CI] = 0.10 [0.01–0.85]). The post-warming features of the survived blastocyst (i.e. degeneration and re-expansion scores and morphological quality) showed no significant association with vitrified blastocyst competence (i.e. live birth) when corrected for pre-vitrification ones (i.e. morphological quality, day of full development and, for untested SET, maternal age at oocyte retrieval). Of note, poor-quality blastocysts pre-vitrification showed a high overall cryo-survival rate post-warming 92.8% (N = 116/125), but the live birth rates were only 2.1% (N = 1/48) and 7.3% (N = 5/68) after untested and euploid SET, respectively.
This study is not randomized and the populations of patients undergoing either non-PGT or PGT-A cycles were different. Centers 1 and 2 adopted different pre-vitrification practices for non-biopsied blastocysts, according to their own laboratory policy. To this regard, multivariate logistic regression analyses were conducted for all outcomes under investigation.
Pre-vitrification features may be used to assist selection of competent embryos, moreover, these results allay concern that trophectoderm biopsy might be associated with impaired blastocyst quality or competence after vitrification/warming.
None.
None.
Introduction
The improvement of oocyte and embryo cryopreservation via vitrification protocols (Rienzi et al., 2017) has allowed the implementation of cycle segmentation to minimize the risk of ovarian hyperstimulation syndrome (OHSS) (Devroey et al., 2011), as well as allowing embryo transfer on a non-stimulated endometrium (Evans et al., 2014). Of note, cycle segmentation efficiently pairs with a single embryo transfer (SET) strategy to reduce the prevalence of multiple pregnancies (Pandian et al., 2013; Evans et al., 2014; Ubaldi et al., 2015). In turn, SET demands the definition of powerful criteria for embryo selection (Gardner et al., 2015). To date, blastocyst transfer (Glujovsky et al., 2016) and euploidy, detected by pre-implantation genetic testing for aneuploidies (PGT-A), have shown the strongest association with implantation (Chen et al., 2015; Dahdouh et al., 2015).
In this scenario, vitrification plays a core role in the management of IVF to (i) cryopreserve supernumerary blastocysts after fresh ET, (ii) adopt a freeze-all policy and/or (iii) conduct genetic assessment during PGT cycles. Nonetheless, the variables that possibly affect the survival rate after warming, especially when trophectoderm biopsy is performed, still need to be thoroughly characterized. The studies published to date have mainly reported that trophectoderm biopsy seems not to impact embryo reproductive competence during fresh cycles (Scott et al., 2013; Cimadomo et al., 2016), but few data have been published concerning blastocyst survival post-warming. Only four papers have investigated this issue, all with a retrospective design (Taylor et al., 2014; Reed et al., 2015; Bradley et al., 2017; Chen et al., 2017). Bradley et al. (2017) reported a decrease in the survival rate, as well as poorer clinical outcomes related to the number of manipulations blastocysts were subjected to: vitrified and biopsied once (survival rate: 98.4%; and ongoing implantation after SET: 54.3%), vitrified twice and biopsied once (97.3%; 47.1%), or biopsied and vitrified twice (93.3%; 31%). Conversely, a similar, previous study (Taylor et al., 2014) that compared the same treatments as in Bradley’s paper, found no association of multiple manipulations with impaired embryo cryo-survival and competence, except for blastocysts cryopreserved twice when the protocol adopted for the first cryopreservation procedure was slow-freezing rather than vitrification. However, the sample size of Taylor’s study was insufficient to draw any clear conclusion upon this issue (N = 116 biopsied and vitrified once; N = 21 biopsied once but cryopreserved twice; and N = 3 biopsied and vitrified twice). More recently, Chen et al. (2017) compared the implantation rates in four groups of blastocysts clustered according to two criteria: vitrified before or later than 3 h after biopsy, and still collapsed or already re-expanded when the vitrification procedure was initiated. The authors claimed that the blastocysts re-expanding and vitrified later than 3 h from biopsy show the highest chance to implant. Lastly, Reed et al. (2015) in a study designed to compare different cryopreservation protocols reported no difference in the cryo-survival rate between biopsied and non-biopsied blastocysts.
More investigations are clearly required to assess all the parameters, procedures and strategies putatively affecting blastocyst cryo-survival after vitrification and competence post-warming in both non-PGT and PGT cycles. To this regard, the aim of the present study was to outline whether and to what extent embryo artificial shrinkage, both laser-assisted and trophectoderm biopsy-induced, corrected for any other putative confounder, impacts blastocyst post-warming behavior in terms of cryo-survival/degeneration (primary outcome), and re-expansion. Furthermore, since blastocyst re-expansion degree, inner cell mass (ICM) and trophectoderm quality post-warming, have been recently proposed as predictive parameters of embryo implantation potential (Desai et al., 2016; Du et al., 2016; Coello et al., 2017), we investigated also the reproductive outcome after SET. To this end, we examined all the consecutive warming cycles performed between June 2016 and August 2017 at two IVF centers in Italy after either non-PGT or PGT-A cycle.
Material and Methods
Study design
This is a cohort study conducted at two IVF centers (Clinica Valle Giulia, G.EN.E.R.A. center for reproductive medicine, Rome, Italy = center 1; and Humanitas Fertility Center, Rozzano, Italy = center 2). All consecutive warming cycles with SET performed between June 2016 and August 2017 were included. Both clinics operated a freeze-all policy. The blastocysts were clustered in three groups according to the pre-vitrification strategy adopted: (i) untested expanded blastocysts vitrified without artificial shrinkage (center 1); (ii) euploid collapsed blastocysts vitrified within 30 min after trophectoderm biopsy (centers 1 and 2); and (iii) untested collapsed blastocysts vitrified after laser-assisted artificial shrinkage (center 2) (Fig. 1). Center 2 internally validated a policy entailing artificial shrinkage pre-vitrification in a study previously published (Levi-Setti et al., 2016). Any putative variable which could influence survival/degeneration and re-expansion after warming, was registered and investigated (maternal age, cause of infertility, protocol of controlled ovarian stimulation (COS), sperm factor, culture media, incubation system, blastocyst quality pre-vitrification, expansion grade pre-vitrification, day of full blastulation, vitrification and warming kits used, vitrification and warming operators). The blastocysts surviving after warming, were scored after 1.5 h according to their degeneration and re-expansion grades. The former defined as 1 (no area of degeneration) or 2 (few areas of degeneration present); the latter defined as 1 (full re-expansion, 80–100%), 2 (partial re-expansion, 20–80%) or 3 (no re-expansion, 0–20%) (see examples in Fig. 1). The survived and re-expanded blastocysts were blindly re-graded also for their post-warming quality. We also monitored the live birth rate after vitrified-warmed SET. Logistic regression analyses were performed also to investigate the variables possibly associated with vitrified-warmed blastocyst reproductive competence. In this investigation we included also the quality post-warming, as well as the degeneration and re-expansion scores. The Institutional Review Board of the clinics approved this study.
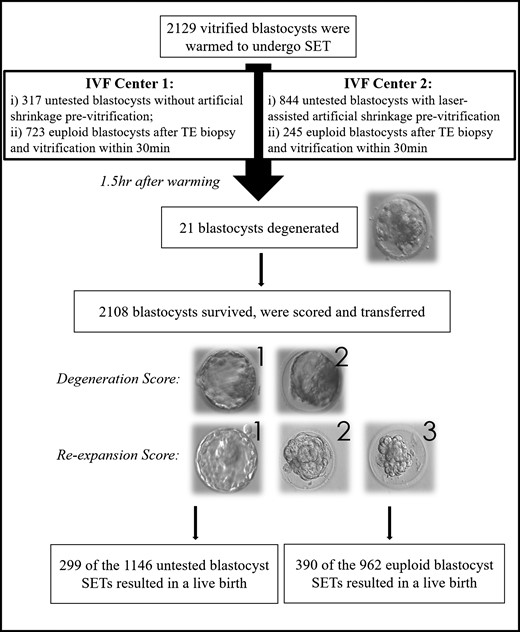
Workflow of the study. Two thousand one hundred and twenty-nine vitrified blastocysts were warmed to undergo single embryo transfer (SET). Three pre-vitrification practices were included: (i) no biopsy and no artificial shrinkage (Center 1 only), (ii) trophectoderm biopsy and vitrification within 30 min (Centers 1 and 2) and (iii) no biopsy but laser-assisted artificial shrinkage (Center 2 only). Twenty-one blastocysts had degenerated 1.5 h after warming. The 2108 surviving blastocysts were scored for their degeneration and re-expansion grades. The former from 1 (no areas of degeneration) to 2 (few areas of degeneration), and the latter from 1 to 3 (fully re-expanded, partially re-expanded or not re-expanded). Overall, 299 untested blastocysts (of the 1146 transferred) and 390 euploid blastocysts (of the 962 transferred) SETs resulted into a live birth. All the variables that may affect degeneration and re-expansion after warming and competence (i.e. live birth) were tested through logistic regression analyses.
Outcomes measures and sample size analysis
The primary outcome was the cryo-survival/degeneration rate after warming and its predictive factors. The secondary outcomes were the re-expansion rate after warming, as well as the live birth rate after vitrified-warmed SET and their predictive factors.
The sample size analysis was performed with the software G*Power v3.1. Specifically, based on our previous experience and knowledge of this subject, we assumed a ~4.5-fold higher degeneration rate after warming when embryo collapse (either via laser-assisted artificial shrinkage or biopsy-induced) is not performed. An outcome, which might be exacerbated by a decreasing blastocyst morphology and more days to reach full development. Therefore, the following parameters were adopted: two-tailed logistic regression analysis (z-test), odds ratio (OR) = 0.22, estimated probability of degeneration post-warming in the absence of collapse pre-vitrification = 0.04, power = 0.9, α error = 0.05, r2 other than ‘embryo collapse’ variable (namely ‘blastocyst quality’ and ‘day of full development’) = 0.01, binomial distribution (either survival or degeneration post-warming), proportion between non-collapsed and collapsed blastocysts = 0.2. The analysis highlighted that at least 2027 blastocysts were required.
IVF procedures
A COS was performed as previously described (Ubaldi et al., 2010, 2015): either an antagonist or an agonist COS strategy was adopted. The cumulus–oocyte complexes were aspirated 36 h after the trigger of ovulation. After 2 h of incubation in a controlled atmosphere (6% CO2 and 5% O2), the oocytes were denuded in HEPES-buffered medium (Irvine Scientific, USA or Quinn’s Advantage, Cooper Surgical Fertility Companies, USA) supplemented with 5% Human Serum Albumin (HSA, Irvine Scientific or Quinn’s Advantage) and containing 20 IU/ml of Hyaluronidase (Hyaluronidase Solution 80 IU/ml, Irvine Scientific or Quinn’s Advantage) and ICSI was performed immediately after denudation as previously described (Rienzi et al., 1998). The presence of two equally sized pronuclei after 16–20 h from insemination was assessed and the embryos were cultured in separate 30 μl drops up to the blastocyst stage (Days 5–7) in an atmosphere containing 5% O2 and 6% CO2 in either a standard benchtop incubator (MINC, Cook Medical, USA) or a time-lapse incubation system (Embryoscope, Vitrolife, Sweden). Either a continuous or a sequential culture media was adopted (Continuous single step medium, CSCM, Irvine Scientific, or Quinn’s Advantage cleavage-blastocyst). Only the latter culture strategy entailed a media change-over on Day 3 of pre-implantation development.
All the blastocysts were categorized based on the day of full blastulation (Day 5, 6 or 7), the level of expansion (not-expanded, fully expanded, hatching, fully hatched), ICM (1 = numerous tightly packed cells, 2 = several and loosely packed cells or 3 = very few cells) and trophectoderm (1 = many cells organized in epithelium, 2 = several cells organized in loose epithelium or 3 = few large cells) morphological quality (this grading system has been adapted from Gardner and Schoolcraft (1999) and previously described by Capalbo et al. (2014); some examples are shown in Supplementary Fig. S1). Four groups of blastocyst morphological quality were defined: excellent (1.1), good (1.2 or 2.1), average (1.3, 3.1 or 2.2) and poor (2.3, 3.2 and 3.3).
During PGT-A cycles, trophectoderm biopsy was performed as previously described (Capalbo et al., 2014) through an approach that does not entail any zona-opening on Day 3 of pre-implantation development: sequential laser-assisted zona-opening and mouth-controlled pipette-assisted trophectoderm fragment retrieval were conducted. Such biopsy method has been previously reported as a standardized procedure at our center (Capalbo et al., 2016). The biopsy was performed on all embryos that developed as viable fully expanded blastocysts, regardless the day of culture and morphological quality. Quantitative polymerase chain reaction (qPCR)-based comprehensive chromosome testing was conducted as described by Treff et al. (2012), and internally validated in our laboratory in 2015 (Capalbo et al., 2015). This methodology was designed to specifically identify constitutive whole-chromosome but not segmental aneuploidies.
Only a freeze-all strategy was adopted. All the blastocysts, either after trophectoderm biopsy (PGT-A cycles) or in its absence (non-PGT cycles), were vitrified. Biopsied blastocysts were cryopreserved within 30 min from the removal of 5–10 cells, while they were still collapsed. Artificial shrinkage was not performed for blastocysts not subject to trophectoderm biopsy at center 1. Laser-assisted artificial shrinkage was instead performed during non-PGT cycles conducted at center 2. Specifically, 5–10 min before vitrification, 1–2 laser pulses were directed to the junctions between trophectoderm cells at a safe distance from the ICM. Vitrification and warming procedures were then performed according to Cobo and colleagues (Cobo et al., 2012). In detail, the blastocysts were equilibrated at room temperature for 14 min in an equilibration solution (ES) containing 7.5% ethylene-glycol (EG) and 7.5% dimethyl-sulfoxide (DMSO) and then transferred in a vitrification solution (VS) with 15% e.g. 15% DMSO, 0.5% sucrose for 1 min. During this phase, the blastocysts were rinsed to dilute the excess of ES. In the last 10 s the blastocysts were placed onto the tip of the Cryolock (Biotech Inc., USA) surrounded by a minimal volume of VS. The devices were lastly plunged into liquid nitrogen. For the warming procedure, the Cryolock were immediately moved from liquid nitrogen into a thawing solution (TS) of 1 M sucrose for 1 min at 37°C. Then, the blastocysts were moved to a dilution solution (DS) of 0.5 M sucrose for 3 min and lastly into a washing solution (WS) for 5 min at room temperature. The blastocysts were then cultured at 37°C for at least 2 h before ET into the same medium used for embryo culture. Two kits of vitrification/warming were used during this study (a variable included in the logistic regression analyses as putative confounder), namely, Kitazato BioPharma Co. (Japan) and Irvine Scientific. Overall, 11 operators were involved in the vitrification and warming procedures from the two clinics (also this variable was included in the logistic regressions). Only SETs were performed, and endometrial preparation and transfer procedures were conducted as previously described (Ubaldi et al., 2015).
Statistical analyses
All data were collected in a relational database (Fertilab Manager, FLM, Italy). Categorical variables are presented as percentages with 95%CI. Continuous variables are presented as mean±SD and range. Chi-squared tests were performed to assess for a distribution of the data significantly different from the expected. Univariate and multivariate regression analyses were performed to correct the results putative confounders upon the outcomes under investigation: blastocyst artificial shrinkage before vitrification (not performed on expanded blastocysts/laser-assisted or biopsy-induced), trophectoderm biopsy before vitrification (not performed/performed); maternal age; cause of infertility; COS protocol; sperm factor; IVF culture media; incubation system; blastocyst morphological quality pre-vitrification; expansion grade pre-biopsy or pre-vitrification; day of full blastulation; kit adopted for vitrification and warming; vitrification operator; warming operator. The logistic regression analyses upon the clinical outcome after vitrified-warmed SET (no live birth achieved/live birth achieved) entailed also the degeneration and re-expansion scores, the blastocyst post-warming morphological quality and the specific sequential number of transfers per patient (first, second, etc.). All statistics were conducted with the software R. A P < 0.05 was considered significant.
Results
This study involved 2129 blastocysts from 1671 patients (among them, 897 underwent a non-PGT and 774 a PGT cycle). In detail, 1671 patients performed at least one vitrified-warmed SET during the study period, 361 of them two (202 after a non-PGT and 159 after a PGT cycle), 75 three (51 after a non-PGT and 24 after a PGT cycle), 15 four (8 after a non-PGT and 7 after a PGT cycle), 6 five (3 after a non-PGT and 3 after a PGT cycle) and 1 six (after a PGT cycle). The specific sequential number of each vitrified-warmed SET was included among the putative confounders of the chance of a blastocyst to result in a live birth. This measure was required to avoid the risk of a cohort effect upon the clinical outcomes.
Blastocyst cryo-survival to vitrification-warming
A total of 2129 blastocysts were warmed. Twenty-one blastocysts degenerated within 1.5 h after warming (1.0%, 95% CI: 0.6–1.5), therefore, the overall survival rate was 99.0% (N = 2108/2129, 95% CI: 98.5–99.4; no areas of degeneration = 88.3%, few areas of degeneration = 10.7%). The logistic regression analyses showed no association with the cause of infertility, the protocol of COS, the sperm factor, the culture media, the incubation system, and the grade of blastocyst expansion pre-vitrification with the degeneration after warming. Neither the use of different kits for vitrification and/or warming, nor the vitrification and warming operators showed differences in the blastocyst cryo-survival rates (Supplementary Fig. S2), but most importantly trophectoderm biopsy did not result into a higher blastocyst degeneration post-warming. No differences were reported among the two IVF centers for the study group they had in common (i.e. collapsed blastocysts vitrified within 30 min from trophectoderm biopsy) in terms of embryo cryo-survival rate: 99.4% (N = 719/723) and 99.2% (N = 243/245) at IVF centers 1 and 2, respectively. Only a worse blastocyst quality pre-vitrification and more days to reach full-blastulation involved a higher risk of degeneration (P < 0.01 and P = 0.04, respectively) (Table I). Conversely, the practice of artificially collapsing the embryo (either laser-assisted or due to trophectoderm biopsy) before vitrification resulted in a lower degeneration post-warming (OR: 0.26, 95% CI: 0.09–0.79, P = 0.02) (Table I). Specifically, the survival rate to warming of untested blastocysts that were vitrified when still expanded was 97.2% (N = 308/317; no areas of degeneration = 87.7%, few areas of degeneration = 9.5%), while blastocysts vitrified within 30 min from trophectoderm biopsy showed a 99.4% survival rate (N = 962/968; no areas of degeneration = 88.4%, few areas of degeneration = 11%), similar to blastocysts vitrified after laser-assisted artificial shrinkage in absence of trophectoderm biopsy which was 99.3% (N = 838/844; no areas of degeneration = 88.4%, few areas of degeneration = 10.9%) (Table II). Table II highlights also the data in these three groups further clustered per day of full-blastulation and blastocyst morphological quality pre-vitrification. Of note, even if entailing the highest risk for degeneration, still poor-quality blastocysts showed an overall cryo-survival rate of 92.8% (N = 116/125), 83.8% if considering only poor-quality blastocysts on Day 7 (N = 31/37).
Logistic regression analysis for the putative predictors of blastocyst degeneration after vitrification-warminga (primary outcome).
| . | Univariate OR (95% CI) . | Multivariate OR (95% CI) . | Adjusted P-value . |
|---|---|---|---|
| Artificial shrinkage before vitrification: not performed (expanded blastocyst) | 0.02 | ||
| Performed (laser-assisted or biopsy-induced) | 0.23 (0.09–0.55) | 0.26 (0.09–0.79) | |
| Trophectoderm biopsy before vitrification: not performed | NS | ||
| Performed | 0.48 (0.18–1.23) | 0.61 (0.19–2.03) | |
| Blastocyst morphological quality pre-vitrification: excellent | <0.01 | ||
| Good | 2.73 (0.68–11.00) | 1.83 (0.44–7.62) | |
| Average | 2.83 (0.70–11.38) | 1.63 (0.38–7.02) | |
| Poor | 18.94 (6.63–54.14) | 11.67 (3.42–39.83) | |
| Days to reach full blastulation: 5 | 0.04 | ||
| 6 | 2.45 (0.78–7.71) | 1.78 (0.52–6.10) | |
| 7 | 12.83 (3.57–46.17) | 4.43 (1.10–20.55) |
| . | Univariate OR (95% CI) . | Multivariate OR (95% CI) . | Adjusted P-value . |
|---|---|---|---|
| Artificial shrinkage before vitrification: not performed (expanded blastocyst) | 0.02 | ||
| Performed (laser-assisted or biopsy-induced) | 0.23 (0.09–0.55) | 0.26 (0.09–0.79) | |
| Trophectoderm biopsy before vitrification: not performed | NS | ||
| Performed | 0.48 (0.18–1.23) | 0.61 (0.19–2.03) | |
| Blastocyst morphological quality pre-vitrification: excellent | <0.01 | ||
| Good | 2.73 (0.68–11.00) | 1.83 (0.44–7.62) | |
| Average | 2.83 (0.70–11.38) | 1.63 (0.38–7.02) | |
| Poor | 18.94 (6.63–54.14) | 11.67 (3.42–39.83) | |
| Days to reach full blastulation: 5 | 0.04 | ||
| 6 | 2.45 (0.78–7.71) | 1.78 (0.52–6.10) | |
| 7 | 12.83 (3.57–46.17) | 4.43 (1.10–20.55) |
OR, odds ratio.
aVariables investigated: artificial shrinkage before vitrification; trophectoderm biopsy before vitrification; maternal age; cause of infertility; ovarian stimulation protocol; sperm factor; IVF culture media; incubation system; blastocyst morphological quality pre-vitrification; expansion grade pre-biopsy or pre-vitrification; day of full blastulation; kit adopted for vitrification and warming; vitrification operator; warming operator
Logistic regression analysis for the putative predictors of blastocyst degeneration after vitrification-warminga (primary outcome).
| . | Univariate OR (95% CI) . | Multivariate OR (95% CI) . | Adjusted P-value . |
|---|---|---|---|
| Artificial shrinkage before vitrification: not performed (expanded blastocyst) | 0.02 | ||
| Performed (laser-assisted or biopsy-induced) | 0.23 (0.09–0.55) | 0.26 (0.09–0.79) | |
| Trophectoderm biopsy before vitrification: not performed | NS | ||
| Performed | 0.48 (0.18–1.23) | 0.61 (0.19–2.03) | |
| Blastocyst morphological quality pre-vitrification: excellent | <0.01 | ||
| Good | 2.73 (0.68–11.00) | 1.83 (0.44–7.62) | |
| Average | 2.83 (0.70–11.38) | 1.63 (0.38–7.02) | |
| Poor | 18.94 (6.63–54.14) | 11.67 (3.42–39.83) | |
| Days to reach full blastulation: 5 | 0.04 | ||
| 6 | 2.45 (0.78–7.71) | 1.78 (0.52–6.10) | |
| 7 | 12.83 (3.57–46.17) | 4.43 (1.10–20.55) |
| . | Univariate OR (95% CI) . | Multivariate OR (95% CI) . | Adjusted P-value . |
|---|---|---|---|
| Artificial shrinkage before vitrification: not performed (expanded blastocyst) | 0.02 | ||
| Performed (laser-assisted or biopsy-induced) | 0.23 (0.09–0.55) | 0.26 (0.09–0.79) | |
| Trophectoderm biopsy before vitrification: not performed | NS | ||
| Performed | 0.48 (0.18–1.23) | 0.61 (0.19–2.03) | |
| Blastocyst morphological quality pre-vitrification: excellent | <0.01 | ||
| Good | 2.73 (0.68–11.00) | 1.83 (0.44–7.62) | |
| Average | 2.83 (0.70–11.38) | 1.63 (0.38–7.02) | |
| Poor | 18.94 (6.63–54.14) | 11.67 (3.42–39.83) | |
| Days to reach full blastulation: 5 | 0.04 | ||
| 6 | 2.45 (0.78–7.71) | 1.78 (0.52–6.10) | |
| 7 | 12.83 (3.57–46.17) | 4.43 (1.10–20.55) |
OR, odds ratio.
aVariables investigated: artificial shrinkage before vitrification; trophectoderm biopsy before vitrification; maternal age; cause of infertility; ovarian stimulation protocol; sperm factor; IVF culture media; incubation system; blastocyst morphological quality pre-vitrification; expansion grade pre-biopsy or pre-vitrification; day of full blastulation; kit adopted for vitrification and warming; vitrification operator; warming operator
| Blastocyst features pre-vitrification . | No biopsy and no artificial shrinkage . | TE biopsy and vitrification within 30 min . | No biopsy and laser-assisted artificial shrinkage . | |
|---|---|---|---|---|
| Day 5 | Excellent | 122/123, 99.2% | 384/384, 100% | 274/275, 99.6% |
| Good | 28/28, 100% | 23/23, 100% | 37/38, 97% | |
| Average | 19/19, 100% | 17/17, 100% | 28/28, 100% | |
| Poor | 0/1, 0% | 6/6, 100% | 3/3, 100% | |
| Day 6 | Excellent | 56/58, 96% | 309/311, 99% | 284/284, 100% |
| Good | 27/29, 93% | 60/60, 100% | 76/76, 100% | |
| Average | 33/34, 97% | 52/52, 100% | 84/86, 98% | |
| Poor | 8/8, 100% | 38/39, 97% | 30/31, 97% | |
| Day 7 | Excellent | 1/1, 100% | 25/25, 100% | 10/10, 100% |
| Good | 3/3, 100% | 11/11, 100% | 3/3, 100% | |
| Average | 7/7, 100% | 13/13, 100% | 6/6, 100% | |
| Poor | 4/6, 67% | 24/27, 89% | 3/4, 75% | |
| Total | 308/317, 97.2% | 962/968, 99.4% | 838/844, 99.3% | |
| Overall | 2108/2129, 99.0% | |||
| Blastocyst features pre-vitrification . | No biopsy and no artificial shrinkage . | TE biopsy and vitrification within 30 min . | No biopsy and laser-assisted artificial shrinkage . | |
|---|---|---|---|---|
| Day 5 | Excellent | 122/123, 99.2% | 384/384, 100% | 274/275, 99.6% |
| Good | 28/28, 100% | 23/23, 100% | 37/38, 97% | |
| Average | 19/19, 100% | 17/17, 100% | 28/28, 100% | |
| Poor | 0/1, 0% | 6/6, 100% | 3/3, 100% | |
| Day 6 | Excellent | 56/58, 96% | 309/311, 99% | 284/284, 100% |
| Good | 27/29, 93% | 60/60, 100% | 76/76, 100% | |
| Average | 33/34, 97% | 52/52, 100% | 84/86, 98% | |
| Poor | 8/8, 100% | 38/39, 97% | 30/31, 97% | |
| Day 7 | Excellent | 1/1, 100% | 25/25, 100% | 10/10, 100% |
| Good | 3/3, 100% | 11/11, 100% | 3/3, 100% | |
| Average | 7/7, 100% | 13/13, 100% | 6/6, 100% | |
| Poor | 4/6, 67% | 24/27, 89% | 3/4, 75% | |
| Total | 308/317, 97.2% | 962/968, 99.4% | 838/844, 99.3% | |
| Overall | 2108/2129, 99.0% | |||
The data are clustered firstly per pre-vitrification practice, i.e. (i) no biopsy and no artificial shrinkage, (ii) trophectoderm biopsy and vitrification within 30 min and (iii) no biopsy but laser-assisted artificial shrinkage), then also per day of full-blastulation and blastocyst morphological quality.
| Blastocyst features pre-vitrification . | No biopsy and no artificial shrinkage . | TE biopsy and vitrification within 30 min . | No biopsy and laser-assisted artificial shrinkage . | |
|---|---|---|---|---|
| Day 5 | Excellent | 122/123, 99.2% | 384/384, 100% | 274/275, 99.6% |
| Good | 28/28, 100% | 23/23, 100% | 37/38, 97% | |
| Average | 19/19, 100% | 17/17, 100% | 28/28, 100% | |
| Poor | 0/1, 0% | 6/6, 100% | 3/3, 100% | |
| Day 6 | Excellent | 56/58, 96% | 309/311, 99% | 284/284, 100% |
| Good | 27/29, 93% | 60/60, 100% | 76/76, 100% | |
| Average | 33/34, 97% | 52/52, 100% | 84/86, 98% | |
| Poor | 8/8, 100% | 38/39, 97% | 30/31, 97% | |
| Day 7 | Excellent | 1/1, 100% | 25/25, 100% | 10/10, 100% |
| Good | 3/3, 100% | 11/11, 100% | 3/3, 100% | |
| Average | 7/7, 100% | 13/13, 100% | 6/6, 100% | |
| Poor | 4/6, 67% | 24/27, 89% | 3/4, 75% | |
| Total | 308/317, 97.2% | 962/968, 99.4% | 838/844, 99.3% | |
| Overall | 2108/2129, 99.0% | |||
| Blastocyst features pre-vitrification . | No biopsy and no artificial shrinkage . | TE biopsy and vitrification within 30 min . | No biopsy and laser-assisted artificial shrinkage . | |
|---|---|---|---|---|
| Day 5 | Excellent | 122/123, 99.2% | 384/384, 100% | 274/275, 99.6% |
| Good | 28/28, 100% | 23/23, 100% | 37/38, 97% | |
| Average | 19/19, 100% | 17/17, 100% | 28/28, 100% | |
| Poor | 0/1, 0% | 6/6, 100% | 3/3, 100% | |
| Day 6 | Excellent | 56/58, 96% | 309/311, 99% | 284/284, 100% |
| Good | 27/29, 93% | 60/60, 100% | 76/76, 100% | |
| Average | 33/34, 97% | 52/52, 100% | 84/86, 98% | |
| Poor | 8/8, 100% | 38/39, 97% | 30/31, 97% | |
| Day 7 | Excellent | 1/1, 100% | 25/25, 100% | 10/10, 100% |
| Good | 3/3, 100% | 11/11, 100% | 3/3, 100% | |
| Average | 7/7, 100% | 13/13, 100% | 6/6, 100% | |
| Poor | 4/6, 67% | 24/27, 89% | 3/4, 75% | |
| Total | 308/317, 97.2% | 962/968, 99.4% | 838/844, 99.3% | |
| Overall | 2108/2129, 99.0% | |||
The data are clustered firstly per pre-vitrification practice, i.e. (i) no biopsy and no artificial shrinkage, (ii) trophectoderm biopsy and vitrification within 30 min and (iii) no biopsy but laser-assisted artificial shrinkage), then also per day of full-blastulation and blastocyst morphological quality.
Blastocyst re-expansion after vitrification-warming
Overall, 2108 blastocysts survived after warming. And 53 blastocysts did not re-expand within 1.5 h (2.5%, 95% CI: 2.0–3.3). The overall re-expansion rate was 97.5% (N = 2055/2108, 95% CI: 96.7–98.1; full = 70.6%, partial = 26.9%). The logistic regression analyses did not highlight any association between trophectoderm biopsy per se and/or embryo artificial shrinkage pre-vitrification with the risk of no re-expansion after warming. Conversely, again a worse blastocyst quality and more days to reach full blastulation, together with the increasing expansion grade pre-vitrification in this case, were significant predictors of this outcome (P < 0.01, P = 0.02 and P < 0.01, respectively) (Supplementary Table SI). Specifically, the re-expansion rate after warming of excellent-, good-, average- and poor-quality blastocysts was 99.0% (N = 1451/1465; full = 77.3%, partial = 21.7%), 98.1% (N = 263/268; full = 66.0%, partial = 32.1%), 94.6% (N = 245/259; full = 56.8%, partial = 37.8%), and 82.8% (N = 96/116; full = 26.8%, partial = 56.0%), respectively. The re-expansion rate for embryos achieving full blastulation on Day 5, 6 or 7 was 99.7% (N = 929/941; full = 72.7%, partial = 27.0%), 97.4% (N = 1030/1057; full = 71.7%, partial = 25.7%) and 87.3% (N = 96/110; full = 49.1%, partial = 38.2%), respectively. Finally, the re-expansion rate for not-expanded, expanded, hatching and fully hatched blastocysts pre-vitrification was 100% (N = 50/50; full = 60.0%, partial = 40.0%), 97.9% (N = 1665/1691; full = 69.2%, partial = 28.7%), 95.5% (N = 338/354; full = 78.3%, partial = 17.2%) and 92.3% (N = 12/13; full = 84.6%, partial = 7.7%), respectively.
Blastocyst competence after vitrification-warming
Logistic regression analyses investigating the parameters correlating with the achievement of a live birth after either untested or euploid blastocyst SET were performed. Importantly, all the post-warming parameters (i.e. degeneration score from 1 to 2, re-expansion score from 1 to 3, and blastocyst post-warming morphological quality) resulted not significant when corrected for the pre-vitrification ones from both analyses (Supplementary Table SI). No significant differences were reported in the life birth rates among the two IVF centers involved in the study for both untested (24.3%, N = 75/308 and 26.7%, N = 224/838 for IVF center 1 and 2, respectively) and euploid SETs (39.9%, N = 287/719 and 42.4%, N = 103/243 for IVF center 1 and 2, respectively). The only variables showing a significant (negative) correlation with vitrified untested blastocyst competence (i.e. live birth) were a worse quality pre-vitrification (P < 0.01), more days to reach full blastulation (P = 0.05) and the increasing maternal age at oocyte retrieval (OR = 0.94, 95% CI: 0.91–0.98, P < 0.01) (Supplementary Table SI). Only the former two parameters maintained their negative predictive power upon vitrified euploid blastocyst competence (P < 0.01 for both these variables) (Supplementary Table SI). The specific sequential number of SET conducted per patient did not show any significant correlation in both the multivariate logistic regression analyses conducted (Supplementary Table SI). Table III shows the clinical outcomes after untested and euploid SET according to day of full-blastulation and blastocyst morphological quality pre-vitrification. The impact of a low morphological quality upon this outcome is even more evident. Poor-quality untested and euploid blastocysts showed an overall live birth rate as low as 2.1% (N = 1/48) and 7.3% (N = 5/68), respectively. In general, also the impact of a slow development to the blastocyst stage was very relevant upon this outcome with Day 7 untested and euploid blastocysts resulting in an overall live birth rate of 8.3% (N = 3/36) and 15.1% (N = 11/73), respectively.
Live birth rates clustered after either untested or euploid single embryo transfer (SET) per blastocyst morphology pre-vitrification and day of full development.
| Blastocyst features pre-vitrification . | Untested blastocyst SET . | Euploid blastocyst SET . | |
|---|---|---|---|
| Day 5 | Excellent | 129/396, 32.6% | 203/384, 52.9% |
| Good | 16/65, 25% | 8/23, 35% | |
| Average | 10/47, 21% | 2/17, 12% | |
| Poor | 1/3, 33% | 1/6, 17% | |
| Day 6 | Excellent | 99/340, 29.1% | 133/309, 43.0% |
| Good | 27/103, 26.2% | 20/60, 33% | |
| Average | 14/117, 12% | 11/52, 21% | |
| Poor | 0/39, 0% | 1/38, 3% | |
| Day 7 | Excellent | 1/11, 9% | 4/25, 16% |
| Good | 1/6, 17% | 2/11, 18% | |
| Average | 1/13, 8% | 2/13, 15% | |
| Poor | 0/6, 0% | 3/24, 13% | |
| Total | 299/1146, 26.1% | 390/962, 40.5% | |
| Blastocyst features pre-vitrification . | Untested blastocyst SET . | Euploid blastocyst SET . | |
|---|---|---|---|
| Day 5 | Excellent | 129/396, 32.6% | 203/384, 52.9% |
| Good | 16/65, 25% | 8/23, 35% | |
| Average | 10/47, 21% | 2/17, 12% | |
| Poor | 1/3, 33% | 1/6, 17% | |
| Day 6 | Excellent | 99/340, 29.1% | 133/309, 43.0% |
| Good | 27/103, 26.2% | 20/60, 33% | |
| Average | 14/117, 12% | 11/52, 21% | |
| Poor | 0/39, 0% | 1/38, 3% | |
| Day 7 | Excellent | 1/11, 9% | 4/25, 16% |
| Good | 1/6, 17% | 2/11, 18% | |
| Average | 1/13, 8% | 2/13, 15% | |
| Poor | 0/6, 0% | 3/24, 13% | |
| Total | 299/1146, 26.1% | 390/962, 40.5% | |
Live birth rates clustered after either untested or euploid single embryo transfer (SET) per blastocyst morphology pre-vitrification and day of full development.
| Blastocyst features pre-vitrification . | Untested blastocyst SET . | Euploid blastocyst SET . | |
|---|---|---|---|
| Day 5 | Excellent | 129/396, 32.6% | 203/384, 52.9% |
| Good | 16/65, 25% | 8/23, 35% | |
| Average | 10/47, 21% | 2/17, 12% | |
| Poor | 1/3, 33% | 1/6, 17% | |
| Day 6 | Excellent | 99/340, 29.1% | 133/309, 43.0% |
| Good | 27/103, 26.2% | 20/60, 33% | |
| Average | 14/117, 12% | 11/52, 21% | |
| Poor | 0/39, 0% | 1/38, 3% | |
| Day 7 | Excellent | 1/11, 9% | 4/25, 16% |
| Good | 1/6, 17% | 2/11, 18% | |
| Average | 1/13, 8% | 2/13, 15% | |
| Poor | 0/6, 0% | 3/24, 13% | |
| Total | 299/1146, 26.1% | 390/962, 40.5% | |
| Blastocyst features pre-vitrification . | Untested blastocyst SET . | Euploid blastocyst SET . | |
|---|---|---|---|
| Day 5 | Excellent | 129/396, 32.6% | 203/384, 52.9% |
| Good | 16/65, 25% | 8/23, 35% | |
| Average | 10/47, 21% | 2/17, 12% | |
| Poor | 1/3, 33% | 1/6, 17% | |
| Day 6 | Excellent | 99/340, 29.1% | 133/309, 43.0% |
| Good | 27/103, 26.2% | 20/60, 33% | |
| Average | 14/117, 12% | 11/52, 21% | |
| Poor | 0/39, 0% | 1/38, 3% | |
| Day 7 | Excellent | 1/11, 9% | 4/25, 16% |
| Good | 1/6, 17% | 2/11, 18% | |
| Average | 1/13, 8% | 2/13, 15% | |
| Poor | 0/6, 0% | 3/24, 13% | |
| Total | 299/1146, 26.1% | 390/962, 40.5% | |
Discussion
Since 2013, when vitrification was acknowledged as a standardized clinical technique (Practice Committees of American Society for Reproductive and Society for Assisted Reproductive, 2013), its implementation and application worldwide has increased. In this study, in a clinical scenario based on a freeze-all and SET policy (with or without aneuploidy testing), we investigated which factors might influence blastocyst cryo-survival after vitrification, as well as its post-warming behavior in terms of re-expansion and competence (i.e. live birth).
We inspected all consecutive warming cycles performed to conduct either an untested or an euploid blastocyst SET. According to the Italian Law (Law 40/2004), that does not allow IVF centers to discard embryos unless proven fully degenerated, all survived blastocysts, even those showing partial areas of degeneration, were transferred independently from their morphological quality and re-expansion level. The overall survival rate after warming was 99%, thus, confirming the high reliability of vitrification (Rienzi et al., 2017). No aspect related with the IVF laboratory, including the use of different kits or the involvement of different operators in the vitrification and/or warming procedures showed any association with blastocyst survival after warming. Especially trophectoderm biopsy did not impair blastocyst post-warming survival and behavior. Conversely, a lower blastocyst post-warming survival was reported associated with a worse morphological quality pre-vitrification and a slower embryo developmental rate. Most importantly, blastocyst artificial shrinkage, both laser-assisted or biopsy-induced, resulted in significantly higher survival rates (99.3 and 99.4%, respectively) than untested blastocysts which were vitrified when still expanded (97.2%), even when correcting for all putative confounders. Besides adding further evidence in favor of the safety of trophectoderm biopsy approach (Scott et al., 2013; Cimadomo et al., 2016), these evidences suggest that the blastocysts benefit from being collapsed before vitrification. To this regard, some previous studies already claimed that such practice is beneficial to limit the degeneration of untested blastocysts after warming. For instance, Mukaida and colleagues reported that micro-needle- and laser-based artificial shrinkage methods, both resulted into higher blastocyst cryo-survival to vitrification than the control (97.2 versus 86%) (Mukaida et al., 2006). An evidence confirmed by Van Landuyt et al. (2015) in a recent randomized controlled trial, where only 2% of total post-warming degeneration was reported when artificial shrinkage was performed, a rate that increased up to 8% in its absence. Our data further strengthen this previously reported evidence and support both the practices of conducting artificial shrinkage during non-PGT cycles and of preventing the re-expansion of a biopsied blastocyst before vitrification. The evidence of a lower cryo-survival rate as the blastocyst quality decreased, as well as the days of culture increased, has been also reported previously (Desai et al., 2016). Possibly, a worse morphological quality along with a longer time to reach full blastulation might subtend more complex indiscernible insults to embryo competence, indirectly requiring a higher energetical expense and metabolic activity. Such scenario might translate in lower energies to invest in embryo cryo-survival, re-expansion after warming as well as implantation: a speculation which well-matches with the ‘quiet embryo hypothesis’, according to which competent embryos are less metabolically active (therefore, more energetically fit) than incompetent ones (Baumann et al., 2007; Leese et al., 2007).
Post-warming re-expansion was not correlated to either embryo artificial shrinkage or trophectoderm biopsy pre-vitrification. On the contrary, blastocyst expansion grade before cryopreservation was highly predictive of this outcome, even if corrected for morphological quality and day of full blastulation (i.e. the only other significant predictors). Precisely, the risk to not re-expand within 1.5 h post-warming was higher for blastocysts hatching or already fully hatched pre-vitrification. Such evidence suggests that possibly the more the volume of the blastocoelic fluid filling the inner cavity of the blastocyst, the higher the risk for putative issues related with its post-warming behavior. Indeed, both the higher content of fluid in this reservoir and the presence of a large opening/total absence of the zona pellucida might modify the processes of dehydration and permeation to cryoprotectants, as already suggested in previous studies (Vanderzwalmen et al., 2002; Mukaida et al., 2006; Ebner et al., 2009). Possibly, the absence of the zona pellucida facilitates the penetration of the cryoprotectant in the cells, which might then require longer after warming to fully expel it and re-acquire their physiological competence. Such speculation might explain also why no correlation was found between the expansion grade pre-vitrification and blastocyst degeneration or live birth rates. Future studies should investigate whether different re-expansion patterns would derive if hatching and fully hatched blastocysts are either exposed to shorter incubation timings in the VS or scored beyond 1.5 h after warming.
Laser-assisted or biopsy-induced artificial shrinkage and/or vitrification post-biopsy conducted within 30 min may not be common practices in many IVF laboratories worldwide. Indeed, very good survival rates might be achieved even in their absence, as reported in this study (>97%), as well as in other previous reports in literature (Cobo et al., 2012; Roy et al., 2014; Chen et al., 2017). Nonetheless, here we demonstrated that these practices are not harmful and might be even beneficial for the blastocysts that need to be vitrified by expert operators. Laser-assisted artificial shrinkage for instance is fast and inexpensive (provided that the IVF laboratory already has a laser-equipped micromanipulator in-house), can be conducted few minutes before vitrification and requires a short training of the operators. Similarly, vitrifying a biopsied blastocyst when it is still collapsed is feasible simply by organizing the workflow so that the vitrification operator would be prepared to perform the procedure soon after the first daily biopsy has been finalized, and then continue vitrifying according to the same order followed by the biopsy operator (clearly the two operators must be different). Here, for the sake of reproducibility among the centers and operators, we set 30 min as deadline; yet, this threshold can be extended. For instance, Chen et al. (2017) reported in their detailed investigation published in 2017, that in more than 96% of cases a blastocyst takes ≥1.5 h to re-expand after trophectoderm biopsy: a time compatible also with the workload of a busy IVF laboratory wishing to prevent biopsied blastocysts to re-expand before vitrification is performed.
When post-warming blastocyst behavior (i.e. degeneration and re-expansion grades) and morphological quality were scored, they showed a correlation with embryo reproductive potential, as already reported previously in different studies (Goto et al., 2011; Desai et al., 2016; Du et al., 2016; Coello et al., 2017). However, for both untested and euploid blastocyst SETs, if corrected for pre-warming parameters, their predictive power became negligible. In other terms, blastocyst post-warming reproductive competence might be estimated based on its pre-vitrification characteristics; namely, morphological quality and day of full blastulation. Conversely, blastocyst expansion grade pre-vitrification (i.e. non-expanded, fully expanded, hatching or fully hatched) did not show any correlation with the achievement of a live birth. Furthermore, neither the specific sequential number of vitrified-warmed SET conducted per patient, when evaluated in a multivariate logistic regression analysis, showed a significant correlation with a successful outcome. In other words, a putative worse outcome in consecutive vitrified-warmed SETs conducted by a patient in the same IVF cycle is again mainly imputable to a poorer morphology and/or slower development of the blastocysts transferred as last of the cohort of embryos produced, rather than to some patient-related features. Yet, this is an intriguing topic that clearly demands further specific investigations. Lastly, maternal age clearly showed an impact upon blastocyst implantation potential only in non-PGT cycles, while it did not correlate with the competence of euploid blastocysts.
The issue of a putative impact of trophectoderm biopsy and vitrification upon blastocyst cryo-survival and competence has been retrospectively assessed previously by other authors with different study designs, procedural strategies, protocols and aims with respect to our investigation. For instance, Bradley et al. reported a decrease in the cryo-survival rate, as well as in the clinical outcomes related with the increasing manipulations of the embryos: vitrified and biopsied once (N = 2130 SETs), vitrified twice and biopsied once (N = 34 SETs), or biopsied and vitrified twice (N = 29 SETs). However, the morphological quality of the blastocysts subject to more manipulations, as well as their day of full blastulation were significantly different (i.e. poorer quality and slower development) from the control group, possibly being themselves responsible for their lower competence (Bradley et al., 2017). A speculation that is supported from our study, where quality and day of full-blastulation were crucial for both a positive cryo-survival and live birth. Indeed, Taylor et al., whose study design entailed the exclusion of poor-quality blastocysts (i.e. <BB according to Gardner and Schoolcraft’s parameters), did not find any difference in the same three groups of blastocysts clustered as in Bradley’s study; they just reported a lower cryo-survival rate for embryos cryopreserved twice and biopsied once, that was imputed to the use of slow-freezing protocol for their first cryopreservation. Nonetheless, the sample size in Taylor’s study, to our knowledge the first to have the merit of shedding light upon this important topic in current IVF, was still limited to give definite indications, especially dealing with the reproductive competence of the blastocysts under investigation (e.g. also double ETs were included and the investigation was overall based upon only 101 ETs). Interestingly, the previously cited study by Chen et al. (2017) reported evidences apparently opposite to ours: the blastocysts re-expanding and being vitrified ≥3 h after trophectoderm biopsy showed the highest chance to implant after warming and transfer. Nonetheless, some points should be taken into account when dealing with Chen’s study: (i) it is a single operator-based retrospective study, (ii) it excluded poor-quality embryos from the analysis, (iii) it included both SETs and double ETs and (iv) only re-expanded blastocysts with >80% of intact cells post-warming were transferred. Therefore, in our opinion, Chen’s paper mainly unveiled a selection parameter useful for IVF centers performing fresh ET after PGT, rather than providing a procedural hint for IVF clinics adopting a freeze-all approach after trophectoderm biopsy. Specifically, the blastocysts pre-vitrification behavior noted by Chen and colleagues and reported as significantly correlated with their implantation potential was not corrected for their post-warming behavior, therefore it cannot be excluded that the latter might have provided the same predictive power as the former. In other terms, to perform vitrification ≥3 h from biopsy when the blastocyst is hopefully re-expanded, besides being time-consuming might also be dispensable. However, targeted studies are required to reach a clear indication related to this issue.
A last important evidence of this study is that, even though poor-quality and/or slow-developing blastocysts were associated with good cryo-survival rates, they resulted in live birth rates per SET ≤15%, also when euploid embryos were transferred. This outcome makes questionable the application of SET when only poor-quality embryos are produced.
Conclusion
In this study we further confirmed vitrification as an efficient cryopreservation protocol which involves a high blastocyst survival after warming (99%). Importantly, no feature related with the daily practice of the IVF laboratory correlated with blastocyst post-warming behavior. This evidence supports that, if high standards are guaranteed, a putative impact of the in vitro culture conditions and IVF-related manipulations might be largely limited. Artificial shrinkage, either laser-assisted or induced after trophectoderm biopsy, positively correlates with blastocyst cryo-survival and, in general, a vitrification-warming cycle seems to be better-tolerated from a collapsed blastocyst.
No correlation exists between blastocyst expansion grade pre-vitrification and its chance to result in a live birth. Conversely, blastocyst post-warming behavior and quality correlate with its reproductive competence; nonetheless this predictive power becomes negligible when corrected for pre-vitrification features. Lastly, here we highlight that poor-quality and slow-developing blastocysts, even if euploid, have a significantly lower chance to result in a live birth. Standing this evidence, double-ET might be discussed with the couple as a putative option when only these embryos are available for a vitrified-warmed transfer.
Authors’ roles
D.C., A.C. and L.R. designed the study. D.C., D.S. and G.O. analyzed the data and drafted the article. All authors contributed to the collection, interpretation and discussion of the data.
Conflict of interest
The authors have no conflict of interest related to this study.
Study funding
None.



