-
PDF
- Split View
-
Views
-
Cite
Cite
Rebecca Deans, Thierry Vancaillie, William Ledger, Jinzhu Liu, Jason A Abbott, Live birth rate and obstetric complications following the hysteroscopic management of intrauterine adhesions including Asherman syndrome, Human Reproduction, Volume 33, Issue 10, October 2018, Pages 1847–1853, https://doi.org/10.1093/humrep/dey237
Close - Share Icon Share
Abstract
What are the live birth rate and risks of obstetric complications following the surgical management of intrauterine adhesions (IUA) such as Asherman syndrome (AS)?
The live birth rate is 63.7%, and obstetric complications including placentation issues, prematurity and postpartum hysterectomy require that pregnancies in women after treatment for IUA should be considered moderate to high risk.
Studies reviewing short-term surgical, menstrual and fertility outcomes following hysteroscopic management are reassuring, with success correlated to the severity of IUA. There are limited data reporting live birth, neonatal and maternal complications.
This retrospective study included all women treated for IUA by hysteroscopic synechiolysis under fluoroscopic guidance in two tertiary University-affiliated hospitals. All women reported at least one pre-treatment symptom including menstrual dysfunction, subfertility or pelvic pain and intended to become pregnant post-treatment. Survival curve analysis was performed for time to pregnancy, and obstetric data were collated from a National Obstetric Database for delivery and neonatal outcomes.
A total of 154 women were included in the study. Surgical intervention involved hysteroscopic synechiolysis under fluoroscopic guidance until cavity restoration was confirmed. Questionnaires regarding fertility and its outcomes were sent to all women undergoing surgery, with analysis of menstrual, fertility rates and outcomes of those pregnancies including risks and complications to the woman and the offspring.
Women were followed up for a minimum of 1 year (range: 1–14 years) from index surgery. The chance of pregnancy was 98/124 (79.0% CI: 63.6, 83.1%) in women wishing to conceive and the chance of a live birth was 79/124 (63.7% CI: 51.3, 70.7%). The chance of a miscarriage was 29/124 (23.4% CI: 18.8, 37.1%). There were 93 live births in 79 women following surgery, with detailed obstetric data available for 85 of these births. They were complicated by abnormal placentation in 15/85 (17.6% CI: 13.0, 30.2%), postpartum hysterectomy in 4/85 (4.7% CI: −0.4, 7.0%), and prematurity in 25/85 (29.4% CI: 17.0, 35.3%) women.
The retrospective nature of the study and extended follow-up time may cause selection and recall bias, however, pregnancy and its outcomes—particularly in women with problems of subfertility—are frequently key milestones, with birthdates readily recalled. Menstrual outcomes are more likely to be subject to recall bias.
Our surgical data are similar to the published literature with reassuring short-term outcomes for menstruation and cavity reconstruction following surgery for IUA. Long-term outcomes including pregnancy rates were higher than published data, however, the obstetric and neonatal complication rates were increased, indicating a continuation of risk beyond infertility and into pregnancy. An altered biochemical or vascular environment is a possible explanation for impaired implantation resulting in poorer reproductive obstetric and neonatal outcomes. The relative rarity of IUA—particularly severe disease—makes prospective data collection difficult. Our data suggest that women with IUA should be treated as moderate—high risk obstetric patients in subsequent pregnancy and counselled appropriately.
No funding and no competing interests.
Introduction
The presence of intrauterine adhesions (IUA) following interventions during any type of pregnancy or intrauterine surgery in the non-gravid uterus is known to impact subsequent pregnancy (Roge et al., 1996; Feng et al., 1999; Thomson et al., 2007; Yu et al., 2008a; Roy et al., 2010). The eponymous Asherman syndrome (AS) is defined as partial or complete obstruction of the uterine cavity by adhesions secondary to trauma of the corpus, with resulting menstrual abnormalities, cyclical lower abdominal pain, infertility, or recurrent pregnancy loss (Asherman, 1950). Contemporary gynaecological surgery incorporates a wide variety of hysteroscopic procedures—both simple and complex—that may also result in IUA, with strong evidence that these too impact subsequent pregnancy (Pelage et al., 2000; Robinson et al., 2008; Sentilhes et al., 2010b; Fernandez et al., 2012; Myers and Hurst, 2012; Xiao et al., 2014).
Clinical studies investigating surgical outcomes of IUAs report favourable menstrual and anatomic restoration, however, repeat surgery or re-formation of IUAs occurs in close to 1/3 of women despite successful initial surgery and subsequent supplementary measures such as adhesion barriers (Hanstede et al., 2015). It is reported that increasing severity of disease is associated with poorer clinical outcomes (Fedele et al., 1986; Pabuccu et al., 1997) and a higher chance of repeat surgery required. Post-operative reproductive function is poorer than menstrual or other clinical outcomes, with pregnancy rates reported to range from 44 to 93% (Yu et al., 2008b; March, 2011). The impact on pregnancy following surgical treatment of IUA is likely related to a deficiency in the residual endometrium (Polishuk et al., 1977; Lo et al., 2008) and myometrial fibroisis that may decrease vascular delivery of steroids to the endometrial tissue (Yaffe et al., 1978) or create an altered endometrial biochemical milieu, affecting implantation (Steinbrech et al., 1999). The objective of this study is to report the live birth rate and other fertility, obstetric and neonatal outcomes following hysteroscopic synechiolysis in women treated for IUAs over a 14-year duration in two referral centres.
Materials and Methods
Ethical approval
Ethics approval for the study was granted by the SESLHD Ethics committee (approval number 07/207 24) to undertake a retrospective study for women treated surgically for IUAs between January 2000 and June 2014 at two tertiary referral hospitals in Sydney, Australia.
Study design
Inclusion criteria were symptomatic women aged between 18 and 51 years at index surgery with the majority wishing further fertility; IUAs diagnosed by hysteroscopy; English speaking or with an adequate interpreter available; and capable of providing informed consent. The exclusion criteria included suspected diagnosis of gynaecological malignancy or its precursors; postmenopausal status at the time of surgery; pre-existing tuberculosis; and no IUAs seen at hysteroscopic surgery.
Previous medical or surgical treatment for IUA was not a contra-indication to entry into the study. Surgery was undertaken by, or under the supervision of two consultant gynaecologists with experience in synechiolysis of IUA using a surgical technique previously described (Thomson et al., 2007). Briefly, this was undertaken with image intensifier control with fibrous adhesions lysed using a Tuouy needle parallel to the hysteroscope, or with the aid of hysteroscopic scissors without electrosurgery. This procedure may be subsequently repeated until the normal anatomy of the uterine cavity is restored in accordance with recommended guidelines (AAGL, 2013). As the surgeons were known to have expertise in the surgical division of IUA, with each having >10 years’ experience in surgery for IUA, referral included a number of women who were considered to have inoperable disease and had failed treatment elsewhere.
The location, extent and type of adhesions were recorded and classified according to the European Society of Hysteroscopy (ESH) classification system (Wamsteker, 1984). Following surgery, women received only conjugated equine oestrogen 2.5 mg/day (Ayerst, Wyeth Australia Pty Ltd., Baulkam Hills, NSW, Australia) for 21 of 28 days, and were discharged the day of surgery. Clinical review was scheduled 6–8 weeks following the index procedure, and repeat hysteroscopy and adhesiolysis was performed if symptoms persisted until there was complete restoration of the endometrial cavity.
A questionnaire was used to collect data, with women asked to recall their menstrual, pain and fertility symptoms prior to, following treatment, and presently. The primary outcome of the study was to determine live birth rate, with other fertility, obstetric and neonatal data recorded. Obstetric outcomes were determined by a 14-year follow-up questionnaire and audit of all women included in a National hospitals’ database. Surgical outcomes including clinical symptoms, the number of procedures and procedural complications are reported secondarily.
Statistical analyses
Comparisons pre- and post-treatment were undertaken using the independent samples t-test and its non-parametric equivalent where appropriate, the latter being used where data did not satisfy the assumption of equal variances (Kolgarov–Smirnov test). Association of dichotomous measures between the groups were assessed utilizing the Chi-squared test, with Fisher’s exact test used as appropriate due to the small sample sizes. Continuous variables were assessed using the Student t-test. P-values are two tailed, unless otherwise indicated, and considered statistically significant if <0.05. The time to pregnancy was assessed using the inverse Kaplan Meier survival curve. Confidence intervals are reported by intention to treat analysis.
Results
For the 14-year study duration, 176 women were identified from the departmental database as having surgery for IUAs, with 154/176 (87.5%), meeting inclusion and exclusion criteria, and having adequate information available for data extraction. Questionnaires were received from 131/154 (85.1%) of these women, whose demographic data, cause of IUAs and ESH grade of adhesions are documented in Table I. The 10 of the 154 women had concurrent laparoscopy to investigate coexisting pathology for pelvic pain and infertility, with 6/154 (3.9%) having biopsy proven endometriosis and all macroscopic disease removed at this procedure, 1/154 woman (0.6%) had pelvic adhesions divided. A total of 259 hysteroscopic procedures were performed in the 154 women and complications are reported per procedure. There were 8/259 (3.1%) complications including maximum fluid deficit reached in 2/259 (0.8%), excessive surgical time in 1/259 (0.4%), uterine perforation 3/259 (1.2%) and infection in 2/259 (0.8%). In all cases where perforation occurred, this was recognized contemporaneously by the passage of radio-opaque contrast into the peritoneal cavity with no sequelae noted after antibiotic administration and observation. There were no serious adverse surgical events recorded.
| . | Median (range) . |
|---|---|
| Age (n = 154) | 36 (19–51) |
| Gravidity (n = 142) | 5 (0–10) |
| Parity (n = 91) | 1 (0–3) |
| Previous pregnancies | |
| Miscarriage (n = 82) | 2 (1–3) |
| Vaginal delivery (n = 60) | 1 (1–4) |
| Caesarean delivery (n = 31) | 1 (1–2) |
| Cause of IUA | N (%) |
| Trimester 1 | |
| Curettage following miscarriage | 71(46.1) |
| Curettage post termination of pregnancy | 7 (4.5) |
| Curettage during ectopic pregnancy treatment | 2 (1.3) |
| Curettage for molar pregnancy | 1 (0.6) |
| Trimester 2 | |
| Curettage post second trimester miscarriage | 1 (0.6) |
| Postpartum causes | |
| Curettage postpartum once | 43 (27.9) |
| Curettage postpartum twice | 5 (3.2) |
| Caesarean delivery | 9 (5.8) |
| Non pregnant causes | |
| Curettage whilst not pregnant | 6 (3.9) |
| Cone biopsy for pre-cancer/cancer | 2 (1.3) |
| Hysteroscopic myomectomy | 2 (1.3) |
| Unknown/unrecorded causes | 5 (3.2) |
| Grade of IUA at index surgery | N (%) |
| Grade I | 32 (20.8) |
| Grade II | 52 (33.8) |
| Grade III | 41 (26.6) |
| Grade IV | 26 (16.9) |
| Unknown/not recorded | 3 (1.9) |
| . | Median (range) . |
|---|---|
| Age (n = 154) | 36 (19–51) |
| Gravidity (n = 142) | 5 (0–10) |
| Parity (n = 91) | 1 (0–3) |
| Previous pregnancies | |
| Miscarriage (n = 82) | 2 (1–3) |
| Vaginal delivery (n = 60) | 1 (1–4) |
| Caesarean delivery (n = 31) | 1 (1–2) |
| Cause of IUA | N (%) |
| Trimester 1 | |
| Curettage following miscarriage | 71(46.1) |
| Curettage post termination of pregnancy | 7 (4.5) |
| Curettage during ectopic pregnancy treatment | 2 (1.3) |
| Curettage for molar pregnancy | 1 (0.6) |
| Trimester 2 | |
| Curettage post second trimester miscarriage | 1 (0.6) |
| Postpartum causes | |
| Curettage postpartum once | 43 (27.9) |
| Curettage postpartum twice | 5 (3.2) |
| Caesarean delivery | 9 (5.8) |
| Non pregnant causes | |
| Curettage whilst not pregnant | 6 (3.9) |
| Cone biopsy for pre-cancer/cancer | 2 (1.3) |
| Hysteroscopic myomectomy | 2 (1.3) |
| Unknown/unrecorded causes | 5 (3.2) |
| Grade of IUA at index surgery | N (%) |
| Grade I | 32 (20.8) |
| Grade II | 52 (33.8) |
| Grade III | 41 (26.6) |
| Grade IV | 26 (16.9) |
| Unknown/not recorded | 3 (1.9) |
| . | Median (range) . |
|---|---|
| Age (n = 154) | 36 (19–51) |
| Gravidity (n = 142) | 5 (0–10) |
| Parity (n = 91) | 1 (0–3) |
| Previous pregnancies | |
| Miscarriage (n = 82) | 2 (1–3) |
| Vaginal delivery (n = 60) | 1 (1–4) |
| Caesarean delivery (n = 31) | 1 (1–2) |
| Cause of IUA | N (%) |
| Trimester 1 | |
| Curettage following miscarriage | 71(46.1) |
| Curettage post termination of pregnancy | 7 (4.5) |
| Curettage during ectopic pregnancy treatment | 2 (1.3) |
| Curettage for molar pregnancy | 1 (0.6) |
| Trimester 2 | |
| Curettage post second trimester miscarriage | 1 (0.6) |
| Postpartum causes | |
| Curettage postpartum once | 43 (27.9) |
| Curettage postpartum twice | 5 (3.2) |
| Caesarean delivery | 9 (5.8) |
| Non pregnant causes | |
| Curettage whilst not pregnant | 6 (3.9) |
| Cone biopsy for pre-cancer/cancer | 2 (1.3) |
| Hysteroscopic myomectomy | 2 (1.3) |
| Unknown/unrecorded causes | 5 (3.2) |
| Grade of IUA at index surgery | N (%) |
| Grade I | 32 (20.8) |
| Grade II | 52 (33.8) |
| Grade III | 41 (26.6) |
| Grade IV | 26 (16.9) |
| Unknown/not recorded | 3 (1.9) |
| . | Median (range) . |
|---|---|
| Age (n = 154) | 36 (19–51) |
| Gravidity (n = 142) | 5 (0–10) |
| Parity (n = 91) | 1 (0–3) |
| Previous pregnancies | |
| Miscarriage (n = 82) | 2 (1–3) |
| Vaginal delivery (n = 60) | 1 (1–4) |
| Caesarean delivery (n = 31) | 1 (1–2) |
| Cause of IUA | N (%) |
| Trimester 1 | |
| Curettage following miscarriage | 71(46.1) |
| Curettage post termination of pregnancy | 7 (4.5) |
| Curettage during ectopic pregnancy treatment | 2 (1.3) |
| Curettage for molar pregnancy | 1 (0.6) |
| Trimester 2 | |
| Curettage post second trimester miscarriage | 1 (0.6) |
| Postpartum causes | |
| Curettage postpartum once | 43 (27.9) |
| Curettage postpartum twice | 5 (3.2) |
| Caesarean delivery | 9 (5.8) |
| Non pregnant causes | |
| Curettage whilst not pregnant | 6 (3.9) |
| Cone biopsy for pre-cancer/cancer | 2 (1.3) |
| Hysteroscopic myomectomy | 2 (1.3) |
| Unknown/unrecorded causes | 5 (3.2) |
| Grade of IUA at index surgery | N (%) |
| Grade I | 32 (20.8) |
| Grade II | 52 (33.8) |
| Grade III | 41 (26.6) |
| Grade IV | 26 (16.9) |
| Unknown/not recorded | 3 (1.9) |
Fertility outcomes
Post-operatively, only 124/154 (80.5%) women in the cohort tried to become pregnant, and fertility outcomes are reported per woman attempting pregnancy. A total of 98/124 (79.0% CI: 63.6–83.1%) achieved at least one pregnancy, with 79/124 (63.7% CI: 51.3–70.7%) women having at least one livebirth and 29/124 (23.4% CI: 18.8–37.1%) having miscarriages. Pregnancy following expectant management occurred in 78/124 (62.9% CI: 50.6–70.1%), with 40/124 (32.3% CI: 25.9–45.5%) requiring assistance to become pregnant and 6/124 (4.8%) women did not report how they became pregnant. Of the 40 women having any medical assistance to achieve pregnancy, 34/40 (85.0%) had IVF/ICSI (with two couples requiring ICSI for male factor subfertility), 2/40 (5.0%) IUI, 1/40 (2.5%) had ovulation induction with clomiphene citrate, 2/40 (5.0%) women used a donor oocyte, and 1/40 (2.5%) the type of assistance was not recorded. Additional treatments utilized to augment endometrial thickness in this group included 25 µg oestradiol patch in 5/40 (12.5%), and aspirin in 1/40 (2.5%) women. The time to become pregnant is demonstrated in Fig. 1. Of the 98 women who achieved pregnancy; 21/98 (21.4%) were grade I IUAs, 36/98 (36.7%) were grade II, 27/98 (27.6%) were grade III, 13/98 (13.3%) were grade IV, and in 1/98 (1.0%) the grade of adhesions was not recorded.
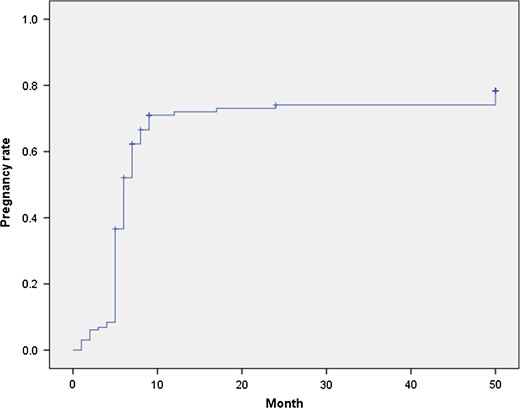
Time to pregnancy. Represents the rate of pregnancy for the cohort according to the time taken to achieve pregnancy in months following surgical management of IUA.
Obstetric outcomes
Overall, there were 93 livebirths in 79 women, with 1 woman reporting an unplanned pregnancy. These women collectively had 157 pregnancies subsequent to index surgery. Figure 2 illustrates the outcomes of all pregnancies. Overall, 66 women had 1 livebirth, 12 women had 2 live births and 1 woman had 3 live births. Detailed obstetric and neonatal outcomes were reported for 71/79 women, and these are shown in Tables II and III. There was one set of twins, accounting for the 72 complete datasets of neonatal outcomes.
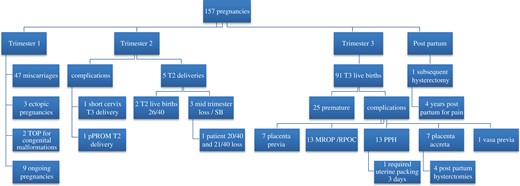
Pregnancy outcomes. Summary of the outcomes for the pregnancies that were reported in the cohort according to trimester that these occurred. T1 = first trimester; T2 = second trimester; T3 = third trimester; TOP = termination of pregnancy; SB = stillbirth; PPH = postpartum haemorrhage; MROP = manual removal of placenta; RPOC = retained products of conception.
Obstetric outcomes from live births with complete datasets in women treated for IUAs.
| . | First live birth following surgery, n (% of total births) . | Second live birth following surgery, n (% of total births) . | Third live birth following surgery, n (% of total births) . | Overall, n (% of total births) . |
|---|---|---|---|---|
| Number of births | 71* (83.5) | 13 (15.3) | 1 (1.2) | 85 (100) |
| Caesarean delivery^ | 49 (69.0) | 8 (61.5) | 1 (100) | 58 (68.2) |
| Antepartum bleeding^ | 2 (2.4) | 0 (0) | 0 (0) | 2 (2.4) |
| Placenta previa^ | 6 (8.5) | 1 (7.7) | 0 (0) | 7 (8.2) |
| Placenta acreta^ | 6 (8.5) | 0 (0) | 1 (100) | 7 (8.2) |
| Vasa previa^ | 1 (1.4) | 0 (0) | 0 (0) | 1 (1.2) |
| Manual removal of placenta^ | 12 (16.9) | 1 (7.7) | 0 (0) | 13 (15.3) |
| Postpartum haemorrhage^ | 12 (16.9) | 1 (7.7) | 0 (0) | 13 (15.3) |
| Blood transfusion^ | 3 (4.2) | 1 (7.7) | 0 (0) | 4 (4.7) |
| Prolonged postpartum bleeding >6 weeks^ | 1 (1.4) | 0 (0) | 0 (0) | 1 (1.2) |
| Postpartum hysterectomy^ | 3 (4.2) | 1 (7.7) | 0 (0) | 4 (4.7) |
| . | First live birth following surgery, n (% of total births) . | Second live birth following surgery, n (% of total births) . | Third live birth following surgery, n (% of total births) . | Overall, n (% of total births) . |
|---|---|---|---|---|
| Number of births | 71* (83.5) | 13 (15.3) | 1 (1.2) | 85 (100) |
| Caesarean delivery^ | 49 (69.0) | 8 (61.5) | 1 (100) | 58 (68.2) |
| Antepartum bleeding^ | 2 (2.4) | 0 (0) | 0 (0) | 2 (2.4) |
| Placenta previa^ | 6 (8.5) | 1 (7.7) | 0 (0) | 7 (8.2) |
| Placenta acreta^ | 6 (8.5) | 0 (0) | 1 (100) | 7 (8.2) |
| Vasa previa^ | 1 (1.4) | 0 (0) | 0 (0) | 1 (1.2) |
| Manual removal of placenta^ | 12 (16.9) | 1 (7.7) | 0 (0) | 13 (15.3) |
| Postpartum haemorrhage^ | 12 (16.9) | 1 (7.7) | 0 (0) | 13 (15.3) |
| Blood transfusion^ | 3 (4.2) | 1 (7.7) | 0 (0) | 4 (4.7) |
| Prolonged postpartum bleeding >6 weeks^ | 1 (1.4) | 0 (0) | 0 (0) | 1 (1.2) |
| Postpartum hysterectomy^ | 3 (4.2) | 1 (7.7) | 0 (0) | 4 (4.7) |
Obstetric outcomes were only available for 71/79 women who achieved livebirth. ^Denotes the number of events as a proportion of first, second or third live births following index surgery.
Obstetric outcomes from live births with complete datasets in women treated for IUAs.
| . | First live birth following surgery, n (% of total births) . | Second live birth following surgery, n (% of total births) . | Third live birth following surgery, n (% of total births) . | Overall, n (% of total births) . |
|---|---|---|---|---|
| Number of births | 71* (83.5) | 13 (15.3) | 1 (1.2) | 85 (100) |
| Caesarean delivery^ | 49 (69.0) | 8 (61.5) | 1 (100) | 58 (68.2) |
| Antepartum bleeding^ | 2 (2.4) | 0 (0) | 0 (0) | 2 (2.4) |
| Placenta previa^ | 6 (8.5) | 1 (7.7) | 0 (0) | 7 (8.2) |
| Placenta acreta^ | 6 (8.5) | 0 (0) | 1 (100) | 7 (8.2) |
| Vasa previa^ | 1 (1.4) | 0 (0) | 0 (0) | 1 (1.2) |
| Manual removal of placenta^ | 12 (16.9) | 1 (7.7) | 0 (0) | 13 (15.3) |
| Postpartum haemorrhage^ | 12 (16.9) | 1 (7.7) | 0 (0) | 13 (15.3) |
| Blood transfusion^ | 3 (4.2) | 1 (7.7) | 0 (0) | 4 (4.7) |
| Prolonged postpartum bleeding >6 weeks^ | 1 (1.4) | 0 (0) | 0 (0) | 1 (1.2) |
| Postpartum hysterectomy^ | 3 (4.2) | 1 (7.7) | 0 (0) | 4 (4.7) |
| . | First live birth following surgery, n (% of total births) . | Second live birth following surgery, n (% of total births) . | Third live birth following surgery, n (% of total births) . | Overall, n (% of total births) . |
|---|---|---|---|---|
| Number of births | 71* (83.5) | 13 (15.3) | 1 (1.2) | 85 (100) |
| Caesarean delivery^ | 49 (69.0) | 8 (61.5) | 1 (100) | 58 (68.2) |
| Antepartum bleeding^ | 2 (2.4) | 0 (0) | 0 (0) | 2 (2.4) |
| Placenta previa^ | 6 (8.5) | 1 (7.7) | 0 (0) | 7 (8.2) |
| Placenta acreta^ | 6 (8.5) | 0 (0) | 1 (100) | 7 (8.2) |
| Vasa previa^ | 1 (1.4) | 0 (0) | 0 (0) | 1 (1.2) |
| Manual removal of placenta^ | 12 (16.9) | 1 (7.7) | 0 (0) | 13 (15.3) |
| Postpartum haemorrhage^ | 12 (16.9) | 1 (7.7) | 0 (0) | 13 (15.3) |
| Blood transfusion^ | 3 (4.2) | 1 (7.7) | 0 (0) | 4 (4.7) |
| Prolonged postpartum bleeding >6 weeks^ | 1 (1.4) | 0 (0) | 0 (0) | 1 (1.2) |
| Postpartum hysterectomy^ | 3 (4.2) | 1 (7.7) | 0 (0) | 4 (4.7) |
Obstetric outcomes were only available for 71/79 women who achieved livebirth. ^Denotes the number of events as a proportion of first, second or third live births following index surgery.
| . | First live birth following surgery . | Second live birth following surgery . | Third live birth following surgery . | Overall . |
|---|---|---|---|---|
| Number of babies, n (% of total babies) | 72* (83.7) | 13 (15.1) | 1 (1.2) | 86 (100) |
| Mean weight, n in kg (range) | 3.08 (0.9–4.35) | 3.13 (2–3.85) | 3.32# | 3.08 (0.9–4.35) |
| Preterm pre-labour rupture of membranes^, n (%) | 1 (1.4) | 0 (0) | 0 (0) | 1 (1.2) |
| Preterm birth <37 weeks gestation^, n (%) | 23 (31.9) | 2 (15.4) | 0 (0) | 25 (29.1) |
| Preterm birth <30 weeks gestation^, n (%) | 3 (4.2) | 0 (0) | 0 (0) | 3 (3.5) |
| Mean gestation of premature births weeks, n (range) | 31 (20–36) | 34# | – | 31 (20–36) |
| Neonatal deaths^, n (%) | 1 (1.4) | 1 (7.7) | 0 (0) | 2 (2.4) |
| . | First live birth following surgery . | Second live birth following surgery . | Third live birth following surgery . | Overall . |
|---|---|---|---|---|
| Number of babies, n (% of total babies) | 72* (83.7) | 13 (15.1) | 1 (1.2) | 86 (100) |
| Mean weight, n in kg (range) | 3.08 (0.9–4.35) | 3.13 (2–3.85) | 3.32# | 3.08 (0.9–4.35) |
| Preterm pre-labour rupture of membranes^, n (%) | 1 (1.4) | 0 (0) | 0 (0) | 1 (1.2) |
| Preterm birth <37 weeks gestation^, n (%) | 23 (31.9) | 2 (15.4) | 0 (0) | 25 (29.1) |
| Preterm birth <30 weeks gestation^, n (%) | 3 (4.2) | 0 (0) | 0 (0) | 3 (3.5) |
| Mean gestation of premature births weeks, n (range) | 31 (20–36) | 34# | – | 31 (20–36) |
| Neonatal deaths^, n (%) | 1 (1.4) | 1 (7.7) | 0 (0) | 2 (2.4) |
72 Neonatal outcomes are reported as one woman had a twin pregnancy.
^Denotes the number of events as a proportion of first, second or third births following index surgery.
#No range given as only one delivery.
| . | First live birth following surgery . | Second live birth following surgery . | Third live birth following surgery . | Overall . |
|---|---|---|---|---|
| Number of babies, n (% of total babies) | 72* (83.7) | 13 (15.1) | 1 (1.2) | 86 (100) |
| Mean weight, n in kg (range) | 3.08 (0.9–4.35) | 3.13 (2–3.85) | 3.32# | 3.08 (0.9–4.35) |
| Preterm pre-labour rupture of membranes^, n (%) | 1 (1.4) | 0 (0) | 0 (0) | 1 (1.2) |
| Preterm birth <37 weeks gestation^, n (%) | 23 (31.9) | 2 (15.4) | 0 (0) | 25 (29.1) |
| Preterm birth <30 weeks gestation^, n (%) | 3 (4.2) | 0 (0) | 0 (0) | 3 (3.5) |
| Mean gestation of premature births weeks, n (range) | 31 (20–36) | 34# | – | 31 (20–36) |
| Neonatal deaths^, n (%) | 1 (1.4) | 1 (7.7) | 0 (0) | 2 (2.4) |
| . | First live birth following surgery . | Second live birth following surgery . | Third live birth following surgery . | Overall . |
|---|---|---|---|---|
| Number of babies, n (% of total babies) | 72* (83.7) | 13 (15.1) | 1 (1.2) | 86 (100) |
| Mean weight, n in kg (range) | 3.08 (0.9–4.35) | 3.13 (2–3.85) | 3.32# | 3.08 (0.9–4.35) |
| Preterm pre-labour rupture of membranes^, n (%) | 1 (1.4) | 0 (0) | 0 (0) | 1 (1.2) |
| Preterm birth <37 weeks gestation^, n (%) | 23 (31.9) | 2 (15.4) | 0 (0) | 25 (29.1) |
| Preterm birth <30 weeks gestation^, n (%) | 3 (4.2) | 0 (0) | 0 (0) | 3 (3.5) |
| Mean gestation of premature births weeks, n (range) | 31 (20–36) | 34# | – | 31 (20–36) |
| Neonatal deaths^, n (%) | 1 (1.4) | 1 (7.7) | 0 (0) | 2 (2.4) |
72 Neonatal outcomes are reported as one woman had a twin pregnancy.
^Denotes the number of events as a proportion of first, second or third births following index surgery.
#No range given as only one delivery.
Clinical outcomes
Pre-operatively, 74/154 (48.1%) women were amenorrhoeic with a statistically non-significant increase in the number of women reporting menstruation post-operatively to 135/154 (87.7%), χ2 = 1.231, P = 0.746. Women with grade IV adhesions had a significantly higher rate of irregular bleeding pattern following surgery than the other grades 8/22 (36.4%) vs 16/108 (14.8%), χ2 = 21.108, P = 0.0001, and amenorrhoeic women were significantly more likely to have grade IV adhesions 15/25 (60.0%) vs 58/124 (46.8%), χ2 = 8.728, P = 0.033. Overall 4/154 women (2.6%) reported periods returning following surgery that subsequently ceased. Periods were not observable in 14 women, with 9 currently pregnant or breastfeeding, and 5 women having a hysterectomy (four postpartum and one due to dysmenorrhoea) following their index surgery. In 9/154 (5.8%), menstrual activity at the time of the questionnaire was not recorded.
There were no differences in the symptom of pain across the grades of IUA, χ2 = 1.761, P = 0.623. For women with dysmenorrhoea, the median duration of pain was 10 months (range: 1–96 months). Of the 61/154 (39.6%) women that had no pain before surgery, 3/61 (4.9%) developed de novo pain following surgery and 58/61 (95.1%) remained pain free. Of the 59/154 (38.3%) who reported pain prior to surgery, 29/59 (49.2%) had persistent pain, and 30/59 (50.8%) had no pain following surgery. In 34/154 (22.1%) women this was unknown. There were significantly fewer women who reported pain after (29/154, 18.8%), compared to before (59/154, 38.3%) surgery (χ2 = 23.520, P = 0.0001).
Discussion
For women with IUAs wishing to become pregnant, live birth rate is the only important clinical outcome, and from this series, 63.7% of women achieved this aim. Menstrual changes that include a change from amenorrhoea and ‘light periods’ to ‘normal’ periods have a more limited role as an outcome, since they do not necessarily correlate with the live birth rate. Indeed the amenorrhoea rate from this series was not statistically significantly improved, although a strong argument may be made for both a clinical improvement (from 48.1% pre-surgery to 87.7% post-surgery) and many women achieved live birth—the ultimate indicator of success. This important difference between clinical and statistical data should not be underestimated (Farland et al., 2016). Whilst the retrospective nature of this study may lead to recall bias, pregnancy outcomes, particularly live births are substantially less likely to be disremembered, whereas the pattern of menstruation and the degree of pain experienced is more subjective and are not the focus of our analyses.
We utilized a National obstetric database to cross-refer obstetric and neonatal outcomes and these data are important to consider when counselling women presenting with infertility and IUA. Despite a high chance of pregnancy success following index surgery in our data, these pregnancies were frequently complicated by placental related morbidity for the mother and offspring. Early data report few obstetric complications in women treated hysteroscopically for AS (Valle and Sciarra, 1988), although contemporary series with rigorous follow-up demonstrate compelling evidence for malplacentatation. These include the of risk of placenta accreta in a subsequent pregnancy to be increased at 2.5% (Roge et al., 1996; Protopapas et al., 1998; Feng et al., 1999; Nasr et al., 2000; Zikopoulos et al., 2004; March, 2008; Yu et al., 2008a) and our results suggest a trebling of this rate at 8.0% as well as placental related neonatal complications such as premature birth and low birth weight compared to the national database (AIHW, 2015). A higher baseline pregnancy rate achieved in our cohort may contribute to this skewing of the data, with women treated with more severe adhesions achieving a pregnancy in the first instance and this pathological process flowing downstream to the feto-maternal interface. Alternatively, this discrepancy may be due to underreporting and publication bias in other reported series.
The postpartum hysterectomy rate from our series is also concerning, affecting 4/85 (4.7%) of deliveries. The extended length of follow-up reported within our series may contribute to this figure and it may be once again explained by the relatively high number of women conceiving within our series compared to others. Importantly, these data highlight the need to adequately counsel women having a procedure for IUAs regarding subsequent pregnancy and its risks and to inform obstetricians to treat any pregnancy as medium-high risk and be aware of all of the potential outcomes and prepare for them.
Asherman’s original description of the syndrome was of IUA and infertility following traumatic postpartum or post abortal curettage (Asherman, 1948, 1950, 1952). Operative hysteroscopic surgery did not exist at that time, nor did procedures such as B-Lynch suture for postpartum haemorrhage and both of these interventions can lead to IUAs (Sentilhes et al., 2010a; Rathat et al., 2011; Ibrahim et al., 2013; Fuglsang, 2014; Kjer, 2014), although the pathophysiology of the adhesion formation may be different. We included all women with IUAs and a desire for pregnancy in our cohort, since their aim is the same—live birth—and although our data are underpowered for meaningful comparison between these two groups, we did not show a significant difference for live birth rate in this series for the different outcomes, with non-pregnancy IUAs having a lower chance of pregnancy than women with classic AS.
Adhesion severity is negatively correlated with subsequent term pregnancy rate, with previous data reporting ~80% success rate for women with mild, 65% moderate and only 30% in women with severe IUAs (Valle and Sciarra, 1988; Yu et al., 2008a,b; Roy et al., 2010), suggesting that higher grade IUAs and its subsequent treatment may lead to marked uterine impairment. Data from our study concur with these findings. This may be in part due to women with severe IUAs requiring multiple procedures to treat disease (Kodaman and Arici, 2007; Thomson et al., 2007) with the combined rate of anatomical restoration from combined large series 640/815 (78.5%, range: 44–93%) (Fedele et al., 1986; Pace et al., 2002; Fernandez and Al-Najjar, 2006; Hanstede et al., 2015), but severity dependent, with restoration reported as only 31/71 (44%) in women with grade 3 or higher IUAs (Fernandez and Al-Najjar, 2006). The initiating event for IUA development is unclear, although a thin endometrium, potentially caused by an impairment of the normal process of endometrial growth (Miwa et al., 2009) has been identified as an independent and critical factor for implantation failure (Shufaro et al., 2008; Gleicher et al., 2011; Zhao et al., 2012; Fang et al., 2016). Since angiogenesis is essential to promote endometrial growth after menstruation and to provide a vascular receptive endometrium for implantation (Smith, 1998, 2000), decreased vascularization due to either an initial surgical insult or treatment for IUAs may impair this pathway. Angiographic studies on women with IUA demonstrates reduced myometrial blood flow, with widespread vascular occlusion that may in part explain the endometrial atrophy, poor receptivity and recurrent miscarriage suffered in this group of women (Polishuk et al., 1977).
Results from this study indicate that hysteroscopic management of IUAs may result in pregnancy rates exceeding 60%, with more severe adhesions having a worse prognosis and requiring more surgical treatments. However, these pregnancies are more likely complicated by premature delivery, and the sequelae of abnormal placentation. Altered biochemical milieu or aberrant vascularization may be at the core of the pathological process, and consequently pregnant women treated for IUAs must be treated as high risk, with adequate antenatal and intrapartum surveillance. Multi-centred collaboration is recommended to undertake adequately powered prospective studies in this important area, but the limitations given the reported variance in individual surgical techniques and low prevalence are recognized.
Acknowledgements
The authors wish to acknowledge Joyce Woo who contributed to the data collection in this project.
Authors’ roles
Each author has contributed to the intellectual planning of the project, analysis of the data, writing and editing of the article.
Funding
No funding sought nor obtained for this project.
Conflict of interest
There is no conflict of interest between the authors of the article, and the research and findings.
References
- pregnancy
- asherman syndrome
- abortion, spontaneous
- fertility
- fluoroscopy
- follow-up
- hysterectomy
- hysteroscopy
- newborn
- infertility
- menstruation
- mothers
- pelvic pain
- placentation
- pregnancy rate
- postpartum period
- reproductive physiological process
- surgical procedures, operative
- obstetrics
- subfertility
- complications of pregnancy, childbirth and the puerperium
- live birth
- premature birth
- birth
- data reporting
- survival curve
- recall bias
- adhesiolysis
- uterine adhesions
- offspring



