-
PDF
- Split View
-
Views
-
Cite
Cite
Agathe Bernet, Alexandre Bastien, Denis Soulet, Olivia Jerczynski, Christian Roy, Maira Bianchi Rodrigues Alves, Cynthia Lecours, Marie-Ève Tremblay, Janice L Bailey, Claude Robert, Clémence Belleannée, Cell-lineage specificity of primary cilia during postnatal epididymal development, Human Reproduction, Volume 33, Issue 10, October 2018, Pages 1829–1838, https://doi.org/10.1093/humrep/dey276
Close - Share Icon Share
Abstract
Where are primary cilia (PC) organelles located during postnatal epididymal development?
Our findings unveil the existence of PC sensory organelles in different epididymal cell types according to postnatal development stage.
Primary cilia are sensory organelles that orchestrate major signaling pathways during organ development and homeostasis. Epididymal PC have been detected in the horses, donkey and mules but their cell-lineage specificity has never been investigated in this organ.
A longitudinal study was performed by examining tissue from n = 3 to n = 10 transgenic mice at different times of postnatal development. Tissues were fixed by intracardiac perfusion and the epididymides collected.
Transmission electron microscopy and confocal microscopy/3D reconstruction were used on a double transgenic mouse model expressing endogenous fluorescence in PC and centrioles (Arl13b-mCherry/Centrin2-GFP). Several PC parameters (i.e. length, orientation relative to the lumen) were quantified by using an image-processing pipeline. Epididymal tissues and serum-free cultures of DC2 immortalized epididymal principal murine cell lines were used to identify primary ciliary signaling components.
We report here a constitutive localization of PC in peritubular myoid cells and a dynamic profiling in epithelial cells throughout postnatal epididymal development. While PC are present at the apical pole of the undifferentiated epithelial cells from birth to puberty, they are absent from the apical pole of the epithelium in adults, where they appear exclusively associated with cytokeratin 5-positive basal cells. We determined that PC from epididymal cells are associated with polycystin 1 (PC1), polycystin 2 (PC2), and Gli-3 Hedgehog signaling transcription factor. No inter-individual variability was observed within each age group.
As our present study is descriptive and performed exclusively in the mouse, future functional studies will be required to unravel the contribution of these organelles in the control of reproductive functions.
Acknowledging the important roles played by PC sensory organelles in organ homeostasis and development in humans, our work opens new avenues of research concerning the cellular control of epididymal functions, which are essential to male fertility.
Study funded by an NSERC operating grant to CB (RGPIN-2015–109194). No competing interest to declare.
Introduction
Postnatal development (PND) of the epididymis is a multistep process synchronized with an increase in plasma androgen concentration, the arrival of testicular fluid flow, and sperm release (Sun and Flickinger, 1979). Proper epididymal PND is essential to generate a fully functional organ that will ensure optimal post-testicular sperm maturation at the time of puberty (see (Rodriguez et al., 2002)). Thus, identification of cellular factors involved in the development of male reproductive functions is a compelling research avenue that may help understand the etiology of some unexplained male infertility issues.
The adult epididymis is a single convoluted tubule divided into three to four main anatomical regions (i.e. initial segment in rodents, caput, corpus and cauda), each displaying distinct morphological features. The lumen of the epididymis is surrounded by a pseudo-stratified epithelium composed of principal, clear and basal cells, whose functions are essential to ensure proper post-testicular sperm maturation. The epididymis becomes fully functional following a series of morphological changes that occur during its PND, particularly prior to puberty. The differentiation and organization of epididymal cells take place over three main stages: the undifferentiated period, the period of differentiation, and the period of expansion (Sun and Flickinger, 1979; Zondek and Zondek, 1980; Abe et al., 1984). During the undifferentiated period (first week in the mouse), the epididymis epithelium is characterized by the presence of columnar cells that lack stereocilia. The period of differentiation (second to fifth week in the mouse) consists of the appearance of differentiated and specialized cells, including clear, principal and basal cells; this period is associated with high cellular proliferation within the initial segment. Finally, the period of expansion (sixth to eighth week in the mouse) features the appearance of spermatozoa within the epididymal lumen, as well as an increase in the size of the epididymis, until cell division arrest at around the 10th week. Different factors such as luminal flow, lumicrine components and androgens have been shown to participate in epididymis PND through epithelial cell differentiation (Orgebin-Crist et al., 1983; Abe et al., 1984). However, the cellular mechanisms involved in this process are unknown. The serendipitous finding of the presence of sensory primary cilia (PC) organelles in the equine epididymis shed light on potential new developmental mechanisms (Arrighi, 2013).
PC are non-motile cilia composed of an axonemal extension and a basal body. This cellular antenna serves as a sensory organelle and a signaling hub to control cell functions important to tissue development and homeostasis (see (Singla and Reiter, 2006; Satir et al., 2010)). Depending on their biological context, PC play two major roles: one as a hedgehog (Hh) signaling mediator, and the other as a mechanosensor through the ciliary Polycystin 1 (PC1) and the Polycystin 2 (PC2) ion channels and transporter proteins (Nauli et al., 2003). The male excurrent duct displays different types of ciliary extensions and microvilli in contact with the surrounding fluid. In the rete testis, the apical surface of the cells bears numerous microvilli as well as a single long cilium—presumably a PC despite the lack of ultrastructural evidence- extending from a centriole pair (Fawcett and Dym, 1974; Dym, 1976). In the efferent ducts, the presence of motile kinocilia and non-motile PC has been reported to protrude from ciliated and non-ciliated cells, respectively (Aire, 1982; Hess, 2015). While motile cilia have been proposed to propel sperm towards the epididymal lumen, the role of non-motile PC in both rete testis and efferent ducts remains unknown. In the epididymis, both non-motile absorptive stereocilia and microplicae are present at the surface of principal and clear cells, respectively (Hermo and Robaire, 2002). With the exception of the serendipitous observation of PC in the equine epididymis reported by Arrighi in 2013 (Arrighi, 2013), no further studies have investigated the characterization and potential role of these biological antennae in epididymis development and homeostasis.
In the present study, we revealed for the first time the presence of primary ciliary components in the epididymis and portrayed their spatio-temporal localization during epididymis PND. Acknowledging that impairment of PC function is associated with male infertility issues and other human diseases referred to as ciliopathies (Hildebrandt et al., 2011), portraying and unraveling the role of PC during epididymal PND may help determine the etiology for unexplained male infertility.
Materials and Methods
Mouse tissues and ethics
Epididymides from C57BL/6 and Tg(CAG-Arl13b/mCherry)1Kvand Tg(CAG-EGFP/CETN2)3-4Jgg/KvandJ (referred to as Arl13b-Cetn2 tg in this study, Jackson Laboratory stock# #027967) were used. The Arl13b-Cetn2 tg mice express both the ciliary component ADP-ribosylation factor-like protein 13B (Arl13b) fused to the monomeric red fluorescence protein mCherry and the centriolar protein Centrin2 (Cetn2) fused to GFP (Bangs et al., 2015). These mice were housed and reproduced in the elite animal facility of the CHU de Quebec research Center. Animal experiments were approved by the ethical committee of the Institutional Review Board of the Centre Hospitalier Universitaire de Québec (CHUQ)(CPAC licenses 2016050 and 12016051, C. Belleannée) and were conducted in accordance with the requirements defined by the Guide for the Care and Use of Laboratory Animals.
Epididymides were obtained from mice killed at different postnatal ages. Postnatal Day 1 (1 dpn) to 28 dpn tissues were fixed by immersion in fixative containing 4% (w/v) paraformaldehyde (PFA) (FisherScientific, NJ, USA), 10 mM sodium periodate (AlfaAesar, USA), 75 mM lysine (FisherScientific, NJ, USA) and 5% (w/v) sucrose (LaboratoireMat, Mtl, Canada) in 0.1 M sodium phosphate buffer (PLP). After 28 dpn, tissues were fixed in situ by perfusion of PLP via the left ventricle (Belleannée et al., 2009).
Immunofluorescence staining
Epididymides from Arl13b-Cetn2 tg mice fixed with PLP were treated for immunofluorescence as previously described (Belleannée et al., 2009). Five- to 25-μm-thick optimum cutting temperature embedded sections were hydrated for 15 min in PBS and treated for 4 min with 1% (w/v) sodium dodecyl sulfate (SDS) and 2% (v/v) Triton X-100 in PBS. Sections were washed in PBS for 5 min and then blocked in PBS containing 1% w/v bovine serum albumin (BSA) for 15 min. Sections were then incubated overnight at 4°C with primary antibodies diluted in DAKO solution (DAKO corporation, North America) and directed against specific markers of epididymal cells and PC components (Supplementary Table SI). When specified, an antigen retrieval step for 10 min at 110°C in citrate buffer was included before incubation with the primary antibodies. After washes, secondary antibodies were applied for 1 h at room temperature. Slides were mounted in Vectashield medium containing 4′,6′-diamidino-2-phenylindole (DAPI; Vector Laboratories, Inc., Burlingame, USA) for imaging.
Immortalized Distal Caput principal cells (DC2) developed from mouse epididymides were kindly provided by Marie-Claire Orgebin-Crist and used for immunofluorescence assays. DC2 cells were cultured as previously described (Araki et al., 2002), plated on fibronectin-coated slides at a density of 125 000 cells per well and fixed with 4% PFA for 10 min. After washes with PBS, blocking was performed for 30 min in PBS solution with 1% w/v BSA and 0.1% v/v Triton X-100. Slides were incubated for 1 h with primary antibody followed by washes, and for 1 h with the appropriate secondary antibody. Slides were mounted in Vectashield medium, as described above.
Confocal imaging
Digital images were acquired by confocal microscopy using an inverted Olympus IX80 microscope equipped with a WaveFX-Borealin-SC Yokagawa spinning disk (Quorum Technologies) and an Orca Flash4.0 camera (Hamamatsu). Image acquisition was performed using Metamorph software (Molecular Devices). Optical Z-sections were acquired for each channel and projected into a single picture using ImageJ. Low (20×) and high (100×) magnification pictures were taken for the three major epididymal segments, the caput, corpus and cauda epididymidis. Three-dimensional animations were generated with Bitplane Imaris software v7.5 (Bitplane, Zurich, Switzerland) from images acquired by an Olympus FV-1000 confocal microscope (Olympus Canada; CFI equipment to DS) equipped with a PLAPON60XOSC objective lens (NA 1.4).
Large data processing
To determine primary cilia features throughout the epididymis, images were acquired on a Zeiss LSM700 confocal microscope with a 40x/0.95 plan-apo lens. The resolution was set to the highest possible according to the lens in use (0.145 μm/pixel). Whole epididymal sections (25 μm thickness) were imaged in the z-axis, generally 15–20 μm at 1 μm interval and large regions of at least 1 mm2 were imaged in the XY plane with mosaic tiling at 10% overlap. Large data processing was carried out with custom ImageJ macro (Rueden et al., 2017) and Matlab code. Centrosomes and cilia were detected using ImageJ’s Find Maxima. We determined the absolute angle of each cilium with a rotating pattern in order to obtain a maximum at the cilium angle relative to the closest tubule edge. Matlab and ImageJ macro to process cilia orientation from epididymal confocal acquisitions are available on https://github.com/alexandrebastien/Cilia.
Transmission electron microscopy
Mice were anesthetized with ketamine (intraperitoneally) and tissues were fixed by intracardiac perfusion with PBS for 2 min followed by 4% (w/v) PFA for 2 min and treated as previously described (Bisht et al., 2016).
Results
Localization of PC in epididymal cells of the adult mouse
Because PC are small, dynamic and present as a solitary antennae on the cell surface, these organelles are difficult to detect within in situ or in vivo whole organ systems. We used the Arl13b-Cetn2 tg mouse model (Bangs et al., 2015) to study the lineage specificity of PC from whole epididymal tissues. Arl13b-positive structures were observed within the epithelium and in the surrounding smooth muscle layer of the epididymis (Fig. 1A). The microtubule component and PC marker acetylated tubulin (Ac-Tub) was observed associated with the sperm flagellum and co-localized with Arl13b-positive cell extension, further confirming the specific detection of non-motile PC from Arl13b-Cetn2 tg mice (Fig. 1BC). By using specific markers of distinct epididymal epithelial cell populations (i.e. keratin-5 for basal cells, aquaporin 9 for principal cells and alpha-actin for smooth muscle cells) we determined the cell-specific localization of PC from adult Arl13b-Cetn2 tg mice (Fig. 2). As demonstrated by labeling with the alpha-actin smooth muscle cell marker, PC were found in the peritubular area associated with the smooth muscle cell centriole (Fig. 2A, inset). The ultrastructure of PC in smooth muscle cells is composed of a basal centriole and an axonemal extension as observed by TEM (Fig. 2B). In addition, Arl13b-positive PC were never observed associated with aquaporin 9-positive principal cells, nor aquaporin 9-negative clear cells from adult mice (Fig. 2C). Finally, we found that PC extended from the centriole of keratin-5-positive basal cells (Fig. 2D, inset). From the whole image acquisitions performed on adult epididymal tissues, all basal cells that were observed within a 25-μm tissue section exposed a primary cilium. While the major part of their cell body is located at the base of the epithelium, basal cells dynamically extend towards the lumen of the epididymis through axiopodia (Shum et al., 2008; Roy et al., 2016). According to basal cell 3D reconstruction from transgenic epididymal tissues, we observed that PC take their origin from the centriole located at the apical pole of the cell body and at the base of the axopodia (Fig. 3). In addition, basal cell-associated PC never cross epididymal tight junctions as determined from 3D reconstruction after ZO-1 staining (Supplementary fig. S1).
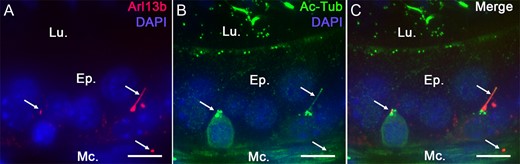
Acetylated tubulin primary cilia (PC) marker co-localizes with Arl13b in Arl13b-Cetn2 tg mice. Epididymal sections from Arl13b-Cetn2 tg mice (A) were stained for the PC marker acetylated tubulin (Ac-Tub) (B). Arl13b-positive cilia extensions colocalize with Ac-Tub (Arrows, A, B and C), which validates this mouse model for the study of PC in the epididymis. Ep.: epithelium; Lu.: lumen; Mc.: myoid cells. Scale bars: 10 μm.
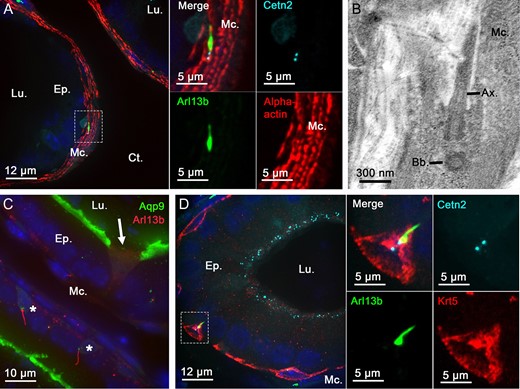
Primary cilia extend from basal and peritubular myoid cells in the epididymis of adult mice. Immunofluorescent staining for alpha-actin myoid cell marker (A and inset), aquaporin 9 (Aqp9) principal cell marker (C), keratin-5 (Krt5) basal cell marker (D and inset) was performed on epididymal sections from adult Arl13b-Cetn2 dTg mice. Primary cilia organelles were exclusively found associated with basal cells (stars) and myoid cells in these tissues. Arrow indicates Aqp9-negative clear cell. Primary cilia structural components, such as the axoneme and basal body, were detected by transmission electron microscopy in epididymal peritubular myoid cells from C57BL/6 mice (B). Ax.: axoneme; Bb.: basal body; Ct.: connective tissue; Ep.: epithelium; Lu.: lumen; Mc.: myoid cells.
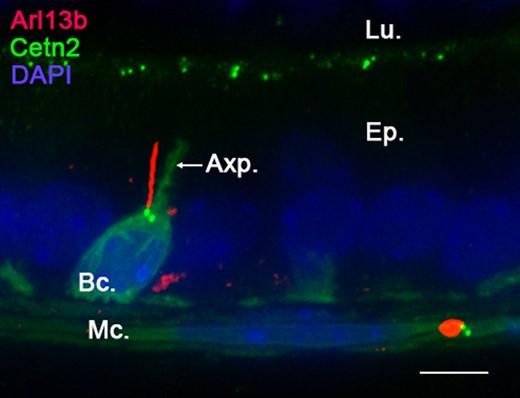
Primary cilia (PC) extend from the basal cell main body. Confocal imaging performed in the caput epididymidis of Arl13b-Cetn2 dTg adult mice showed a basal cell with a characteristic axiopodia (Axp.) extending from its main cell body. Arl13b-positive PC extend from centrin2-positive centrioles located at the base of the axiopodia. No PC extension was found within the axiopodial extension in the caput, corpus or cauda epididymidis. This figure is representative of acquisitions performed on n = 10 mice. Bc.: basal cell; Ep.: epithelium; Lu.: lumen; Mc.: myoid cells. Scale bar: 10 μm.
Localization of PC along the adult epididymis
The epididymis is a highly segmented tubule with each segment displaying distinct features in terms of cell populations, cell composition and cell functions. We investigated the properties of basal cell PC throughout the different segments of the epididymis from Arl13b-Cetn2 tg mice (i.e. initial segment (IS), caput, corpus and cauda) by whole tissue confocal imaging and digital image processing (Fig. 4). Primary cilia were observed associated with peritubular myoid and basal cells in all segments of the epididymis, with a higher proportion in the basal cells from the distal regions (Fig. 4A, Supplementary fig. S2). In the IS, basal cell PC appeared as short 2–3 μm extensions directed towards the base of the epithelium, according to GFP-positive centriole localization (Figs. 4A, B). In the other segments, i.e. the caput, corpus and cauda, PC appeared as longer 5–10 μm extensions, presenting with erratic directions. Furthermore, myoid cell PC presented no obvious variation from one segment to another in terms of length (5–6 μm) or orientation (generally perpendicular to the intratubular axis). In addition to their localization throughout the basal and peritubular myoid cells of the epididymis, PC were also associated with basal cells in the vas deferens (VD) (Fig. 4A, VD) and were observed as tremendously long extensions in the efferent ducts (ED) (Fig. 4A, ED). In ED, whose unilaminar epithelium exclusively consists of ciliated and non-ciliated cells, numerous centrin2-positive centrioles were observed at the apical surface of ciliated cells. Moreover, elongated Arl13b-positive PC (15–40 μm) were found associated with centrioles located in non-ciliated cells of the ED.
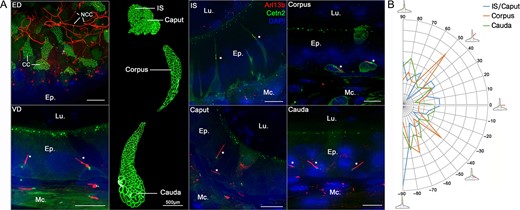
Primary cilia (PC) are present in the efferent ducts (ED), the vas deferens (VD), and the different segments of the mouse epididymis. Confocal imaging was performed in the ED, VD and different segments of the epididymis from Arl13b-Cetn2 dTg adult mice. Ciliated cells (CC) from the ED were characterized by the presence of numerous centrioles at the apical pole of the cells. Elongated PC (15–40 μm) were detected at the apical surface of non-ciliated cells (NCC). PC were found associated with basal cells (stars) and peritubular myoid cells in all segments of the epididymis and in the VD (A). Following confocal imaging acquisitions, the angle of basal cell PC relative to the closest tubule edge was determined by image processing in each epididymal region, i.e. IS/Caput (n = 899 cells), corpus (n = 371 cells) and cauda (n = 1065 cells) from three mice. Proportions of PC with distinct angles were distributed between −90° for cilia directed towards the base of the epithelium to 90o for cilia directed towards the lumen (B). Ep.: epithelium. Lu.: Lumen. Mc.: myoid cell. IS: initial segment. Scale bars when non specified: 10 μm.
Profiling of PC orientation throughout epididymis postnatal development
The epithelium of the epididymis undergoes important changes during the different PND stages (Sun and Flickinger, 1979). We explored the dynamics of PC exposure that accompany epididymis epithelium development just after birth (at 5 dpn), at pre- and post-pubertal ages (at 30 and 42 dpn, respectively), and at the adult stage (at 42 to 268 dpn) (Fig. 5). The Arl13b-Cetn2 tg mouse model displays the same pattern of epididymal development as previously described in wild-type mice (Tajiri et al., 2012) (Fig. 5, Top). For instance, H & E staining performed on the epididymis at the 5 dpn stage shows undifferentiated columnar cells of the epididymal epithelium surrounding an empty lumen. At 30 dpn, we observed a pseudo-stratified epithelium and the presence of basal cells. The lumen was filled with non-cellular material. At 42 dpn and 268 dpn, the pseudo-stratified epithelium surrounded large lumina filled with spermatozoa. At an early stage of dpn (Fig. 5, at 5 dpn Bottom and Supplementary Movie 1), PC were observed by confocal microscopy as short Arl13b-positive extensions associated with centrioles, located at the apical surface of non-differentiated columnar cells. Primary cilia extensions were extensively observed associated with myoid cells surrounding the epithelium. At 30 dpn, prior to puberty, PC were found at the apical surface of the epithelium associated with differentiated cells, potential clear or principal cells, and associated with basal cells. After puberty, at 42 dpn (Fig. 5, at 42 dpn and Supplementary Movie 2) and 268 dpn, PC extensions were exclusively found in basal cells within the epithelium as well as in surrounding myoid cells. Thus, according to the PND stage of the mouse epididymis, PC were localized at different positions along the epididymal epithelium, i.e. facing the lumen during the undifferentiated period, facing the lumen and at the base of the epithelium during the period of differentiation, and exclusively at the base of the epithelium during the expansion phase. Primary cilia were observed in myoid cells at all PND stages studied. According to ZO-1 immunostaining of the apical pole of the epididymis (Fig. 6), PC extended from the epithelial cell surface at an early stage of PND (at 5 dpn) and were in direct contact with the intraluminal compartment.
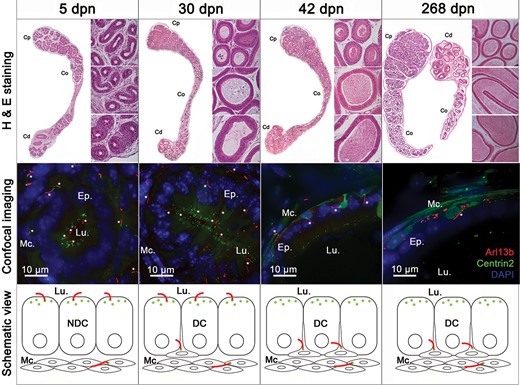
Primary cilia (PC) are observed at different stages of postnatal epididymal development. Hematoxylin and eosin staining (H & E) and confocal imaging were performed on epididymal sections from Arl13b-Cetn2 dTg mice at different stages of postnatal development, i.e. at 5, 30, 42 and 268 days postnatal (dpn). PC were detected from different epididymal tubules at each stage of postnatal development (stars). Schematic representation of PC location is shown throughout postnatal development with centrioles displayed in green and PC in red. Cp, Co, Cd: caput, corpus, cauda. DC: differentiated cells; NDC: non-differentiated cells; Lu.: lumen; Mc.: myoid cells.
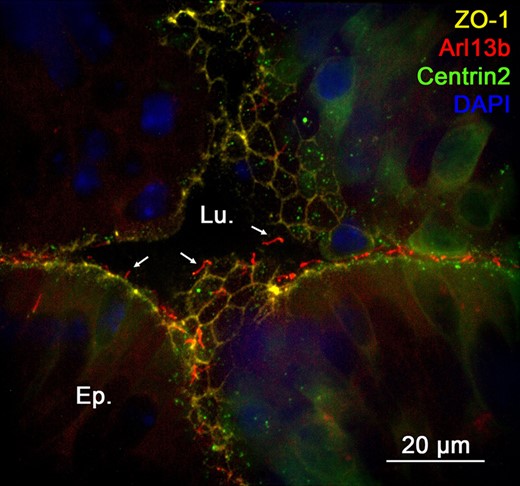
Primary cilia are in contact with the intraluminal compartment of the epididymis at an early stage of postnatal development. Immunofluorescent staining for the tight junction protein-1 (ZO-1) was performed in the cauda epididymidis of Arl13b-Cetn2 dTg mice at 5 days postnatal (dpn). Primary cilia extend from the apical pole of the cells towards the intraluminal compartment (arrows). Lu.:lumen; Ep.:epithelium.
Overview of PC-dependent pathways expressed in the epididymis
By analogy with other model systems, we investigated the functional signaling components associated with PC in the epididymis. We first explored the expression level of PC1 and PC2 (Fig. 7), two major players in the PC-dependent fluid-flow mechanosensation. At dpn 7, PC1 was detected at the apical pole of undifferentiated cells as well as in peritubular myoid cells in the different epididymal segments of Arl13b-Cetn2 tg mice (Fig. 7A). At this postnatal stage, a stronger signal was observed in the vas deferens (Fig. 7A, VD). In tissues from adult mice, PC1 was intensively detected in the cytoplasm and the apical pole of the epithelium in the initial segment/caput of the epididymis. While PC1 intensity transitionally decreased in the corpus compared with the caput, it was drastically higher in the apical membrane of epithelial cells from the cauda as well as in the vas deferens (VD). PC1 was also detected, but to a lower extent, in the peritubular myoid cells. While PC2 could not be detected on epididymal sections by IHC under our experimental conditions (not shown), we observed a co-localization between PC2 and the PC marker Ac-Tub in the DC2 epididymal principal cell line (Fig. 7B, arrows).
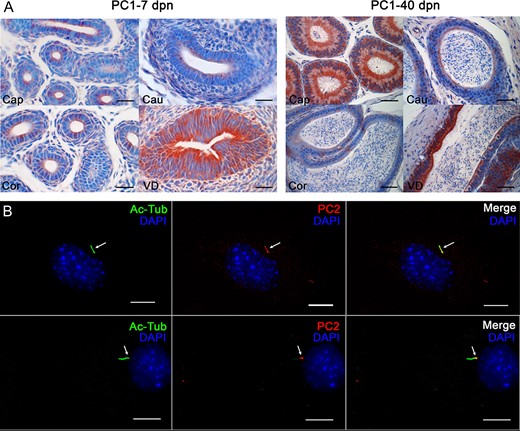
Expression of Polycystin 1 (PC1) and Polycystin 2 (PC2) in epididymal cells and in the mouse epididymis. (A) Immunohistochemical staining for PC1 in the epididymis of Arl13b-Cetn2 dTg mice was performed at 7 and 40 days postnatal (dpn). Cap.: caput; Cor.:corpus; Cau.:cauda; VD: Vas deferens. Scale bars: 50 μm. (B) Immunofluorescent staining for PC2 and the PC marker acetylated tubulin (Ac-Tub) was performed on DC2 cells. PC2 co-localizes with Ac-Tub-positive primary ciliary extension (arrows). Scale bars: 5 μm
We next investigated the expression of GLI-3, a downstream transcriptional factor of the Hh pathway (Fig. 8). In the adult epididymis of Arl13b-Cetn2 tg mice, GLI-3 was observed as discontinuous staining at the apical pole of the epithelium in the corpus and cauda epididymidis. In addition, strong GLI-3 staining was observed in the nucleus of different epithelial cell populations in all segments of the organ. This staining was particularly intense in basal cell nuclei (Fig. 8, stars and insets).
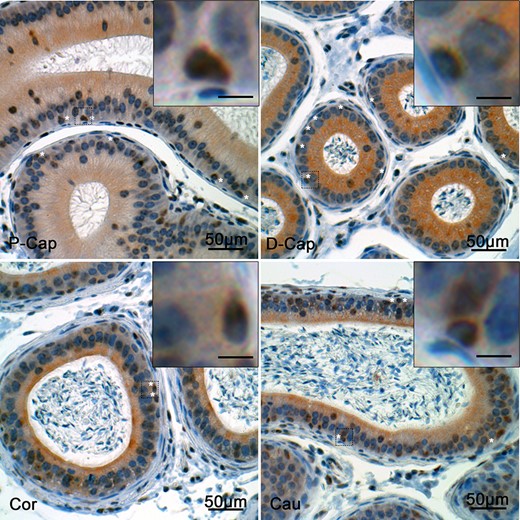
Expression of Gli-3 in the mouse epididymis. Immunohistochemical staining for Gli-3 was performed in the epididymis of Arl13b-Cetn2 dTg mice at 40 days postnatal (dpn). Gli-3 was detected in the basal cell nuclei (stars) from the different epididymal segments. P-Cap: proximal caput; D-Cap: distal caput; Cor.:corpus; Cau.:cauda. Inset scale bars: 10 μm
Discussion
Impairment of PC formation in humans is responsible for a broad range of clinical syndromes—including male infertility—and diseases referred to as ciliopathies (van der Linden et al., 1995; Hildebrandt et al., 2011; Kanagarajah et al., 2012). Acknowledging the potential of PC in the diagnosis of unexplained male infertility, we have portrayed for the first time the cell-lineage specificity of PC in the epididymis, the male reproductive system organ that governs post-testicular sperm maturation and sustains male fertility. In light of the spatio-temporal changes of PC observed during PND, and the functional components associated with these antenna (i.e. PC1/PC2 and Gli-3), we propose a novel putative mechanism of intercellular communication involving PC in the control of epididymal functions (Fig. 9).
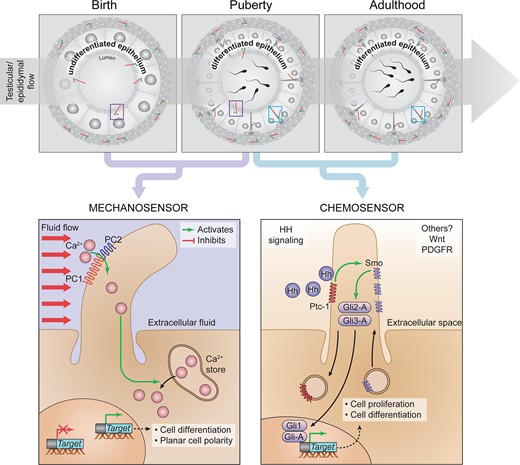
Cell-lineage specificity and hypothetical roles of PC from the epithelium of the epididymis during postnatal development. From birth to puberty, PC are in direct contact with the intraluminal compartment of the epididymis, where they could be responsive to shear-flow mechanosensation through PC1/PC2, as described in the kidney. In the latter, shear-flow triggers calcium entry and the subsequent regulation of target gene expression. From puberty, PC are exclusively associated with basal cells in the epithelium, where they may transduce Hedgehog (Hh) signaling pathway. Comprehensively this pathway involves Protein patched homolog 1 (ptch1) and Smoothened (Smo) ciliary receptors, as well as the GLI family transcription factors (Gli-1, 2 and 3) to regulate cell proliferation and differentiation.
Consistent with the observation made by Arrighi in 2013 (Arrighi, 2013), we determined that PC were exclusively associated with basal cells in the epididymal epithelium of adult mice. Basal cells from several other stratified and pseudo-stratified epithelia expose PC with distinct signaling functions. For instance, basal cells from the olfactory epithelium have PC that control basal cell activation, proliferation and differentiation, and as such are necessary for regeneration of the epithelium following injury (Joiner et al., 2015). In addition, PC associated with basal cells from the mammary gland are involved in the acquisition of their stem cell properties via Hh signaling, thereby controlling mammary tissue outgrowth and basal cell tumor development (Guen et al., 2017). Although basal cells from the epididymis do not seem to be progenitors of the other cell populations during early development (Murashima et al., 2011; Shum et al., 2013), these cells share common properties with adult stem cells and were recently proposed to differentiate into columnar cells as a mechanism of epididymal epithelium regeneration (Mandon et al., 2015). In that regards, basal cells from the epididymis may participate in epithelial regeneration via PC signaling, in a similar manner to that of other epithelia.
Primary cilia coordinate Hh signal-transduction machinery whose components—either agonists or downstream effectors—are found enriched in basal cells of the rat epididymis (Mandon et al., 2015) and is particularly relevant to the control of epididymal homeostasis and post-testicular maturation events occurring in this organ (Turner et al., 2006). For instance, blockage of the Hh signaling pathway following cyclopamine administration triggers a significant decrease in Gli-1 and Gli-3 transcription factor expression as well as a significant decrease in epididymal sperm motility (Turner et al., 2006). Whether or not this phenotype relies on PC-dependent Hh signaling remains to be established. Complementary in vivo studies on basal cell PC will further define the contribution of this organelle to epididymal physiology, and ultimately to sperm maturation and male fertility.
Furthermore, PC were found constantly associated with myoid cells surrounding the epididymal epithelium throughout postnatal development. While nothing is known with regards to the role played by myoid cell PC in the epididymis, recent reports indicate that these organelles participate in airway smooth muscle cells contractions in the context of airway remodeling (Trempus et al., 2017). By analogy, smooth muscle cell PC found in the epididymis may mediate communication between mesenchymal cells and the extracellular matrix during organ elongation.
Finally, the fact that PC are observed at the apical pole of the epithelium from early stages of PND to puberty and colocalize with polycystins (PC1 and PC2), suggests that these organelles might play a mechanosensory role prior to puberty. This type of PC-dependent mechanosignalling is broadly documented in the kidney, which shares the same embryonic origin as the epididymis and presents numerous functional similarities with it (Nauli et al., 2003; Mohammed et al., 2017). In this organ, the shear-flow exerted at the surface of the cells triggers the physical bending of PC and the subsequent PC1 dependent-transepithelial transport of calcium to control tissue morphogenesis (Nauli et al., 2003). Approximately 40 years ago, Sun and Flickinger proposed that the flow of testicular-derived fluid entering the epididymis before sperm production may stimulate epithelial cell proliferation and differentiation in the epididymis (Sun and Flickinger, 1979). Building on our new findings, it is now conceivable that PC are strategically positioned in direct contact with the lumen of the epididymis to initiate shear-flow dependent epithelium maturation and to sense the first wave of spermatozoa at puberty to sustain maturation.
In conclusion, our study reveals for the first time the cell-lineage specificity of PC along the epididymis at different stages of PND. Considering that non-genetic environmental insults, such as lithium treatment and folic acid uptake, have been shown to alter cilia length and functions(Miyoshi et al., 2009; Toriyama et al., 2017), portraying and unraveling the role of PC in human will open new avenues concerning the diagnosis of unexplained male infertility cases.
Acknowledgements
We thank Pr Sylvie Breton, PhD, for providing the aquaporin nine antibody, as well as Pr Marie-Claire Orgebin-Crist, PhD, for providing the epididymal DC2 cell line. We would like to acknowledge the contribution of Sabine Elowe, PhD, for confocal imaging support, and Christine Légaré, MSc, for her technical support.
Author contributions
Experiments were conceived and designed by A.Ba, A.Be, C.B., C.R. and D.S. Contributed to critical reagent, resources and expertize; C.B., C.R., D.S., J.B., M.E.T. Experiments were performed by A.B., A.B., C.B., C.L., C.R., D.S., O.J., M.B.R. Data were processed and analyzed by A.B., A.B., C.B., C.R., D.S., M.B.R. and supervised by C.B., C.R., D.S., J.B. and M.E.T. The manuscript was written by A.B., C.R. and C.B. and critically reviewed by all authors.
Funding
This work was supported by an Canadian Institutes of Health Research (CIHR) grant (RGPIN-2018-119871) and an Fonds de recherche du Québec – Santé (FRQS)-Junior 1 salary award to C.B., Natural Sciences and Engineering Research Council of Canada (NSERC) grant to M.E.T. (RGPIN-2014-05308), NSERC grant to C.R. (RGPIN-2017-04775) and an FRQS-Junior 2 award to D.S. A.B. is a recipient of a Centre de recherche en reproduction, développement et santé intergénérationnelle (CRDSI) fellowship. M.B.R. is a recipient of a Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) fellowship. M.E.T. is a Canada Research Chair (Tier 2).
Conflict of interest
The authors declare no competing or financial interests.



