-
PDF
- Split View
-
Views
-
Cite
Cite
Abha Maheshwari, Priya Bhide, Jyotsna Pundir, Siladitya Bhattacharya, Routine serum thyroid-stimulating hormone testing—optimizing pre-conception health or generating toxic knowledge?, Human Reproduction, Volume 32, Issue 9, September 2017, Pages 1779–1785, https://doi.org/10.1093/humrep/dex240
Close - Share Icon Share
Abstract
Monitoring subclinical hypothyroidism (SCH) in women is believed to be important in terms of preventing overt hypothyroidism and optimizing the health and cognitive development of their children. Current systematic reviews have suggested an association between maternal SCH and adverse obstetric and neonatal outcomes. However, initiating the administration of thyroxine during pregnancy has failed to demonstrate appreciable health benefits. Hence there are calls by professional endocrine societies for optimizing serum thyroid-stimulating hormone (TSH) levels pre-conception. The strategy of ensuring that serum TSH levels are below 2.5 mIU/l during the pre-conception period has generated considerable uncertainty partly because the recommended level of <2.5 mIU/l is lower than those previously used to define the condition and partly due to uncertainty about the best screening programme clinicians can use in this context. Recalibrating the expected normal peri-conceptional range of serum TSH (<2.5 mIU/l), will have a significant impact on clinical services due to an inevitable increase in numbers of women diagnosed with SCH who will need to be investigated, treated and monitored. Serum TSH fulfils the criteria for a screening test and oral thyroxine is an inexpensive drug. Therefore, there is no reason to believe that screening cannot be undertaken in all women planning to conceive. Yet this approach will miss women whose pregnancies are unplanned and generate anxiety, further tests and many more prescriptions for thyroxine, coupled with the need for lifelong monitoring in affected women. A number of existing and ongoing randomized trials have evaluated the use of thyroxine in women with infertility or miscarriage with detectable thyroid auto-antibodies. These are unlikely to answer the question whether routine pre-conception testing for SCH in asymptomatic women is beneficial. Routine screening of women at risk of pregnancy and optimization of their thyroid status could result in significant health benefits for their offspring. Alternatively this approach could prove to be an expensive way of generating toxic knowledge resulting in anxiety, increased drug use and potential harm. Only large, appropriately designed studies can reveal the answer.
Introduction
The World Health Organization (WHO) considers iodine deficiency to be ‘the single most important preventable cause of brain damage’ worldwide (WHO et al. 2007). Iodine is a vital component of the thyroid hormones, thyroxine (T4) and triiodothyronine (T3), which are required for brain and neurological development. These hormones are required for the proliferation and migration of neurones, development of the brain cytoarchitecture and glial differentiation and migration. A lack of adequate thyroid hormone has deleterious effects on foetal brain development.
It is believed that iodine deficiency in pregnancy is associated with a spectrum of disability in the offspring ranging from severe outcomes, such as motor ability cretinism—characterized by profound intellectual impairment, deaf-mutism and motor rigidity, to subtle impairment of IQ. The UK-based Avon Longitudinal Study of Parents and Children (ALSPAC) found an association between low iodine status in early pregnancy (urinary iodine-to-creatinine ratio < 150 μg/g) and lower verbal IQ and reading scores in the offspring (Bath et al., 2013).
Iodine requirements increase in pregnancy due to an increase in both renal iodine excretion as well as levels of circulating thyroxine binding proteins. Thyroid hormone production also rises—partly due to increased secretion of placental human chorionic gonadotrophin which has a stimulatory effect on the thyroid gland. However, these compensatory mechanisms are inadequate in the presence of existing thyroid deficiency or high levels of thyroid antibodies which can cause euthyroid women to develop subclinical hypothyroidism (SCH) and those with SCH to develop overt hypothyroid (OH).
The deleterious effects of OH are well established (Zimmermann, 2007), but even subclinical hypothyroidism can affect neuro-developmental processes and cause cognitive impairment (Haddow et al., 1999; Abalovich et al., 2002; Mannisto et al., 2009; Su et al., 2011). Endocrinologists [Association of American Clinical Endocrinologists (AACE), American Thyroid Association (ATA) and the Endocrine Society guidelines (ESG)] recommend levothyroxine replacement in all women with Subclinical Hypothyroidism (SCH) defined as TSH > 2.5 mIU/l in first trimester, regardless of TPO Ab (Thyroid peroxidise) status (Garber et al., 2012). Recent ATA guidelines (Alexander et al., 2017) recommend that women with SCH undergoing IVF or ICSI should be treated with levothyroxine with a goal to achieve serum TSH levels of <2.5 mU/l. Ideally, the objective should be the same in every woman trying to conceive, and not just those undergoing fertility treatment. However, routine screening for TSH is not recommended by any of the national/international societies including American College of Obstetrics and Gynaecology (ACOG)/American Thyroid Association and NHS, UK (https://cpdscreening.phe.org.uk/timeline). This has led to a serious dilemma for clinicians resulting in conflicting advice for patients.
In this paper we evaluate the benefits and disadvantages of routine TSH screening in all women who are trying to conceive with the intention of treating those diagnosed with subclinical hypothyroidism.
TSH as a screening test
Screening is defined as a systematic and active search for a health condition by means of testing otherwise healthy people. As shown in Table I, according to the Wilson and Jungner (1968) criteria, TSH has the potential to be a good screening test provided it improves offspring health.
| Criteria . | Applicability to TSH . |
|---|---|
| The condition being screened for, should be an important health problem | SCH is a common clinical problem; Gharib et al. (2005) |
| The natural history of the condition should be well understood | This is well defined in females - annual rate of progression from SCH to OH was 2.6% in patients negative for thyroid antibodies, but 4.3% if TPO Abs were present; Razvi et al. (2010) |
| There should be a detectable early stage | SCH precedes the development of OH |
| Treatment at an early stage should be of more benefit than at a later stage | In pregnancy pre-conception optimization is thought to be better especially for neurodevelopment of the offspring |
| A suitable test should be devised for the early stage | TSH detects SCH at early stage |
| The test should be acceptable | TSH is a simple blood test, It may be difficult to identify all women in pre-conception stage but in an era of contraception, a fair proportion can be identified |
| Intervals for repeating the test should be determined | Usual guidance is once a year |
| Adequate health service provision should be made for the extra clinical workload resulting from screening | Thyroxine replacement is a simple treatment |
| The risks, both physical and psychological, should be less than the benefits | There is minimal risk of taking a simple blood test |
| The costs should be balanced against the benefits | As TSH is an inexpensive test and thyroxine is an inexpensive medication, there is a potential that benefits will overweigh the costs even if a few preterm birth, miscarriage or cognitive impairment is prevented |
| Criteria . | Applicability to TSH . |
|---|---|
| The condition being screened for, should be an important health problem | SCH is a common clinical problem; Gharib et al. (2005) |
| The natural history of the condition should be well understood | This is well defined in females - annual rate of progression from SCH to OH was 2.6% in patients negative for thyroid antibodies, but 4.3% if TPO Abs were present; Razvi et al. (2010) |
| There should be a detectable early stage | SCH precedes the development of OH |
| Treatment at an early stage should be of more benefit than at a later stage | In pregnancy pre-conception optimization is thought to be better especially for neurodevelopment of the offspring |
| A suitable test should be devised for the early stage | TSH detects SCH at early stage |
| The test should be acceptable | TSH is a simple blood test, It may be difficult to identify all women in pre-conception stage but in an era of contraception, a fair proportion can be identified |
| Intervals for repeating the test should be determined | Usual guidance is once a year |
| Adequate health service provision should be made for the extra clinical workload resulting from screening | Thyroxine replacement is a simple treatment |
| The risks, both physical and psychological, should be less than the benefits | There is minimal risk of taking a simple blood test |
| The costs should be balanced against the benefits | As TSH is an inexpensive test and thyroxine is an inexpensive medication, there is a potential that benefits will overweigh the costs even if a few preterm birth, miscarriage or cognitive impairment is prevented |
SCH, subclinical hypothyroidism; TSH, thyroid-stimulating hormone; TPO, thyroid peroxidise; OH, overt hypothyroid.
| Criteria . | Applicability to TSH . |
|---|---|
| The condition being screened for, should be an important health problem | SCH is a common clinical problem; Gharib et al. (2005) |
| The natural history of the condition should be well understood | This is well defined in females - annual rate of progression from SCH to OH was 2.6% in patients negative for thyroid antibodies, but 4.3% if TPO Abs were present; Razvi et al. (2010) |
| There should be a detectable early stage | SCH precedes the development of OH |
| Treatment at an early stage should be of more benefit than at a later stage | In pregnancy pre-conception optimization is thought to be better especially for neurodevelopment of the offspring |
| A suitable test should be devised for the early stage | TSH detects SCH at early stage |
| The test should be acceptable | TSH is a simple blood test, It may be difficult to identify all women in pre-conception stage but in an era of contraception, a fair proportion can be identified |
| Intervals for repeating the test should be determined | Usual guidance is once a year |
| Adequate health service provision should be made for the extra clinical workload resulting from screening | Thyroxine replacement is a simple treatment |
| The risks, both physical and psychological, should be less than the benefits | There is minimal risk of taking a simple blood test |
| The costs should be balanced against the benefits | As TSH is an inexpensive test and thyroxine is an inexpensive medication, there is a potential that benefits will overweigh the costs even if a few preterm birth, miscarriage or cognitive impairment is prevented |
| Criteria . | Applicability to TSH . |
|---|---|
| The condition being screened for, should be an important health problem | SCH is a common clinical problem; Gharib et al. (2005) |
| The natural history of the condition should be well understood | This is well defined in females - annual rate of progression from SCH to OH was 2.6% in patients negative for thyroid antibodies, but 4.3% if TPO Abs were present; Razvi et al. (2010) |
| There should be a detectable early stage | SCH precedes the development of OH |
| Treatment at an early stage should be of more benefit than at a later stage | In pregnancy pre-conception optimization is thought to be better especially for neurodevelopment of the offspring |
| A suitable test should be devised for the early stage | TSH detects SCH at early stage |
| The test should be acceptable | TSH is a simple blood test, It may be difficult to identify all women in pre-conception stage but in an era of contraception, a fair proportion can be identified |
| Intervals for repeating the test should be determined | Usual guidance is once a year |
| Adequate health service provision should be made for the extra clinical workload resulting from screening | Thyroxine replacement is a simple treatment |
| The risks, both physical and psychological, should be less than the benefits | There is minimal risk of taking a simple blood test |
| The costs should be balanced against the benefits | As TSH is an inexpensive test and thyroxine is an inexpensive medication, there is a potential that benefits will overweigh the costs even if a few preterm birth, miscarriage or cognitive impairment is prevented |
SCH, subclinical hypothyroidism; TSH, thyroid-stimulating hormone; TPO, thyroid peroxidise; OH, overt hypothyroid.
Whom should we screen?
ATA recommends that all newly pregnant patients or those seeking pregnancy should undergo clinical evaluation and those with any of the risk factors shown in Table II should be tested for serum TSH levels (Recommendation 97; Alexander et al., 2017). However, previous studies have suggested that a case finding approach misses a large proportion of women with elevated TSH (Chang et al., 2011) and is not cost-effective (Thung et al., 2009; Dosiou et al., 2012) when compared to a strategy of universal screening. Hence it has been suggested that screening for all women could be cost-effective especially in areas of iodine deficiency such as UK (Bath et al., 2013).
Risk factors for targeted case finding criteria for subclinical hypothyroidism (Alexander et al., 2017).
| (1) Age > 30 years |
| (2) Personal history of thyroid dysfunction |
| (3) Prior head or neck irradiation |
| (4) Prior thyroid surgery |
| (5) Family history |
| (6) Symptoms |
| (7) Presence of Goitre |
| (8) TPO Ab positivity |
| (9) Autoimmunity |
| (10) Infertility |
| (11) Miscarriage or preterm delivery |
| (12) Iodine deficient population |
| (13) Medications and iodinated contrast media |
| (14) Morbid obesity (BMI > 40 kg/m2) |
| (1) Age > 30 years |
| (2) Personal history of thyroid dysfunction |
| (3) Prior head or neck irradiation |
| (4) Prior thyroid surgery |
| (5) Family history |
| (6) Symptoms |
| (7) Presence of Goitre |
| (8) TPO Ab positivity |
| (9) Autoimmunity |
| (10) Infertility |
| (11) Miscarriage or preterm delivery |
| (12) Iodine deficient population |
| (13) Medications and iodinated contrast media |
| (14) Morbid obesity (BMI > 40 kg/m2) |
Risk factors for targeted case finding criteria for subclinical hypothyroidism (Alexander et al., 2017).
| (1) Age > 30 years |
| (2) Personal history of thyroid dysfunction |
| (3) Prior head or neck irradiation |
| (4) Prior thyroid surgery |
| (5) Family history |
| (6) Symptoms |
| (7) Presence of Goitre |
| (8) TPO Ab positivity |
| (9) Autoimmunity |
| (10) Infertility |
| (11) Miscarriage or preterm delivery |
| (12) Iodine deficient population |
| (13) Medications and iodinated contrast media |
| (14) Morbid obesity (BMI > 40 kg/m2) |
| (1) Age > 30 years |
| (2) Personal history of thyroid dysfunction |
| (3) Prior head or neck irradiation |
| (4) Prior thyroid surgery |
| (5) Family history |
| (6) Symptoms |
| (7) Presence of Goitre |
| (8) TPO Ab positivity |
| (9) Autoimmunity |
| (10) Infertility |
| (11) Miscarriage or preterm delivery |
| (12) Iodine deficient population |
| (13) Medications and iodinated contrast media |
| (14) Morbid obesity (BMI > 40 kg/m2) |
When should we screen?
Screening for SCH can be done either prior to conception or once pregnancy has been established. A meta-analysis of three trials in women undergoing IVF/ICSI reported that pre-conception screening followed by treatment of those with subclinical hypothyroidism resulted in reduction in miscarriage rate (Velkeniers et al., 2013). There is no reason to suppose that these results are not applicable to a non IVF population.
In a joint Statement on Management from the American Association of Clinical Endocrinologists, the American Thyroid Association and The Endocrine Society (Gharib et al., 2005), routine screening was recommended for subclinical thyroid dysfunction in adults, including pregnant women and those contemplating pregnancy. This was based on the fact that the potential benefits of early detection and treatment of subclinical thyroid dysfunction significantly outweigh the potential side effects that could result from early diagnosis and therapy. However, routine pre-conception testing for all is not recommended currently by any of the relevant professional bodies (ATA, ACOG, RCOG)—primarily because there are no data from large scale randomized trials on the general population to support this.
How do we define subclinical hypothyroidism?
Although, it is believed that treatment of subclinical hypothyroidism will improve outcomes, there is no consensus about what the condition actually is. ATA recommends that population-based trimester-specific reference ranges for serum TSH, based on local population data should be used to define SCH in pregnancy (Alexander et al., 2017).
Published and ongoing randomized trials show a wide variation in the criteria for making a diagnosis of SCH (Table III). The uses of various combinations of TSH and TPO antibody levels have added to the prevailing sense of confusion in this field.
Existing and ongoing trials on thyroxine replacement in women with subclinical hypothyroidism and pregnancy and neonatal outcomes.
| Study . | Population . | Definition of subclinical hypothyroidism . | Primary outcome . | Results . |
|---|---|---|---|---|
| Published studies (on women undergoing IVF/ICSI) | ||||
| Negro et al. (2005) | Women undergoing first cycle of IVF/ICSI | TSH between 0.27 and 4.2 mIU/l and TPO positive | Delivery rate | Higher rate of miscarriage in TPO +Ab group; no difference in pregnancy rateLT4 treatment do not affect delivery rate |
| Abdel Rahman et al. (2010) | Women undergoing first cycle of IVF/ICSI with SCH | TSH 0.27–4.2 mIU/l | Delivery rate | Thyroid supplementation was associated with lower miscarriage rate, higher clinical pregnancy and delivery rate in women with subclinical hypothyroidism undergoing assisted reproduction |
| Kim et al. (2011) | Women undergoing first cycle of IVF/ICSI with SCH | TSH 0.27–4.2 mIU/l | Delivery rate | Thyroid supplementation was associated with lower miscarriage rate, in women with subclinical hypothyroidism undergoing Assisted Reproduction |
| Published studies (on women who were pregnant) | ||||
| Zhou et al. (2015) Trial was aborted due to funding withdrawal | Women with a singleton pregnancy of fewer than 20 weeks in mild/moderate iodine deficiency area | Childhood neurodevelopment assessed at 18 months by using Bayley Scales | Iodine supplementation in pregnancy did not result in better childhood neurodevelopment | |
| Casey et al. (2017) | women with a singleton pregnancy before 20 weeks of gestation | TSH ≥ 4.00 mIU/l and T4 level—11 to 24 pmol/l | IQ score at 5 years of age | Treatment for subclinical hypothyroidism or hypothyroxinemia beginning between 8 and 20 weeks of gestation did not result in significantly better cognitive outcomes in children through 5 years of age than no treatment for those conditions |
| Lazarus et al. (2012) | Pregnant women with gestational age < 15 weeks and 6 days | TSH above the 97.5th percentile or free T4 below the 2.5th percentile, or both | IQ at 3 years of age | Antenatal screening (at a median gestational age of 12 weeks 3 days) and maternal treatment for hypothyroidism did not result in improved cognitive function in children at 3 years of age |
| Thyroxine prescribed at 15 weeks of gestation | ||||
| Ongoing trials | ||||
| TABLET Recruitment complete | History of miscarriage/having fertility treatment | TSH between 0.44 and 3.6 mIU/l and TPO positive | Live birth beyond 34 weeks | Awaited |
| T4 LIFE Ongoing | History of recurrent miscarriage (2 miscarriages) | TPO positive and TSH < 5 mIU/l | Live birth beyond 24 weeks | Awaited |
| Study . | Population . | Definition of subclinical hypothyroidism . | Primary outcome . | Results . |
|---|---|---|---|---|
| Published studies (on women undergoing IVF/ICSI) | ||||
| Negro et al. (2005) | Women undergoing first cycle of IVF/ICSI | TSH between 0.27 and 4.2 mIU/l and TPO positive | Delivery rate | Higher rate of miscarriage in TPO +Ab group; no difference in pregnancy rateLT4 treatment do not affect delivery rate |
| Abdel Rahman et al. (2010) | Women undergoing first cycle of IVF/ICSI with SCH | TSH 0.27–4.2 mIU/l | Delivery rate | Thyroid supplementation was associated with lower miscarriage rate, higher clinical pregnancy and delivery rate in women with subclinical hypothyroidism undergoing assisted reproduction |
| Kim et al. (2011) | Women undergoing first cycle of IVF/ICSI with SCH | TSH 0.27–4.2 mIU/l | Delivery rate | Thyroid supplementation was associated with lower miscarriage rate, in women with subclinical hypothyroidism undergoing Assisted Reproduction |
| Published studies (on women who were pregnant) | ||||
| Zhou et al. (2015) Trial was aborted due to funding withdrawal | Women with a singleton pregnancy of fewer than 20 weeks in mild/moderate iodine deficiency area | Childhood neurodevelopment assessed at 18 months by using Bayley Scales | Iodine supplementation in pregnancy did not result in better childhood neurodevelopment | |
| Casey et al. (2017) | women with a singleton pregnancy before 20 weeks of gestation | TSH ≥ 4.00 mIU/l and T4 level—11 to 24 pmol/l | IQ score at 5 years of age | Treatment for subclinical hypothyroidism or hypothyroxinemia beginning between 8 and 20 weeks of gestation did not result in significantly better cognitive outcomes in children through 5 years of age than no treatment for those conditions |
| Lazarus et al. (2012) | Pregnant women with gestational age < 15 weeks and 6 days | TSH above the 97.5th percentile or free T4 below the 2.5th percentile, or both | IQ at 3 years of age | Antenatal screening (at a median gestational age of 12 weeks 3 days) and maternal treatment for hypothyroidism did not result in improved cognitive function in children at 3 years of age |
| Thyroxine prescribed at 15 weeks of gestation | ||||
| Ongoing trials | ||||
| TABLET Recruitment complete | History of miscarriage/having fertility treatment | TSH between 0.44 and 3.6 mIU/l and TPO positive | Live birth beyond 34 weeks | Awaited |
| T4 LIFE Ongoing | History of recurrent miscarriage (2 miscarriages) | TPO positive and TSH < 5 mIU/l | Live birth beyond 24 weeks | Awaited |
LT4, levothyroxine.
Existing and ongoing trials on thyroxine replacement in women with subclinical hypothyroidism and pregnancy and neonatal outcomes.
| Study . | Population . | Definition of subclinical hypothyroidism . | Primary outcome . | Results . |
|---|---|---|---|---|
| Published studies (on women undergoing IVF/ICSI) | ||||
| Negro et al. (2005) | Women undergoing first cycle of IVF/ICSI | TSH between 0.27 and 4.2 mIU/l and TPO positive | Delivery rate | Higher rate of miscarriage in TPO +Ab group; no difference in pregnancy rateLT4 treatment do not affect delivery rate |
| Abdel Rahman et al. (2010) | Women undergoing first cycle of IVF/ICSI with SCH | TSH 0.27–4.2 mIU/l | Delivery rate | Thyroid supplementation was associated with lower miscarriage rate, higher clinical pregnancy and delivery rate in women with subclinical hypothyroidism undergoing assisted reproduction |
| Kim et al. (2011) | Women undergoing first cycle of IVF/ICSI with SCH | TSH 0.27–4.2 mIU/l | Delivery rate | Thyroid supplementation was associated with lower miscarriage rate, in women with subclinical hypothyroidism undergoing Assisted Reproduction |
| Published studies (on women who were pregnant) | ||||
| Zhou et al. (2015) Trial was aborted due to funding withdrawal | Women with a singleton pregnancy of fewer than 20 weeks in mild/moderate iodine deficiency area | Childhood neurodevelopment assessed at 18 months by using Bayley Scales | Iodine supplementation in pregnancy did not result in better childhood neurodevelopment | |
| Casey et al. (2017) | women with a singleton pregnancy before 20 weeks of gestation | TSH ≥ 4.00 mIU/l and T4 level—11 to 24 pmol/l | IQ score at 5 years of age | Treatment for subclinical hypothyroidism or hypothyroxinemia beginning between 8 and 20 weeks of gestation did not result in significantly better cognitive outcomes in children through 5 years of age than no treatment for those conditions |
| Lazarus et al. (2012) | Pregnant women with gestational age < 15 weeks and 6 days | TSH above the 97.5th percentile or free T4 below the 2.5th percentile, or both | IQ at 3 years of age | Antenatal screening (at a median gestational age of 12 weeks 3 days) and maternal treatment for hypothyroidism did not result in improved cognitive function in children at 3 years of age |
| Thyroxine prescribed at 15 weeks of gestation | ||||
| Ongoing trials | ||||
| TABLET Recruitment complete | History of miscarriage/having fertility treatment | TSH between 0.44 and 3.6 mIU/l and TPO positive | Live birth beyond 34 weeks | Awaited |
| T4 LIFE Ongoing | History of recurrent miscarriage (2 miscarriages) | TPO positive and TSH < 5 mIU/l | Live birth beyond 24 weeks | Awaited |
| Study . | Population . | Definition of subclinical hypothyroidism . | Primary outcome . | Results . |
|---|---|---|---|---|
| Published studies (on women undergoing IVF/ICSI) | ||||
| Negro et al. (2005) | Women undergoing first cycle of IVF/ICSI | TSH between 0.27 and 4.2 mIU/l and TPO positive | Delivery rate | Higher rate of miscarriage in TPO +Ab group; no difference in pregnancy rateLT4 treatment do not affect delivery rate |
| Abdel Rahman et al. (2010) | Women undergoing first cycle of IVF/ICSI with SCH | TSH 0.27–4.2 mIU/l | Delivery rate | Thyroid supplementation was associated with lower miscarriage rate, higher clinical pregnancy and delivery rate in women with subclinical hypothyroidism undergoing assisted reproduction |
| Kim et al. (2011) | Women undergoing first cycle of IVF/ICSI with SCH | TSH 0.27–4.2 mIU/l | Delivery rate | Thyroid supplementation was associated with lower miscarriage rate, in women with subclinical hypothyroidism undergoing Assisted Reproduction |
| Published studies (on women who were pregnant) | ||||
| Zhou et al. (2015) Trial was aborted due to funding withdrawal | Women with a singleton pregnancy of fewer than 20 weeks in mild/moderate iodine deficiency area | Childhood neurodevelopment assessed at 18 months by using Bayley Scales | Iodine supplementation in pregnancy did not result in better childhood neurodevelopment | |
| Casey et al. (2017) | women with a singleton pregnancy before 20 weeks of gestation | TSH ≥ 4.00 mIU/l and T4 level—11 to 24 pmol/l | IQ score at 5 years of age | Treatment for subclinical hypothyroidism or hypothyroxinemia beginning between 8 and 20 weeks of gestation did not result in significantly better cognitive outcomes in children through 5 years of age than no treatment for those conditions |
| Lazarus et al. (2012) | Pregnant women with gestational age < 15 weeks and 6 days | TSH above the 97.5th percentile or free T4 below the 2.5th percentile, or both | IQ at 3 years of age | Antenatal screening (at a median gestational age of 12 weeks 3 days) and maternal treatment for hypothyroidism did not result in improved cognitive function in children at 3 years of age |
| Thyroxine prescribed at 15 weeks of gestation | ||||
| Ongoing trials | ||||
| TABLET Recruitment complete | History of miscarriage/having fertility treatment | TSH between 0.44 and 3.6 mIU/l and TPO positive | Live birth beyond 34 weeks | Awaited |
| T4 LIFE Ongoing | History of recurrent miscarriage (2 miscarriages) | TPO positive and TSH < 5 mIU/l | Live birth beyond 24 weeks | Awaited |
LT4, levothyroxine.
Risk of routine testing
The potential pitfalls of universal screening followed by treatment include increased health costs and concerns about safety. Patients started on levothyroxine therapy need to be monitored closely to ensure that they are euthyroid. This can lead to multiple appointments at primary or secondary care settings, create anxiety and delay attempts to conceive or seek fertility treatment. The full economic cost of such a strategy (routine screening and treatment) is unknown and will need to be explored in future studies.
Levothyroxine is a commonly used drug in obstetric-thyroid clinics, and has a well-established safety profile. Three randomized studies (Negro et al., 2005; Abdel Rahman et al., 2010; Kim et al., 2011) did not raise safety concerns for the mother or the baby—such as hyperthyroidism from overtreatment with levothyroxine. A literature review found that the potential risk of levothyroxine was limited to the development of subclinical hyperthyroidism in the mother, which can occur in 14–21% of those treated (Surks et al., 2004). In contrast other report suggests that thyroxine replacement is associated with fewer miscarriages but increases the risk of preterm delivery and gestational diabetes (Maraka et al., 2017). These concerns about safety of both mother and baby associated with pre-conception use of thyroxine means that treatment needs to be closely monitored. This will inevitably increase anxiety levels and drive up healthcare and societal costs.
How good is the existing evidence base?
Although it has been suggested that maternal hypothyroidism can affect cognitive outcome in the offspring, two large randomized trials (Lazarus et al., 2012; Casey et al., 2017) have not demonstrated any improvement in cognitive function in children of women diagnosed with SCH in whom levothyroxine was commenced between 15 and 16 weeks of gestation. This could be due to the fact that, for optimal development of the foetal brain, adequate levels of thyroxine are critical during the early weeks of pregnancy (well before most women would have had their TSH levels checked).
Despite the evidence from these two randomized trials, experts continue to endorse the recent guidelines of the American Thyroid Association (Cooper and Pearce, 2017). This is because, it is perceived that the early initiation of low-dose levothyroxine therapy for subclinical hypothyroidism is inexpensive, unlikely to be harmful and ‘may’ be beneficial. In addition, selective screening does not seem to be cost-effective and the risk matrix used is imprecise, thus excluding many potentially affected women. On the other hand, universal screening may be seen by experts as practicing defensive medicine which could be expensive and wasteful.
In the IVF/ICSI setting, screening and treatment of subclinical hypothyroidism, prior to commencing treatment has been shown to be beneficial in terms of reducing miscarriage rates (Negro et al., 2005; Abdel Rahman et al., 2010; Kim et al., 2011). There are no published randomized controlled trials for ‘routine’ pre-conception testing of the general population followed by treatment of subclinical hypothyroidism and its impact on obstetric and neonatal outcomes and cognitive function.
What evidence will ongoing trials provide?
Currently there are two ongoing trials [TABLET (ISRCTN: 15948785) and T4 Life (NTR 3364)] which use a case finding approach in women with a history of miscarriage or infertility with positive TPO antibodies. Primary outcomes for both are live birth beyond 34 weeks (Table III). Neither of them will answer the question whether routine pre-conception testing followed by treatment of hypothyroidism to attain a pre-conception TSH of <2.5 mu/l will improve the cognitive function.
Research implications
Assuming that serum TSH is a potentially suitable screening test, thyroxine an inexpensive medication and SCH a preventable cause of intellectual impairment, the case for routine screening followed by treatment seems persuasive, but there a number of issues which need further elucidation such as costs, side effects, safety and crucially, efficacy.
We propose that in the absence of an agreement on routine pre-conception testing of TSH, uncertainty regarding treatment of SCH based on the current definition, and lack of robust evidence regarding the clinical as well as cost-effectiveness of such a strategy, an appropriately powered large multicentre screening trial is urgently needed to provide a definitive answer to clinicians, policymakers as well as patients. To have genuine public health impact, the population in such a trial should comprise women who are trying to conceive (as opposed to a case finding approach focussed on women with a history of infertility or miscarriage); the intervention should be screening of the eligible population and treatment with levothyroxine for those with SCH (defined as TSH > 2.5 mu/l). The primary outcome should be cognitive status in the offspring (Table IV) with an expected improvement in IQ scores, as suggested by previous trials (minimum of a five point improvement in median IQ score in intervention group).
| Population | All women trying to conceive (irrespective of the duration) will be invited for TSH screening (irrespective of attending any clinic—infertility, miscarriage, early pregnancy, sexual reproductive health, GP surgeries/no clinic) |
| Intervention | TSH testing and prescribing Thyroxine if TSH is >2.5 mIU/l, irrespective of antibody status |
| Control | TSH testing and prescribing Placebo if TSH is >2.5 mIU/l, irrespective of antibody status |
| Outcome |
|
| Population | All women trying to conceive (irrespective of the duration) will be invited for TSH screening (irrespective of attending any clinic—infertility, miscarriage, early pregnancy, sexual reproductive health, GP surgeries/no clinic) |
| Intervention | TSH testing and prescribing Thyroxine if TSH is >2.5 mIU/l, irrespective of antibody status |
| Control | TSH testing and prescribing Placebo if TSH is >2.5 mIU/l, irrespective of antibody status |
| Outcome |
|
| Population | All women trying to conceive (irrespective of the duration) will be invited for TSH screening (irrespective of attending any clinic—infertility, miscarriage, early pregnancy, sexual reproductive health, GP surgeries/no clinic) |
| Intervention | TSH testing and prescribing Thyroxine if TSH is >2.5 mIU/l, irrespective of antibody status |
| Control | TSH testing and prescribing Placebo if TSH is >2.5 mIU/l, irrespective of antibody status |
| Outcome |
|
| Population | All women trying to conceive (irrespective of the duration) will be invited for TSH screening (irrespective of attending any clinic—infertility, miscarriage, early pregnancy, sexual reproductive health, GP surgeries/no clinic) |
| Intervention | TSH testing and prescribing Thyroxine if TSH is >2.5 mIU/l, irrespective of antibody status |
| Control | TSH testing and prescribing Placebo if TSH is >2.5 mIU/l, irrespective of antibody status |
| Outcome |
|
Pre-conception screening should be feasible in the era where access to contraception is universal and at least 60% pregnancies are planned (Sedgh et al. 2014). Potential participants could include women who plan to come off contraception, seek pre-conception advice or attend fertility clinics and early pregnancy units. Sexually active women could also be recruited from opportunistically from primary care and sexual reproductive health settings where they might attend for other reasons. Advertisements in the media and public places and use of social media could be used for recruitment to ensure a representative sample of pre-conception women. Although women who have unplanned pregnancies will not be included in such a trial, they would not constitute a population who are likely to attend screening even if this strategy were to be proven to be effective With wider availability of contraception, the proportion of unplanned pregnancy should decline. A policy of universal pre-conception screening can only be recommended if such a trial is able to convincingly demonstrate the clinical and cost-effectiveness of such an approach.
The risk of not undertaking such a trial is gradual shift in practice, tacit acceptance of over-medicalization, potential risks and real healthcare costs - all of which will continue to challenge our commitment to evidence based medicine.
Clinical implications
Till such time that a trial is completed, a pragmatic approach will be for every unit to liaise with their local endocrinologists to have a standardized protocol. An example is given in Fig. 1.
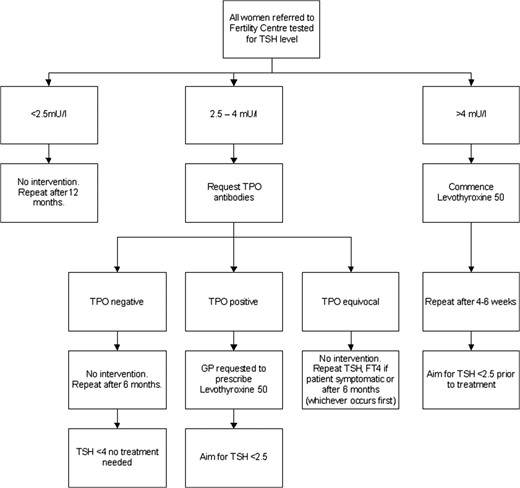
Suggested management for pre-conception optimization of thyroxine.
Conclusions
Thyroid deficiency is believed to be a preventable cause of cognitive impairment. Existing studies have proven that routine screening in pregnancy and commencing thyroxine thereafter does not improve cognitive function. Currently ongoing studies will provide further clarity on whether a case finding approach with pre-conception treatment improves pregnancy outcomes. Previous studies have suggested that case finding approach might miss a significant proportion of those who may benefit from intervention. There is continuing uncertainty regarding clinical and cost-effectiveness of routine pre-conception testing and treatment of those with SCH to attain pre-conception TSH to <2.5 mu/l. Variation in guidance from different professional bodies will continue unless there is good quality evidence generated by appropriately powered randomized controlled trials.
Authors’ roles
A.M. and S.B. conceived the manuscript. J.P. and P.B. did the literature searches. All authors contributed to all versions of the manuscript
Funding
No external funding was sought to prepare this manuscript.
Conflict of interest
No authors have any conflict of interest in relation to this manuscript.



