-
PDF
- Split View
-
Views
-
Cite
Cite
Chi-Wai Wong, Kevin K.W. Lam, Cheuk-Lun Lee, William S.B. Yeung, Wei E. Zhao, Pak-Chung Ho, Jian-Ping Ou, Philip C.N. Chiu, The roles of protein disulphide isomerase family A, member 3 (ERp57) and surface thiol/disulphide exchange in human spermatozoa–zona pellucida binding, Human Reproduction, Volume 32, Issue 4, April 2017, Pages 733–742, https://doi.org/10.1093/humrep/dex007
Close - Share Icon Share
Abstract
Are multimeric sperm plasma membrane protein complexes, ERp57 and sperm surface thiol content involved in human spermatozoa–zona pellucida (ZP) interaction?
ERp57 is a component of a multimeric spermatozoa–ZP receptor complex involved in regulation of human spermatozoa–ZP binding via up-regulation of sperm surface thiol content.
A spermatozoon acquires its fertilization capacity within the female reproductive tract by capacitation. Spermatozoa–ZP receptor is suggested to be a composite structure that is assembled into a functional complex during capacitation. Sperm surface thiol content is elevated during capacitation. ERp57 is a protein disulphide isomerase that modulates the thiol-disulphide status of proteins.
The binding ability and components of protein complexes in extracted membrane protein fractions of spermatozoa were studied. The roles of capacitation, thiol-disulphide reagent treatments and ERp57 on sperm functions and sperm surface thiol content were assessed.
Spermatozoa were obtained from semen samples from normozoospermic men. Human oocytes were obtained from an assisted reproduction programme. Blue native polyacrylamide gel electrophoresis, western ligand blotting and mass spectrometry were used to identify the components of solubilized ZP/ZP3-binding complexes. The localization and expression of sperm surface thiol and ERp57 were studied by immunostaining and sperm surface protein biotinylation followed by western blotting. Sperm functions were assessed by standard assays.
Several ZP-binding complexes were isolated from the cell membrane of capacitated spermatozoa. ERp57 was a component of one of these complexes. Capacitation significantly increased the sperm surface thiol content, acrosomal thiol distribution and ERp57 expression on sperm surface. Sperm surface thiol and ERp57 immunoreactivity were localized to the acrosomal region of spermatozoa, a region responsible for ZP-binding. Up-regulation of the surface thiol content or ERp57 surface expression in vitro stimulated ZP-binding capacity of human spermatozoa. Blocking of ERp57 function by specific antibody or inhibitors against ERp57 reduced the surface thiol content and ZP-binding capacity of human spermatozoa.
N/A
The mechanisms by which up-regulation of surface thiol content stimulates spermatozoa–ZP binding have not been depicted.
Thiol-disulphide exchange is a crucial event in capacitation. ERp57 modulates the event and the subsequent fertilization process. Modulation of the surface thiol content of the spermatozoa of subfertile men may help to increase fertilization rate in assisted reproduction.
This work was supported by The Hong Kong Research Grant Council Grant HKU764611 and HKU764512M to P.C.N.C. The authors have no competing interests.
Introduction
Freshly ejaculated mammalian spermatozoa cannot fertilize an oocyte and require a maturational event termed capacitation that occurs during in vivo migration of the spermatozoa in the female reproductive tract to be competent in fertilization. During capacitation, the sperm plasma membrane undergoes dramatic changes and reorganization (Nixon et al., 2007). Fertilization starts with binding of the capacitated spermatozoon to the zona pellucida (ZP) of an oocyte. Human ZP composes of four glycoproteins, ZP1, ZP2, ZP3 and ZP4 (Lefievre et al., 2004). ZP3 is the primary binding site and the major inducers of acrosome reaction of human spermatozoa (Chiu et al., 2008a, 2008b).
The identity of human spermatozoa–ZP receptor(s) remains controversial. Several candidates including zona receptor kinase, alpha-d-mannosidase, sperm agglutination antigen 1 and fucosyltransferase 5 (FUT5) have been proposed for human spermatozoa (Chiu et al., 2014). However, none of them is solely responsible for mediating spermatozoa–ZP interaction, prompting a revision of the traditional concept of having a single, constitutively expressed spermatozoa–ZP receptor. Recent evidences indicate that the spermatozoa–ZP receptor is a composite structure involving coordinated actions of multiple molecules that are assembled into a functional complex during capacitation (Nixon et al., 2007; Chiu et al., 2014).
Sperm surface thiol content is elevated during capacitation (de Lamirande and Gagnon, 1998). Thiol oxidoreductases are a group of protein chaperones that catalyse the formation, reduction and rearrangement of disulphide bonds in mammalian cells. Among these enzymes, the protein disulphide isomerase (PDI) family is the most extensively studied enzymes important in modulating thiol-disulphide exchange (Maattanen et al., 2006). Apart from highly enriched in the endoplasmic reticulum (ER), mounting evidences show that plasma membrane also contains PDIs such as PDI family A, member 3 (PDIA3 also known as ERp57). In the ER, PDIs act predominantly as thiol oxidase and/or isomerase. On the cell surface, however, they act as reductase (Maattanen et al., 2006). Thiol-disulphide status is crucial to protein structure, function, folding and localization. Interestingly, the thiol/disulphide contents of human spermatozoa are associated with sperm maturation (Mercado et al., 1976) and infertility (Ramos et al., 2008), though little is known about the role of thiol status on sperm functions. Two previous studies reported a global increase in sperm plasma membrane thiol content after capacitation and an enhancement of capacitation by surface reductants (de Lamirande and Gagnon, 1998). The significance of sperm thiol status in fertilization is unknown.
Defective spermatozoa–ZP binding causes infertility and occurs in ~80% of oligospermic men (Liu and Baker, 2004). It is a major cause of low fertilization rates in clinical IVF (Liu and Baker, 2000), consistent with a meta-analysis showing high predictive power of spermatozoa–ZP-binding assay on fertilization outcome (Oehninger et al., 2000). Despite the importance of spermatozoa–ZP binding, its regulatory mechanisms are largely unknown. To solve this problem, successful identification of the sperm surface ZP-receptors is crucial. This study investigated the involvement of multimeric plasma membrane protein complexes and thiol/disulphide exchange in mediating human spermatozoa–ZP binding.
Materials and Methods
Ethical approval
The Institutional Review Board of the University of Hong Kong/Hospital Authority Hong Kong West Cluster approved the research protocol. Informed consent was obtained from donors of oocytes and semen samples.
Semen samples
Spermatozoa from men attending the infertility clinic at Queen Mary Hospital with normal semen parameters according to the World Health Organization criteria (WHO, 2010) were processed by AllGrad (Life Global, CT, USA) density gradient centrifugation. The processed spermatozoa were suspended in Earle's Balanced Salt Solution (EBSS; Sigma, St. Louis, MO, USA) pH 7.4, supplemented with 0.3% bovine serum albumin (BSA) (EBSS/0.3% BSA). Capacitated spermatozoa were prepared by incubating the processed spermatozoa in EBSS supplemented with 3% BSA (EBSS/3% BSA) at 37°C for 3 h in 5% CO2 before resuspension in EBSS/0.3% BSA (Chiu et al., 2005). The percentage of capacitated spermatozoa as determined by chlortetracycline staining (Chiu et al., 2005) was 51.2 ± 3.6% (N = 10).
Human oocyte collection
Unfertilized oocytes after intracytoplasmic sperm injection were obtained from the assisted reproduction programme at the Queen Mary Hospital. They were stored in phosphate buffered saline (PBS) containing 1.5 M MgCl2, 0.1% polyvinylpyrrolidone-36000, 40 mM 4-(2-hydroxyethyl)-1-piperazineethanesulphonic acid (HEPES), pH 7.2 at 4°C before use.
Radioiodination of ZP glycoprotein
Twenty-five micrograms of human solubilized ZP or purified ZP3 (Chiu et al., 2008b) in 0.01 ml of 0.05 M PBS (pH 7.4) was mixed thoroughly with 2 mCi of carrier-free sodium-125I (20 μl; GE Healthcare, Chicago, IL, USA) and freshly prepared chloramine T (Sigma) (100 μg in 0.02 ml PBS) for radioiodination. After 60 s, sodium metabisulphite (Sigma) (300 μg in 0.05 ml) was used to stop the reaction. Free 125I was removed by passing the mixture through a 10 ml desalting column.
Extraction of sperm membrane proteins
Sperm plasma membrane proteins of 50 × 106 capacitated AllGrad-processed spermatozoa in 0.1 ml of PBS were extracted by a detergent-free ProteoExtract® Transmembrane Protein Extraction Kit (TM-PEK; Novagen, Billerica, MA, USA) (Huang et al., 2013). The purities of the samples were determined by western blotting using antibodies against sperm membrane (flotillin-2) (Cross, 2004) and cytosolic protein markers (lactate dehydrogenase C, LDHC) (Martínez-Heredia et al., 2006).
Western ligand blotting of sperm protein complexes
Protein complexes in the membrane protein fractions were resolved by blue native gel (BNG) electrophoresis (Wittig and Schagger, 2008) (Supplementary Data Methods). The 125I-ZP/ZP3-binding abilities of the protein complexes were studied by western ligand blotting (Redgrove et al., 2011) (Supplementary Data Methods). The components of the protein complexes were studied by BNG/SDS-PAGE (Redgrove et al., 2011) and mass spectrometry (Supplementary Data Methods).
Expression and localization of sperm ERp57
SDS-PAGE resolved sperm membrane proteins were blotted on a polyvinylidene difluoride membrane. Western blotting was performed using a mouse monoclonal anti-ERp57 antibody (1:1000; Abcam, Cambridge, UK). Anti-tubulin antibody was used to account for sample loading.
For immunostaining, EBSS-washed human spermatozoa were incubated with the anti-ERp57 antibody (1:100) overnight at 4°C and washed. The bound antibodies were detected by Alexa Fluor 488-conjugated goat anti-mouse IgG (1:1000; Invitrogen, Waltham, MA, USA), and examined under a fluorescence microscope (Zeiss, Oberkochen, Germany) with ×600 magnification.
Biotinylation of total sperm surface proteins was performed by incubating ice-cold PBS-washed spermatozoa in PBS with freshly prepared EZ-Link Sulpho-NHS-SS-Biotin according to the manufacturer's instructions (Thermo Scientific, Inc., Waltham, IL, USA) (Supplementary Data Methods). The biotinylated spermatozoa were lysed in Cytobuster protein extraction reagent (Novagen) containing protease inhibitors (Calbiochem, San Diego, CA, USA). The biotinylated sperm surface proteins were captured by streptavidin agarose beads (Millipore, Billerica, MA, USA), and subjected to SDS-PAGE and western blotting using the anti-ERp57 antibody (Supplementary Data Methods). Biotin-conjugated irrelevant goat IgG (1:100; Abcam) was included as a control.
Biotinylation of the sperm surface thiol
The sperm surface thiol was biotinylated with a cell impermeant thiol-reactive maleimide derivative, 3-(N-maleimidylpropionyl)-biocytin (MPB; Sigma) (de Lamirande and Gagnon, 1998). MPB acts specifically with sulphhydryl groups or reduced disulphide bonds and does not label proteins lacking a free sulphhydryl group at a pH 6.5–7.5. EBSS-washed spermatozoa 1 ml of EBSS were incubated with 1 mM of MPB at 37°C for 30 min. Uncoupled MPB was removed by washing with EBSS/0.3% BSA. Sperm surface thiol semi-quantification and localization were then performed (Supplementary Data Methods).
Determination of sperm functions
Sperm functions including viability, motility, acrosome reaction and ZP binding were determined by trypan blue exclusion staining, computer-assisted sperm analysis, FITC-labelled peanut (Pisum sativum) agglutinin (FITC-PSA; Sigma) staining and hemizona-binding assay, respectively (Supplementary Data Methods).
Effects of thiol-disulphide modulating reagents on sperm surface thiol status and sperm functions
Spermatozoa were treated with different thiol-disulphide modulating reagents (Supplementary Data Table S1). The distribution and amount of surface thiol, and sperm functions of the treated spermatozoa were examined using the above assays.
The role of ERp57 in regulating sperm surface thiol status and sperm functions
Uncapacitated spermatozoa were capacitated in EBSS/3% BSA in the presence of rabbit polyclonal anti-ERp57 functional blocking antibody (1:10 000–1:10; Abcam) at 37°C for 3 h. Anti-ERp57 antibody pre-absorbed with 10-fold molar excess of recombinant ERp57 (Abcam) and isotopic antibody were used as controls. The sperm thiol content, sperm viability and motility, acrosomal status and ZP-binding capacity were evaluated.
Data analysis
All the data were expressed as mean ± SEM, and were analysed by SigmaPlot 11.0 (Systat Software, Inc., San Jose, CA, USA) and SigmaStat 2.03 (Jandel Scientific, San Rafael, CA, USA). Non-parametric analysis of variance on rank test was used for multiple comparisons followed by Mann–Whitney U-test as the post-test. A probability of <0.05 was considered statistically significant.
Results
ERp57 is a component of a spermatozoa–ZP receptor complex
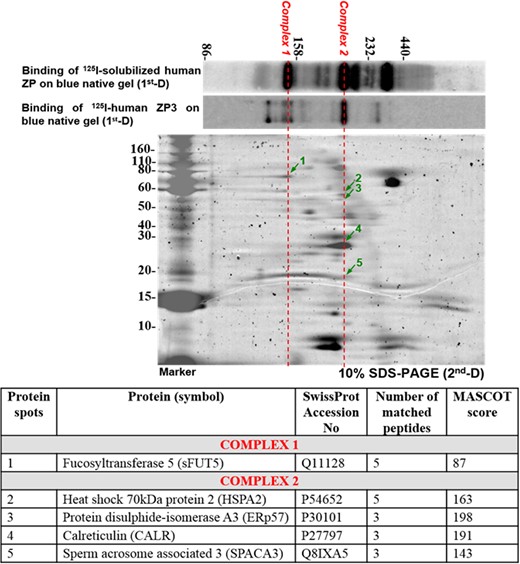
Two-dimensional BNG/SDS-PAGE of capacitated sperm plasma membrane proteins purified by ProteoExtract® Transmembrane Protein Extraction Kit. Arrows indicate components of complexes that bind to solubilized ZP/ZP3. The results shown are representative of three replicate experiments. Lower: Identification of individual components of the solubilized ZP/ZP3-interacting sperm protein complexes by mass spectrometry. BNG, blue native gel; ZP, zona pellucida.
Western ligand blotting using solubilized human ZP or isolated ZP3 with purity >92% (Chiu et al., 2008a) was used to investigate the ZP-binding abilities of the BNG-resolved protein complexes. Several protein complexes bound iodinated solubilized human ZP. At least two predominant protein complexes bound to ZP3 (Fig. 1). Mass spectrometric analysis identified sFUT5 as a component of one major solubilized ZP/ZP3-bound complex (Complex 1; Fig. 1) (N = 3). The components of the other solubilized ZP/ZP3-bound complexes were heat shock 70 kDa protein 2 (HSPA2), ERp57, calreticulin (CALR) and sperm acrosome associated 3 (SPACA3) (Complex 2; Fig. 1).
Expression of ERp57 protein in human spermatozoa
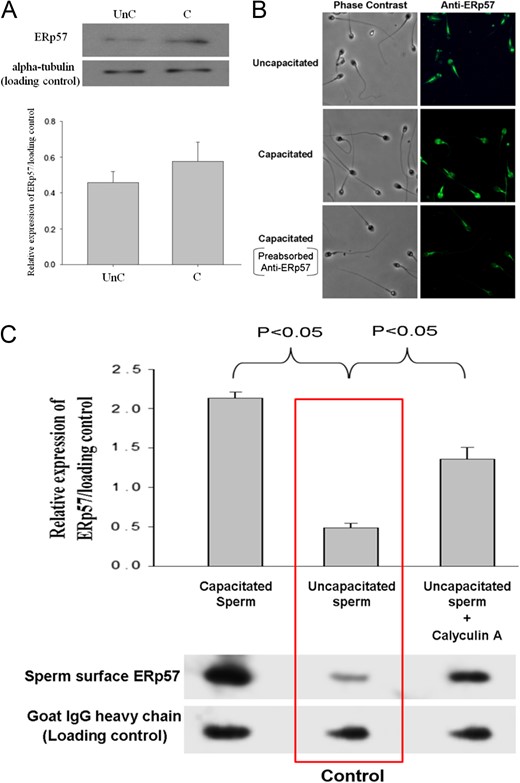
Expression of ERp57 in human spermatozoa. (A) Western blot analysis of total sperm protein extract probed with anti-ERp57 antibody (1:1000). Lower: Semi-quantitative comparison of ERp57 expression between uncapacitated (UnC) and capacitated (C) spermatozoa in western blotting by densitometric analysis. Data represent the mean ± SEM (N = 4). (B) Immuno-localization of ERp57 in human spermatozoa with or without capacitation. Spermatozoa were incubated with 1:100 mouse monoclonal anti-ERp57 antibody. They were visualized using Alexa Fluor 488-conjugated goat anti-mouse IgG. Anti-ERp57 antibody pre-absorbed with 10-fold molar excess recombinant ERp57 (Abcam) was used as the control. The results shown are representative of four replicate experiments examined under a fluorescence microscope with ×600 magnification. (C) Sperm surface expression of ERp57. Human spermatozoa (2 × 106) with or without 0.1 μM calyculin A treatment were resuspended in PBS containing 10 mM of membrane impermeable EZ-Link Sulpho-NHS-SS-Biotin, which labelled the sperm surface proteins. Lower: The biotinylated proteins were purified and resolved on SDS-PAGE followed by western blotting using anti-ERp57 antibody. The results shown are representative of four replicate experiments. Upper: Semi-quantitative comparison of ERp57 expression between sperm samples in western blotting by densitometric analysis was shown. Data represent the mean ± SEM (N = 4).
Western blot analysis of the streptavidin-purified biotinylated sperm surface protein demonstrated that sperm surface ERp57 expression was significantly (P < 0.05) induced by capacitation (Fig. 2C). Calyculin A is known to increase cell surface translocation of ERp57 (Obeid, 2008). Similar to the capacitation process, calyculin A treatment significantly (P < 0.05) enhanced the ERp57 translocation to the sperm surface in uncapacitated spermatozoa (Fig. 2C).
Effects of thiol-disulphide modulating reagents on sperm functions
ERp57 is a thiol oxidoreductase. We proposed that changes in the surface thiol status altered ZP-binding capacity of human spermatozoa. Therefore, spermatozoa were treated with different thiol-disulphide modulating reagents (Supplementary Data Table S1) at concentrations without significant effect on sperm viability (Supplementary Data Table SII), motility (Supplementary Data Table SIII), spontaneous and the A23187-induced acrosome reaction (Supplementary Data Table SIV) when compared to the untreated control.
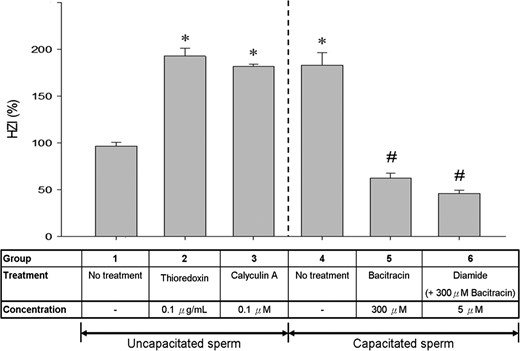
Comparison of HZI of spermatozoa incubated in the presence or absence of different thiol-disulphide modulating reagents. Bars represent the mean ± SEM of the results of 10 hemizona assays. Bars represent the mean ± SEM of the results of five HZA using five ZP and five different spermatozoa samples (N = 5). *P < 0.05 when compared with the uncapacitated control (Group 1). #P < 0.05 when compared with the capacitated control (Group 4). HZI, hemizona-binding index; HZA, hemizona-binding assay.
Effects of thiol-disulphide modulating reagents on sperm surface thiol content
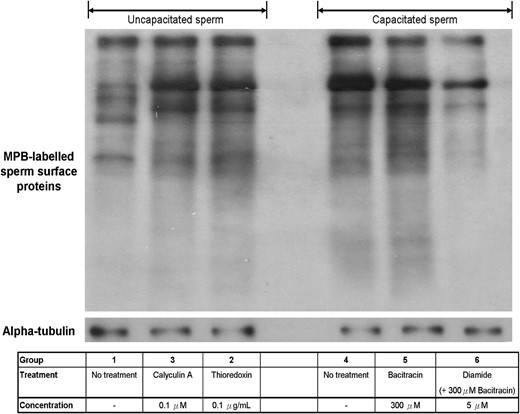
Semi-quantification of the human sperm surface thiol content. Cell surface thiol of human spermatozoa was labelled with MPB and quantified by western blotting. The results shown are representative of seven replicate experiments. MPB, 3-(N-maleimidylpropionyl)-biocytin.
Effects of thiol-disulphide modulating reagents on location of sperm surface thiol
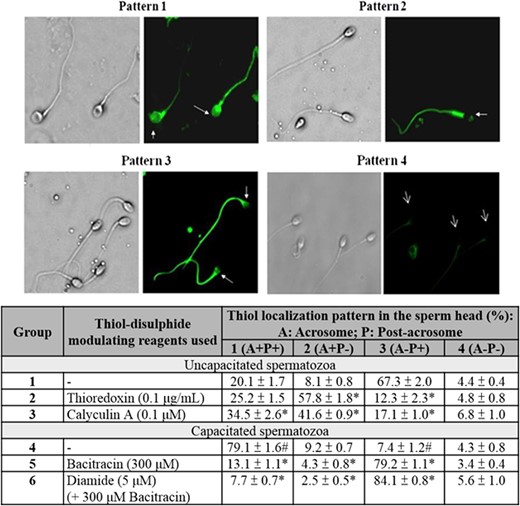
Localization of cell surface thiol on human spermatozoa. Sperm surface thiols were biotinylated with MPB. They were then visualized using streptavidin-FITC. The results shown are representative of five replicate experiments. Lower: Quantification of MPB-labelled spermatozoa with different staining patterns. Data represent the mean ± SEM (N = 5). #P < 0.05 when compared between uncapacitated (Group 1) and capacitated spermatozoa without treatment (Group 4). *P < 0.05 when compared with the corresponding control (Group 1 or Group 4) without treatment.
The percentage of MPB-labelled spermatozoa with acrosomal staining (Pattern 1 + 2) was significantly (P < 0.05) higher in the capacitated population than in the uncapacitated population (Fig. 5), suggesting an up-regulation of thiol content/reduced surface protein expression at the acrosomal region after capacitation. Similar stimulatory effect was observed in the uncapacitated spermatozoa after thioredoxin (Group 2) or calyculin A treatment (Group 3) (Fig. 5). On the other hand, capacitated spermatozoa treated with bacitracin in the presence (Group 6) or absence of diamide (Group 5) showed significant (P < 0.05) reduction in the amount of acrosomal thiol relative to the control (Group 4).
Role of ERp57 on spermatozoa–ZP binding and capacitation-induced sperm surface thiols up-regulation
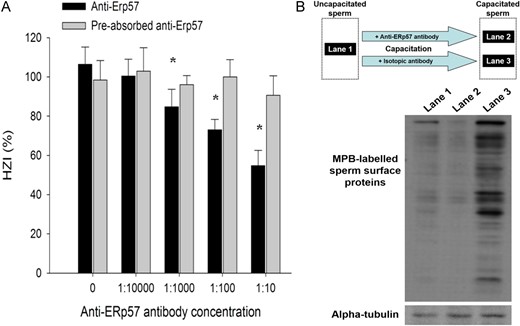
Effect of function blocking anti-ERp57 antibody on ZP-binding capacity and sperm surface thiol content of human spermatozoa. (A) Uncapacitated spermatozoa were capacitated in EBSS/3% BSA (control) or in EBSS/3% BSA containing different concentrations of anti-ERp57 antibody with or without recombinant ERp57 preabsorption. The ZP-binding ability of capacitated spermatozoa after treatment was determined by the HZA. The results were expressed as HZI (%). Each point represents the mean ± SEM of the results of 10 HZA using 10 ZP and 10 different spermatozoa samples (N = 10). *P < 0.05 when compared with the corresponding control without treatment. (B) Uncapacitated spermatozoa (Lane 1) were capacitated in EBSS/3% BSA containing 1:100 anti-ERp57 antibody (Lane 2) or isotopic antibody (Lane 3). Cell surface thiol of human spermatozoa (2 × 106/ml) was then labelled with MPB and semi-quantified by western blotting. The results shown are representative of five replicate experiments.
To demonstrate the role of ERp57 on sperm surface thiol content, spermatozoa were capacitated in the presence of anti-ERp57 functional blocking antibody. After capacitation, the sperm surface thiols were labelled by MPB followed by analysis of the MPB-labelled proteins (Fig. 6B). Consistent with Fig. 4, capacitation significantly increased the sperm surface thiol content (Fig. 6B; Lane 1 vs Lane 3). The increase in sperm surface thiol content was abolished by the inclusion of anti-ERp57 antibody during capacitation (Fig. 6B; Lane 1 vs Lane 2), indicating that capacitation involved the ERp57-mediated reduction of surface protein on spermatozoa.
Discussion
In this study, we demonstrated at least two protein complexes with ZP/ZP3-binding capacities on capacitated spermatozoa. One of which contains sFUT5 and the other one contains HSPA2, SPACA3, CALR and ERp57. The results provide evidence on the existence of functional multi-molecular ZP receptor complexes on capacitated human spermatozoa.
BNG has been applied to characterize the protein complexes on spermatozoa of a number of species including humans (Redgrove et al., 2012, 2013), mice (Dun et al., 2011) and pigs (Kongmanas et al., 2015). A human spermatozoa–ZP receptor complex consisting of arylsulphatase A, HSPA2 and sperm adhesion molecule 1 (SPAM1) is translocated to the acrosomal region during capacitation (Redgrove et al., 2012, 2013). We failed to identify arylsulphatase A and SPAM1 in the present ZP receptor complex. The discrepancy is probably due to difference in sample preparation for BNG/SDS-PAGE. We used TM-PEK extraction reagent, whereas n-dodecyl β-d-maltoside (a water soluble non-ionic detergent) was used in the other studies (Redgrove et al., 2012, 2013) to extract native proteins. The detergent used can significantly affect the conformation and components of the isolated protein complex (Wittig and Schagger, 2009). In order to demonstrate preservation of biological activities of our isolated sperm protein complexes, we performed western ligand blotting assay using solubilized ZP and purified ZP3, in addition to the use of functional blocking antibodies only (Redgrove et al., 2013). sFUT5 is a ZP-binding protein on the acrosomal region of human spermatozoa (Chiu et al., 2007). Its identification in one of the human ZP/ZP3-binding protein complexes supports the usefulness of the present protocol.
ERp57 is a 57 kDa member of the PDI family. ERp57 increases the cell surface thiol content of a variety of cell types such as fibrosarcoma cells (Jiang et al., 1999) and lymphocytes (Lawrence et al., 1996). Plasma membrane of human spermatozoa possesses ERp57 (Wittig and Schagger, 2008). Its functional role in fertilization has never been reported.
This study is the first to demonstrate an association between sperm surface thiol content and ZP-binding capacity of human spermatozoa with four observations. First, capacitated spermatozoa had a higher ZP-binding capacity and sperm surface thiol content when compared to uncapacitated spermatozoa, consistent with other studies (de Lamirande and Gagnon, 1998). Second, capacitation induced an increase in thiol content at the acrosomal region of human spermatozoa, a region important for ZP-binding (Chiu et al., 2008b). Third, the ZP-binding capacity of uncapacitated spermatozoa was up-regulated by increasing the sperm surface thiol content (by thioredoxin) or PDI surface expression (by calyculin A). Finally, inhibition of the sperm surface PDI activity (by bacitracin) and/or thiol formation (by diamide) suppressed the ZP-binding capacity of capacitated spermatozoa.
Calyculin A is a protein phosphatase ½A inhibitor. It promotes cell surface expression of ERp57 via inhibition of protein phosphatase 1 (Obeid, 2008). Calyculin A treatment increased the sperm surface thiol content and the ZP-binding capacity of uncapacitated spermatozoa. Since the initiation of human sperm capacitation is associated with a decrease in the phosphatase activity (e.g. phosphatase 2A) (Signorelli et al., 2012), the calyculin A-induced spermatozoa–ZP binding may also be mediated by the stimulatory effect of calyculin A on sperm capacitation, instead of PDI cell surface translocation. Therefore, the experiment was repeated with a PDI inhibitor, bacitracin (Mandel et al., 1993), to confirm the role of sperm surface thiol formation on ZP-binding.
In this study, we further demonstrated for the first time that the sperm surface ERp57 is important in regulating the sperm surface thiol content and spermatozoa–ZP binding. Consistent with previous findings (Zhang et al., 2007), we localized the ERp57 expression to the head, midpiece and tail region of human spermatozoa. The molecule is translocated to the sperm surface at the acrosomal region during in vitro capacitation. Since the acrosomal region is responsible for spermatozoa–ZP binding, the up-regulation of acrosomal ERp57 expression highlights the importance of ERp57 in the process. Indeed, hemizona-binding assay clearly demonstrated that anti-ERp57 antibody dose-dependently suppressed the ZP-binding capacity of capacitated spermatozoa.
Sperm ERp57 is also involved in human gamete fusion (Zhang et al., 2007). Thus, sperm ERp57 may have multiple roles in fertilization. Consistently, the expression level of ERp57 on human spermatozoa was associated with IVF outcome; the expression of ERp57 was dramatically lower in IVF patients with low fertilization rates when compared with those with high fertilization rates or fertile sperm donors (Zhang et al., 2007).
There is no significant difference in the total ERp57 expression between uncapacitated and capacitated spermatozoa. However, western blot analysis of the biotinylated sperm surface proteins indicated an up-regulation of the sperm surface ERp57 expression after capacitation. Although the mechanism for the capacitation-induced relocalization is unknown, the induction of ERp57 surface relocalization by calyculin A in uncapacitated spermatozoa indicates that the event may be associated with phosphatase inhibition. Capacitation is regulated by protein phosphorylation, which depends on balanced actions of protein kinases and phosphatases. In fact, the onset of capacitation in human spermatozoa is associated with a rapid decrease in the phosphatase activity (Signorelli et al., 2012). Interestingly, inhibition of protein phosphatase enhances changes in phospholipid distribution and lipid architecture of the sperm plasma membrane, which are events necessary for relocalization of the sperm surface protein (Visconti et al., 2011). Taken together, capacitation-induced ERp57 relocalization is likely to be mediated by phosphatase inhibition followed by plasma membrane reorganization on human spermatozoa.
This study demonstrated that increased thiol content on sperm surface via thiol-disulphide exchange is involved in the capacitation-induced acquisition of sperm fertilizing capability. How does sperm surface thiol formation stimulate spermatozoa–ZP binding is unknown. One possibility is that the thiol-disulphide exchange of sperm surface protein may modulate the intracellular signalling pathway, and thereby the capacitation process and the ZP-binding capacity. In fact, thioredoxin treatment promotes capacitation via enhancing tyrosine phosphorylation (de Lamirande and Gagnon, 1998), which has been implicated in spermatozoa–ZP binding and acrosome reaction (Urner and Sakkas, 2003; Naz and Rajesh, 2004). Since thiol-disulphide exchange reaction modifies reversibly the activity of cell surface receptor tyrosine kinases (Clark and Konstantopoulos, 1993), it is possible that the increased thiol content on sperm surface may up-regulate the ZP-binding capacity via its modulatory effect on the activities of sperm signalling components (e.g. receptor tyrosine kinase).
Our results suggest that increase in sperm surface thiol groups may enhance fertilization in assisted reproduction. In fact, a positive role of thiol modulation of sperm proteins on IVF or fertilizing ability of different mammalian spermatozoa have been demonstrated with the use of reduced glutathione (Gadea et al., 2013).
Taken together, our results provide direct evidence for the expression of a ZP-binding multimeric protein complex on the plasma membrane of capacitated human spermatozoa. ERp57 is a component of the complex. In addition, we demonstrated the roles of ERp57 and thiol-disulphide exchange in mediating human spermatozoa–ZP binding.
Supplementary data
Supplementary data are available at Human Reproduction online.
Authors’ roles
C.W.W., C.L.L., P.C.N.C. study design; researched data; data analysis; contributed to discussion; and wrote, reviewed and edited the manuscript. K.K.W.L., W.S.B.Y., P.C.H. study design; contributed to discussion; reviewed and edited the manuscript. J.P.O., W.E.Z. study design; researched data; reviewed and edited the manuscript.
Funding
The Hong Kong Research Grant Council (Grant HKU764611 and HKU764512M to P.C.N.C).
Conflict of interest
None declared.



