-
PDF
- Split View
-
Views
-
Cite
Cite
Hang-Wun Raymond Li, Ying-Xing Li, Tian-Tian Li, Hongjie Fan, Ernest Hung-Yu Ng, William Shu-Biu Yeung, Pak-Chung Ho, Kai-Fai Lee, Effect of ulipristal acetate and mifepristone at emergency contraception dose on the embryo-endometrial attachment using an in vitro human trophoblastic spheroid and endometrial cell co-culture model, Human Reproduction, Volume 32, Issue 12, December 2017, Pages 2414–2422, https://doi.org/10.1093/humrep/dex328
Close - Share Icon Share
Abstract
Do both ulipristal acetate (UPA) and mifepristone inhibit embryo-endometrial attachment at concentrations corresponding to the emergency contraception (EC) dose?
Both UPA and mifepristone at concentrations corresponding to the EC dose do not have an inhibitory effect on embryo implantation, although mifepristone at a higher concentration appeared to have such an effect.
Levonorgestrel is commonly used for EC, but it only acts through inhibition of ovulation. UPA and mifepristone have higher efficacy as EC compared to levonorgestrel; while there is some suggestion that mifepristone may interfere with implantation, whether UPA has post-ovulatory action in inhibiting implantation is yet to be confirmed.
An in vitro experimental study using trophoblastic spheroids made from JAr cell line as the embryo surrogate, and the Ishikawa cell line and primary human endometrial cells cultured to monolayer as the endometrial surrogate. The primary endometrial cells were collected from nine volunteer women in the mid-luteal phase with consent.
The study was conducted in a university gynaecology unit. The JAr and Ishikawa cell lines (or primary endometrial cells) were treated with graded concentrations of UPA (0, 0.04, 0.4 and 4 μM) or mifepristone (0, 0.1, 1 and 10 μM) for 24 h. Embryo-endometrial attachment was studied using an in vitro JAr spheroid-endometrial co-culture model. Expressions of progesterone receptor, β-catenin and glycogen synthase kinase 3 β (GSK-3β) were studied with real-time RT-PCR and Western blotting, respectively.
In the Ishikawa experiments, there was no significant difference in the JAr spheroid attachment rate after treatment with UPA at 0 (93.0%), 0.04 (93.6%), 0.4 (93.4%) and 4 (91.4%) μM concentrations (P > 0.05); the attachment rate was reduced after treatment with mifepristone only at 10 μM (79.8%, P < 0.0001) but not at 0.1 (92.1%) or 1.0 (95.2%) μM concentrations. In the primary endometrial cell experiments, again no significant difference was observed in the JAr spheroid attachment rate after treatment with UPA 4 μM (42.6%) compared to control (46.5%, P > 0.05). Both UPA and mifepristone could significantly up-regulate progesterone receptor expression. There was no significant alteration in expression of β-catenin and GSK-3β after treatment with UPA 4 μM or mifepristone 10 μM (P > 0.05).
The co-culture model is only a surrogate which may not fully represent the complicated process of embryo implantation in vivo, although there is no existing perfect model for studying implantation in vitro which fully resembles the latter.
The lack of inhibitory effect on embryo implantation by UPA and possibly mifepristone at concentrations corresponding to the EC dose is an important information for contraceptive counseling.
We had free supply of the UPA compound used in this study from Laboratoire HRA Pharma. This work was supported by a Seed Fund from the Centre of Reproduction, Development and Growth, Faculty of Medicine, The University of Hong Kong, Hong Kong.
Introduction
Researchers in the area of emergency contraception (EC) have been trying to find agents that are more effective and less restrictive in the timing of use after unprotected sexual intercourse. The most popular method for EC currently available for oral use consists of a single dose of 1.5 mg levonorgestrel to be taken within 72 h of unprotected sexual intercourse (Li et al., 2014; Faculty of Sexual and Reproductive Healthcare, 2017). Studies have shown that levonorgestrel is effective as EC only when administered before, but not after, ovulation (Novikova et al., 2007; Noe et al., 2010). Levonorgestrel inhibits or delays follicular development and ovulation if administered before onset of the LH surge but has no effect on implantation (Lalitkumar et al., 2007; Meng et al., 2009).
Mifepristone is an anti-progestogen which has also been developed as an effective EC. A Cochrane review suggested that a single dose of 25–50 mg mifepristone (and possibly at the 10 mg dose although evidence is less) taken up to 120 h after unprotected sexual intercourse is the most effective regimen for oral EC (Shen et al., 2017). Its higher efficacy may be due to the fact that mifepristone not only inhibits ovulation but also interferes with implantation (Lalitkumar et al., 2007; Meng et al., 2009).
More recently, ulipristal acetate (UPA), another selective progesterone receptor modulator, has been introduced for EC. Meta-analysis of two randomized controlled trials revealed that UPA had significantly lower failure rate compared to levonorgestrel (Glasier et al., 2010). It is possible that the better efficacy of UPA could be attributed to broader mechanisms of action. Apart from its effect in delaying ovulation (Stratton et al., 2000; Brache et al., 2010), it also alters endometrial histology (Stratton et al., 2000, 2010) although it is uncertain whether this may translate into inhibition of implantation.
We conducted this study to investigate the effect of UPA and mifepristone on embryo-endometrial attachment and on some of the molecular markers of implantation using a human JAr spheroid and endometrial cell co-culture model.
Materials and Methods
The effect of mifepristone and UPA on embryo-endometrial attachment was studied using a two-dimensional in vitro trophoblastic spheroid-endometrial cell attachment model as described previously (Liu et al., 2010). This model used the JAr (human choriocarcinoma) cell line made into spheroids as the embryo surrogate. In our previously published model, the Ishikawa endometrial adenocarcinoma cell line plated and cultured to a confluent monolayer was used as the endometrial surrogate. In the current study, the attachment model was replicated in addition using a similar monolayer of primary endometrial cells obtained from human volunteers which serves as the more physiological endometrial surrogate.
JAr and Ishikawa cell culture
The Ishikawa human endometrial adenocarcinoma cell line (ECACC 99040201, ATCC, Manassas, VA, USA) was cultured in minimal essential medium (MEM, Sigma, USA), and the JAr human choriocarcinoma cell line (HTB-144, ATCC) was maintained in RPMI 1640 medium (Sigma); both cultures were maintained at 37°C under 5% CO2 in air, and the culture media was supplemented with 10% (v/v) fetal bovine serum (FBS, Invitrogen, Carlsbad, CA, USA), 2 mM L-glutamate, 100 units/ml penicillin and 100 units/ml streptomycin.
Mifepristone and UPA treatment and JAr spheroid-Ishikawa cell line co-culture
The JAr and Ishikawa cells were treated, respectively, with graded concentrations of mifepristone at 0, 0.1, 1.0 and 10.0 μM in DMEM/F12 containing 10% charcoal-stripped FBS (cs-FBS) in separate wells for 24 h. In a pharmacokinetic study in Southern Chinese subjects, the peak serum concentration of mifepristone after a 25 mg oral dose was 842.4 ng/ml (i.e. 1.96 μM) (Tang et al., 2009). Hence the treatment conditions covered a five-time range above this. Another set of the JAr and Ishikawa cell lines was treated with graded concentrations of UPA (Laboratoire HRA Pharma, France) at 0, 0.04, 0.4 and 4.0 μM, respectively. The concentration range of UPA used covered ten-times above and below the peak serum drug level after oral administration of the 30 mg UPA tablet, i.e. 0.4 μM (Snow et al., 2011). A parallel arm treated with 5.0 μM methotrexate (MTX) instead of mifepristone or UPA was also included to serve as the positive control for JAr spheroid attachment inhibition.
The treated JAr cells were then trypsinized, washed and seeded (2–3 × 105 cells per well) in a 6-well plate and shaken at 106 rpm overnight for spheroid generation. The Ishikawa cells were seeded at 2 × 105 cells per well in 12-well plate during the above treatment. After treatment, the wells containing the Ishikawa cells were washed with PBS and refilled with fresh DMEM/F12 containing 1% bovine serum albumin (BSA).
JAr spheroids with diameter of 60–200 μm were selected and 30 spheroids were gently added onto Ishikawa monolayer with a glass pipette under microscopic visualization. The co-culture was maintained for 1 h at 37°C under 5% CO2 in air and then subjected to vigorous shaking at 140 rpm for 10 min. The medium was removed and refilled. The number of attached spheroids was counted under microscope. Attachment rate was defined as the ratio of the number of attached spheroids to the total number of spheroids seeded. Results from 19 independent repeats of the experiment were pooled for analysis.
Preparation of the primary endometrial cells
Primary endometrial cells were obtained by endometrial aspiration from nine volunteer women with regular monthly menstrual cycles. Endometrial aspirates were performed using Pipelle sampler after written consent during the mid-luteal phase of their natural menstrual cycles (7 days post-LH surge). These were women suffering from subfertility without obvious ovulatory or uterine factors who were awaiting assisted reproduction treatment in the Centre of Assisted Reproduction and Embryology, The University of Hong, Queen Mary Hospital, Hong Kong. Women who gave history of pregnancy or usage of hormonal medication within 3 months were not recruited. Ethics approval was obtained from the Institutional Review Board, The University of Hong Kong/Hospital Authority Hong Kong West Cluster (approval number: UW11-150). The tissue was stored at 4°C in Hank's balanced salt solution (HBSS, Sigma) until further processing.
The endometrial tissue was minced to less than 1 mm in diameter, re-suspended and digested for 1 h in a shaking water bath at 37°C with 0.5 mg/ml Type I A collagenase (Sigma) and 150 μg/ml deoxyribonuclease I (Worthington, Gene Company, USA) in DMEM/F12 containing 1% BSA. After a 1 h-digestion, the mixture was filtered through a 100 μm cell strainer (BD, Falcon,). The cells in the filtrate were sedimented at 1500 rpm for 5 min and re-suspended in DMEM/F12 containing 10% FBS (Gibco) to stop the digestion. The above treatment was repeated once for the undigested tissues. The filtrate was passed through a 40μm cell strainer and centrifuged at 1500 rpm for 5 min to separate the glandular epithelial cells from the stromal cells by size; the glandular epithelial cells retaining on the strainer were re-suspended with DMEM/F12 containing 10% FBS, and further seeded on plates coated with BD Matrigel (1:3 dilution in serum free DMEM/F12 medium, coated for 1 h at 37°C and washed before seeding). The epithelial cells were cultured in DMEM/F12 (without phenol red) supplemented with 10% FBS, 100 units/ml penicillin and streptomycin, 2 mM L-glutamate, and 1 ml/l Insulin-Transferrin-Selenium. 17β-estradiol at 500 pM and progesterone at 40 nM concentration were added into the culture system to mimic the mid-luteal phase hormone level. The medium was changed every other day. About 4–5 days were allowed for the cells to reach confluence before they were used for further treatment and co-culture. Immunofluorescence using cytokeratin (anti-cytokeratin 8.12, 1:20, Sigma), which stains cytokeratin 13 and 16, as the marker was applied to verify the purity of epithelial cells. The stromal cells were cultured similarly and the spent conditioned medium was collected for use in subsequent co-culture as described below.
UPA treatment and JAr spheroid-primary endometrial cell co-culture
JAr cells were treated with 4.0 μM UPA in 0.1% ethanol for 24 h before being made into spheroids as described above. Similarly, the primary endometrial cell layer was incubated with 4.0 μM UPA in 0.1% ethanol for 24 h. The treated JAr spheroids were then co-cultured with the primary epithelial cell layer for 3 h at 37°C in DMEM/F12 (without phenol red) with 10% cs-FBS, 100 units/ml penicillin and streptomycin, 2 mM L-glutamate and 1 ml/l Insulin-Transferrin-Selenium, supplemented with 500 pM 17β-estradiol and 40 nM progesterone to mimic the mid-luteal phase condition. Approximately 30 spheroids were added to each well of epithelial monolayer. After 3 h of co-culture, the plate was then shaken at 140 rpm for 10 min. The unattached spheroids were removed and the attachment rate was counted. Because of relative paucity of primary endometrial cells available for experimental use, UPA treatment was only studied at concentrations of 0 and 4 μM. Results were pooled from seven independent repeats of the experiment for analysis.
Cell proliferation assay
The effect of mifepristone and UPA treatment on cell proliferation rate was studied by the CyQUANT® NF cell proliferation assay kit (Invitrogen) which is based on measurement of cellular DNA content via fluorescent dye binding. Ishikawa and JAr cells were seeded in a 96-well plate at density of 1000 cells per well in MEM. Before further treatment, the DMEM/F12 with 10% cs-FBS was used to ‘wash out’ the hormonal effect of normal FBS for 24 h. MTX at 5.0 μM, mifepristone at 4.0 μM, and UPA at 0.04, 0.4 and 4.0 μM concentrations (in 0.1% ethanol) were added to the cells in six replicates. The medium was removed after 24 and 48 h. After washing the cells with PBS, carefully avoiding detachment of the cultured cells, 70 μl of dye binding solution was added to each well and incubated at 37°C for 45 min. Intensity of fluorescence of each well was measured by a fluorescence reader with excitation at 485 nm and emission at 530 nm.The results were presented as percentages of control treatment.
Quantitative reverse transcription-polymerase chain reaction (qRT-PCR)
After treatment with UPA and mifepristone for 24 h, total RNA was extracted from the Ishikawa cells using Absolutely RNA Microprep Kit (Agilent Technologies, USA) according to the supplier's protocol. The purity of RNA was assessed by spectrophotometry (A260/A280). Briefly, 0.5 μg total RNA was reverse transcribed using TaqMan reverse transcription reagent kit (Applied Biosystems, Foster City, USA), and the cDNA was subjected to quantitative PCR analysis using ABI 7500 Sequence Detector (PE Applied Biosystem, Foster City, California, USA). All the TaqMan probes (PGR: Hs00172183_m1 and 18 S: 4310893E) were purchased from Applied Biosystems. A standard PCR cycling protocol was performed as described below: 95°C for 10 min for initial activation, 40 cycles of denaturation, annealing and amplification at 95°C for 15 s and 60°C for 60 s. The 2-ΔΔCT relative quantification method was applied to quantify the data relative to 18S rRNA expression.
Protein extraction and Western blotting
JAr and Ishikawa cells were collected and subjected to Western blotting after 24 h of the various treatments. The medium was removed and the cells were washed with PBS. Radio-immunoprecipitation (RIPA) solution (1×PBS, 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% SDS) containing 7 μl/ml protease inhibitor cocktail (Calbiochem) was applied to dissolve the cell pellet. The mixture was centrifuged at 16 800 g for 10 min to remove cell debris. Protein concentration was determined using Bradford assay (Coomassie plus Bradford protein assay solution) and spectrophotometer (BioRad, Hercules, CA, USA). Equal amount of total protein was prepared in 1× SDS loading buffer, heat-denatured and resolved in 8% SDS-PAGE. The protein was then transferred onto nitrocellulose membrane, blocked with 0.5% non-fat milk in PBST (blocking solution) and probed with affinity purified mouse anti-human β-catenin (1:5000, BD Bioscience), and mouse anti-glycogen synthase kinase 3 (anti-GSK-3β, 1:2000, BD Bioscience) or mouse anti-β-actin (1:10 000, Sigma) in blocking solution overnight at 4°C. After washing with PBST 5 times for 5 min each, anti-mouse or anti-rabbit horse reddish peroxidase conjugated secondary antibodies (1:5000, GE Healthcare) were added and incubated for at least 1 h. The signal was visualized by enhanced chemiluminescence reagent (AbFrontier, Seoul, Korea) and developed in X-ray films. Western blot images were quantified by ImageJ software (http://imagej.nih.gov/ij/).
Statistical analysis
The JAr spheroid attachment rate was compared between treatment groups using Fisher's Exact test. Continuous variables were compared between treatment groups using Friedman's test or the Kruskal–Wallis test as specified in the results. Statistical analysis was performed using the GraphPad Prism 6.0 software.
Results
Establishment of the JAr spheroid-endometrial cell co-culture model
The purity of the primary endometrial cell monolayers was verified using immunofluorescence staining with cytokeratin and vimentin as the epithelial and stromal cell markers, respectively. The cultured epithelial cells demonstrated strongly positive cytoplasmic staining for cytokeratin and negative staining for vimentin (Fig. 1). Attachment of the treated JAr spheroids to the Ishikawa cell monolayer or the primary endometrial cell monolayer is illustrated in Fig. 2.

Verification of the purity of the isolated endometrial epithelial cells by immunofluorescence staining. The purified endometrial epithelial cells were stained with DAPI (A and D), anti-cytokeratin antibody (B), and anti-vimentin antibody (E) for detecting epithelial and stromal origin of cells, respectively. Merged images suggested that the cells are mainly of epithelial original (C and F) (100×).
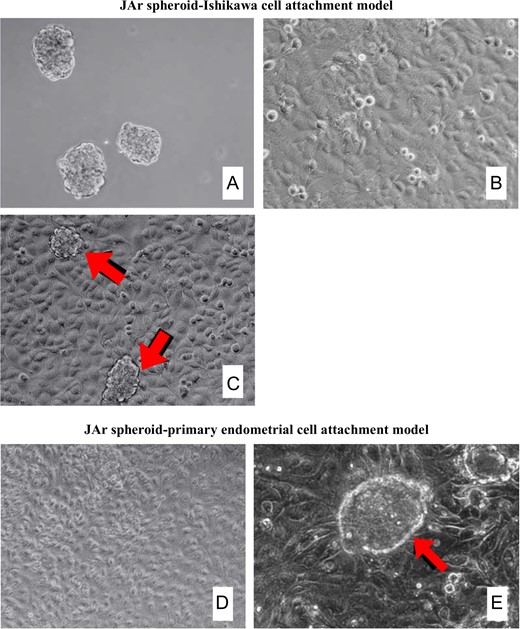
Co-culture of JAr spheroids and epithelial cell monolayer. (A) JAr spheroids with a size range of 100–150 μm was generated by shaking at 106 rpm for 18 h. (B) Ishikawa epithelial monolayer after 3–5 days of culture. (C) JAr spheroids attached firmly onto Ishikawa epithelial monolayer (arrow). (D) Human primary endometrial epithelial cell monolayer after culture to confluence. (E) JAr spheroids attached firmly onto primary endometrial cell monolayer (arrow). Magnification 100× (A–D), 200× (E).
Effect of mifepristone and UPA on viability and proliferation of JAr and Ishikawa cells
Treatment of both JAr and Ishikawa cell lines with UPA at 0.04, 0.4 and 4.0 μM concentrations, as well as mifepristone at 4.0 μM did not result in significant alteration in cell proliferation (as determined by the CyQUANT NF® cell proliferation assay) (P > 0.05) (Supplementary Fig. S1).
Effect of mifepristone and UPA treatment on the JAr spheroid attachment rate
In the Ishikawa experiments, there was no significant difference (P > 0.05) in the JAr spheroid attachment rate after treatment with UPA at 0.04 μM (93.6%), 0.4 μM (93.4%) and 4.0 μM (91.4%) concentrations (control at 93.0%). In contrast, a significantly reduced attachment rate was observed after treatment with mifepristone at 10.0 μM (79.8%, P < 0.0001) but not at 0.1 μM (92.1%) nor 1 μM (95.2%) concentrations (P > 0.05) (Fig. 3A).

Trophoblastic spheroid-endometrial cell attachment model. (A) Effect of ulipristal acetate (UPA) and mifepristone (MIFE) on JAr spheroid attachment to Ishikawa endometrial adenocarcinoma cell line. Treatment with 0.04, 0.4 and 4 μM UPA did not result in significant change in attachment rate compared to control (P > 0.05). Attachment rate was significantly reduced after treatment with MIFE at 10 μM (P < 0.0001), but not at lower concentrations. Attachment rate was also significantly inhibited (P < 0.0001) after treatment with 5 μM methotrexate (MTX) which was used as the positive control. (B) Effect of UPA treatment on JAr spheroid attachment to human primary endometrial cells. Attachment rate was not significantly different from control (P > 0.05) after treatment with 4 μM UPA, but was significantly reduced (P = 0.0055) after treatment with 5 μM methotrexate (MTX) which was used as the positive control. The number of attached spheroids to the total number of spheroids added were shown in each bar. *denotes statistically significant difference from control.
Because of the limited availability of endometrial tissue, we only studied the effect of UPA at 4.0 μM concentration on JAr spheroid attachment onto primary endometrial cells. No significant difference was observed in the JAr spheroid attachment rate between the treatment group (UPA 4.0 μM, 42.6%) compared to the control (without UPA treatment, 46.5%, P > 0.05) (Fig. 3B).In both Ishikawa cell line and primary endometrial cell experiments, significant suppression of spheroid attachment rate (P < 0.01) was observed in the positive controls with 5.0 μM MTX treatment (Fig. 3A and B).
Effect of UPA on progesterone receptor mRNA expression and the Wnt/β-catenin signaling pathway in Ishikawa cells
Treatment with both UPA and mifepristone significantly up-regulated the mRNA expression of the progesterone receptor gene in Ishikawa cells (Fig. 4, upper panel).
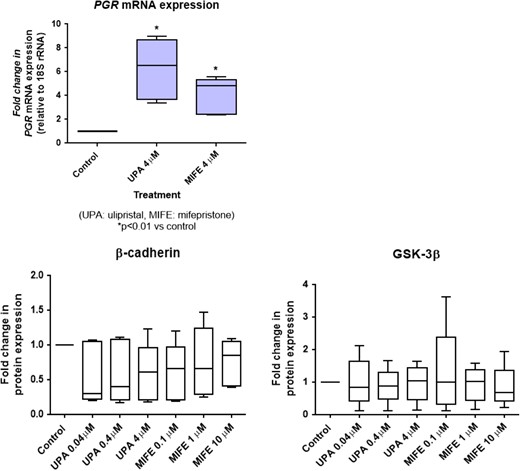
Effect of ulipristal acetate (UPA) and mifepristone (MIFE) on progesterone receptor expression (upper panel) and the Wnt/β-catenin signal transduction pathway (lower panels) in JAr cells. JAr cells were treated with either UPA or MIFE at the respective doses for 24 h. The progesterone receptor mRNA expression was determined by real-time reverse transcription and polymerase chain reaction, and was significantly increased (P < 0.05) upon treatment with UPA and MIFE compared to control. The expression levels of β-catenin and GSK-3β were detected by Western blotting and quantified by ImageJ software. The box-whisker plots showed no significant changes (P > 0.05) in these molecules in all treatment conditions (the boxes show the median and the 25th and 75th percentiles, whereas the whiskers show the minimum and maximum). The experiments were repeated five times.
After treatment with UPA at 0.04, 0.4 and 4.0 μM concentrations, or with mifepristone at 0.1, 1 and 10.0 μM concentrations for 24 h, the expression levels of β-catenin and GSK-3β in Ishikawa cell lines did not show significant change as determined by Western blotting (Fig. 4, lower panel).
Discussion
The present study demonstrated that in vitro treatment with UPA at concentrations up to 4.0 μM had no significant effect on embryo-endometrial attachment in a JAr spheroid-endometrial cell co-culture model. There has been another report showing that 4.0 μM UPA did not affect embryo-endometrial attachment using 20 human embryos and a three-dimensional endometrial co-culture model (Berger et al., 2015). Our results replicated and verified the same conclusion on a larger sample size utilizing thousands of JAr spheroids in a slightly different co-culture model with UPA treatment over a range of concentrations. This JAr spheroid model for studying endometrial receptivity has been validated in previously published reports to be able to differentiate endometrial substrates with different receptivities (Liu et al., 2010; Kodithuwakku et al., 2011). In view of unavailability of actual human blastocysts for such experimental use due to ethical reason, the JAr spheroids may represent a reasonable embryo surrogate, although the two may not be fully identical.
In this study, we took 0.4 μM as the pharmacological concentration of UPA, which corresponded to the peak serum concentration after ingestion of a 30 mg UPA oral tablet (Snow et al., 2011). Our in vitro treatment doses covered a 10-times range above and below this pharmacological concentration in serum, although whether or not this represents the actual tissue concentration in the endometrium after UPA intake is uncertain. Taking into consideration the variation in pharmacokinetics between individuals, we set our drug treatment concentrations to cover an adequately wide margin in this study. After a single dose intake as EC, the peak concentration in the circulation is only maintained over a short duration with a half-life of 36 h (Snow et al., 2011). In addition, UPA binds with high affinity to albumin and red blood cells in the circulation. Hence, it is likely that the actual bioactive concentration of UPA at the tissue level, to which the embryo and endometrium are exposed in vivo, is lower than the peak serum concentration. Therefore, it is reasonable to believe that the UPA 30 mg tablet in usual pharmacological use does not suppress implantation.
On the other hand, our results showed that mifepristone at 10.0 μM, but not lower concentrations, could significantly inhibit attachment of the JAr spheroid to endometrial tissue. The 10.0 μM concentration is about five times the peak serum concentration after a 25 mg oral dose of mifepristone (Tang et al., 2009), the recommended EC dose.
Due to obvious practical and ethical reasons, human embryo implantation cannot be studied in vivo; this is mostly studied with surrogate biomarkers of endometrial receptivity or on in vitro models. The in vitro co-culture model used here has been validated in our previously published studies for studying embryo attachment, the essential initial step of embryo implantation (Liu et al., 2010). The Ishikawa cell monolayer, which is a human endometrial adenocarcinoma cell line, was used as the endometrial surrogate. In the present work, in order to verify the results using a more physiological endometrial surrogate, we replicated the UPA experiment in a parallel set-up using primary endometrial cells, which basically reached the same conclusion. We only worked on the highest treatment dose of UPA at 4.0 μM with the primary endometrial cells because of the relative paucity of such human tissue obtained. In view of lack of significant difference from the control at this concentration, it was sufficient to deduce the same outcome with lower concentrations. We did not repeat the experiment on mifepristone treatment using primary endometrial cell co-culture because of the scarce availability of human endometrial tissue and that an inhibitory effect of mifepristone at 10 μM (equivalent to the highest concentration in our experimental range) has already been reported previously (Lalitkumar et al., 2007).We compared the results of treatment with UPA to that with mifepristone in the JAr spheroid-Ishikawa cell line model. Mifepristone, structurally similar to UPA, is another progesterone receptor modulator considered as one of the most effective emergency contraceptive agents (Shen et al., 2017), which is yet having very limited availability for such use. Our results demonstrated a dose-dependent effect of mifepristone on embryo attachment, which was significantly reduced at 10.0 μM but not at 1.0 μM or lower doses. Pharmacokinetic studies reported that the peak serum concentrations after a single 25 mg and 600 mg oral dose were around 2 μM and 5 μM, respectively (Sarkar, 2002; Tang et al., 2009). Therefore, 10.0 μM is actually a supra-pharmacological dose for mifepristone. However, it is worth to note that in another recent report, even a lower dose of mifepristone at 0.5 μM, which corresponded to the circulating level after a daily oral dose of 5 mg, was able to inhibit human embryo attachment onto primary endometrial cells cultured in a three-dimensional in vitro model (Boggavarapu et al., 2016). Whether UPA might have similar dose-dependent effect in inhibiting embryo-endometrial attachment at a concentration higher than the current pharmacological dose is uncertain. Such dose-response relationship may be worth further exploration to alleviate uncertainties and concerns about the endometrial effect of the drug at different dose ranges. Nonetheless, the available results inferred a difference in the scope of action between UPA and mifepristone at the EC dose.
We excluded alteration in proliferation of the JAr and/or Ishikawa cells as possible confounders of our JAr attachment results by the CyQUANT NF cell proliferation assay. We then explored the effect of UPA and mifepristone on progesterone receptor expression and some molecular markers of implantation. We demonstrated that both UPA and mifepristone could significantly up-regulate the expression of progesterone receptor mRNA, a finding which was compatible with a recent report (Whitaker et al., 2017). We did not elaborate on the effects on the different isoforms of the progesterone receptor, which was not the main aim of this study, although this may worth exploration in future studies. The Wnt/β-catenin signaling pathway is a progesterone-dependent pathway which plays important roles during implantation. It is reported that Wnt/β-catenin signaling is required for the early peri-conception processes in human including embryo implantation, stromal decidualization, embryo development and placenta formation. Accumulating evidence showed that progesterone and estrogen modulate the Wnt/β-catenin signaling to maintain homeostasis, balancing estrogen-induced proliferation and progesterone-induced differentiation. Many studies have reported that Wnt/β-catenin signaling is induced by estrogen while inhibited by progesterone (Wang et al., 2010; van der Horst et al., 2012; Tepekoy et al., 2015). Our results revealed that the expression of Wnt/β-catenin signaling pathway molecules (total β-catenin and its inhibitor, GSK-3β) was not affected in either Ishikawa cells after either UPA or mifepristone exposure. The inhibitory effect of higher doses of mifepristone on embryo-endometrial attachment may be mediated through other pathways.
The main mechanism of action of UPA (Gemzell-Danielsson et al., 2013) is believed to be inhibition or postponement of ovulation when it is taken before the LH peak of the cycle. However, a post-ovulatory action on either sperm or tubal function or endometrial receptivity can theoretically expand the window of efficacy of the drug particularly if it is taken after ovulation has occurred. Practically it is important because contraceptive accidents may happen at random timing prompting the women to seek for EC at any part of the cycle. Our published work has shown that UPA at pharmacological dose could have possible effect in suppressing progesterone-induced acrosome reaction, hyper-activation and calcium influx in human spermatozoa (Ko et al., 2014), as well as reducing ciliary beat frequency and muscular contraction in the human Fallopian tube (Li et al., 2014).The mechanism of action of an EC is one of the main issues influencing its acceptability; an earlier study in Scotland reported that women do have more positive attitude towards contraceptive methods acting on ovulation than those acting on implantation (Glasier et al., 1999), although a very recent study by the same group revealed that about 80% of respondents accepted an EC that worked by preventing or disrupting implantation (Willetts et al., 2017). Although it was defined by a Judicial Review in the United Kingdom in 2002 that conception is established at the time of embryo implantation but not at fertilization, there is still diversity in views and acceptance over the issue regarding the actual moment of life establishment based on religious or personal grounds (Keenan, 2011; ESHRE Capri Workshop Group, 2015). Hence, the present study provided some important evidence for contraceptive counseling.
In conclusion, our results suggested that both UPA and mifepristone at concentrations corresponding to the EC dose do not have inhibitory effect on embryo implantation, although mifepristone at a higher concentration appeared to have such an effect.
Supplementary data
Supplementary data are available at Human Reproduction online.
Acknowledgements
We would like to thank Dr. Tracy Yeung and other colleagues of the Reproductive Medicine Team, Queen Mary Hospital, Hong Kong, for assistance in endometrial sample collection. We also thank Mr. Ka-Leung Kwok, Miss Tsz-Yan Cheung and Miss Hilda Tsang for assistance in part of the experimental work. We also thank Laboratoire HRA Pharma for the free supply of the UPA compound for the experiments in this study.
Authors’ roles
H.W.R.L., E.H.Y.N., W.S.B.Y., P.C.H. and K.F.L. conceived and designed the study. E.H.Y.N. supervised human subject recruitment for endometrial samples. Y.X.L. performed most of the experiments, with some contribution by T.T.L., H.F. and K.F.L. H.W.R.L., Y.X.L., H.F. and K.F.L. analyzed the results. H.W.R.L. drafted the manuscript, which was edited and finally approved by all authors.
Funding
This work was supported by a Seed Fund to the first author from the Centre of Reproduction, Development and Growth, Faculty of Medicine, The University of Hong Kong, Hong Kong.
Conflict of interest
None declared.
References
Author notes
Equal contribution.



