-
PDF
- Split View
-
Views
-
Cite
Cite
Clémentine Wambergue, Raoudha Zouari, Selima Fourati Ben Mustapha, Guillaume Martinez, Françoise Devillard, Sylviane Hennebicq, Véronique Satre, Sophie Brouillet, Lazhar Halouani, Ouafi Marrakchi, Mounir Makni, Habib Latrous, Mahmoud Kharouf, Florence Amblard, Christophe Arnoult, Pierre F. Ray, Charles Coutton, Patients with multiple morphological abnormalities of the sperm flagella due to DNAH1 mutations have a good prognosis following intracytoplasmic sperm injection, Human Reproduction, Volume 31, Issue 6, June 2016, Pages 1164–1172, https://doi.org/10.1093/humrep/dew083
Close - Share Icon Share
Abstract
Does DNAH1 status influence intracytoplasmic sperm injection (ICSI) outcomes for patients with multiple morphological abnormalities of the sperm flagella (MMAF)?
Despite a highly abnormal morphology, sperm from MMAF patients with DNAH1 mutations have a low aneuploidy rate and good nuclear quality, leading to good embryonic development following ICSI and a high pregnancy rate.
Teratozoospermia represents a heterogeneous group including a wide range of phenotypes. Among all these qualitative defects, a flagellar phenotype called MMAF is characterized by a mosaic of morphological abnormalities of the flagellum, including coiled, bent, irregular, short or/and absent flagella, mainly due to the absence of the axonemal central pair microtubules. We previously demonstrated that homozygous mutations in the DNAH1 gene, encoding an inner arm heavy chain dynein, are frequently found in patients with MMAF (28% of the patients from the initial cohort). Numerous studies have reported an increased rate of aneuploidy and a poor sperm nuclear quality related to sperm flagellar abnormalities, which could impede ICSI outcome. Moreover, success rates after ICSI may be influenced by the type of ultrastructural flagellar defects and/or by the gene defects carried by the patients.
This retrospective cohort study included 6 infertile males with MMAF due to deleterious homozygous DNAH1 mutations and their respective spouses, who underwent 9 ISCI cycles, with 16 embryos being transferred. ICSI results were compared with two control populations of 13 MMAF men without DNAH1 mutations and an aged-matched control group of 1431 non-MMAF couples. All ICSI attempts took place between 2000 and 2012.
Clinical and biological data were collected from patients treated for infertility at the CPSR les Jasmins in Tunis (Tunisia). We compared the ICSI outcomes obtained with couples including DNAH1 mutated and nonmutated patients and non-MMAF couples. For the analysis of the chromosomal status, fluorescence in situ hybridization (FISH) analyses were performed on sperm cells from 3 DNAH1-mutated patients and from 29 fertile control subjects. Sperm chromatin condensation and DNA fragmentation were evaluated using aniline blue staining and TUNEL assays, respectively, on sperm cells from 3 DNAH1-mutated men and 6 fertile controls.
There was a significantly increased proportion of disomy XY and 18 in sperm from DNAH1 mutated patients compared with fertile controls (1.52 versus 0.28%, P = 0.0001 and 0.64 versus 0.09%, P = 0.0001). However, there were no statistically significant differences among sperm from the two groups in their frequencies of either 13, 21, XX or YY disomy or diploidy. Measures of DNA compaction and fragmentation demonstrated a good nuclear sperm quality among DNAH1 mutated men. The overall fertilization, pregnancy and delivery rates of couples including DNAH1 mutated men were of 70.8, 50.0 and 37.5%, respectively. There were no statistically significant differences in any of these parameters compared with the two control groups (P > 0.05).
A limitation of this study is the small number of DNAH1-mutated patients available and the low number of genes identified in MMAF. Further genetic studies are warranted to identify other MMAF-inducing genes to better characterize the genetic etiology of the MMAF phenotype and to improve the management of patients diagnosed with flagellar defects.
MMAF patients with DNAH1 mutations have low aneuploidy rates and good nuclear sperm quality, explaining the high pregnancy rate obtained with these patients. Good ICSI results were obtained for both MMAF groups (DNAH1 mutated and nonmutated), suggesting that patients presenting with asthenozoospermia due to flagellar defects have a good ICSI prognosis irrespective of their genotype. The majority of MMAF cases currently remain idiopathic with no genetic cause yet identified. In depth genetic analysis of these patients using next generation sequencing should reveal new causal genes. Subsequent genotype phenotype analyses could improve advice and care provided to MMAF patients.
None of the authors have any competing interest. This work is part of the project ‘Identification and Characterization of Genes Involved in Infertility (ICG2I)’, funded by the program GENOPAT 2009 from the French Research Agency (ANR) and the MAS-Flagella project, financed by the French ANR and the Direction Générale de l'Offre de Soins (DGOS).
Introduction
Teratozoospermia is a semen defect defined by the World Health Organization as the presence in the ejaculate of more than 96% morphologically abnormal spermatozoa (WHO, 2010). Teratozoospermia represents a heterogeneous group including a wide range of phenotypes affecting solely or simultaneously the head, neck, midpiece or tail of the spermatozoa. Among all these qualitative defects, a flagellar phenotype called multiple morphological abnormalities of the sperm flagella (MMAF) has been described (Ben Khelifa et al., 2014). This phenotype, previously reported as dysplasia of the fibrous sheath or short/stump tail syndromes, is characterized by a mosaic of morphological abnormalities of the flagellum including coiled, bent, irregular, short or/and absent flagella due to numerous ultrastructural defects of the axoneme and fibrous sheath (Chemes and Rawe, 2003; Coutton et al., 2015). Mammalian sperm flagella are composed of a central structure called the axoneme surrounded by the mitochondrial sheath (only in the midpiece), the fibrous sheath and the outer dense fibers (Fig. 1). The axoneme is composed of an intricate network of microtubules (nine peripheral microtubule doublets and a central pair (CP)), radial spokes, dynein arms and several other compounds. On each microtubule doublet, inner and outer dynein arms are fixed, producing the flagella beating force and therefore the motility of the spermatozoa. Recently, in a cohort of 18 unrelated MMAF patients, a homozygous frameshift mutation in DNAH1 was found in 5 cases (28%) (Ben Khelifa et al., 2014). DNAH1 is a gene coding for an inner dynein arm heavy chain which is believed to reinforce the link between the radial spokes and the outer doublet (Fig. 1). The radial spokes are believed to be involved in the positioning/stabilization of the central doublets. When DNAH1 is absent or dysfunctional, the axoneme is grossly disorganized, often with a ‘9 + 0’ structure (Ben Khelifa et al., 2014). Such central pair defects (9 + 0 structure) have previously been described as the most frequent ultrastructural abnormalities observed in genetically uncharacterized MMAF patients (Chemes and Rawe, 2003).
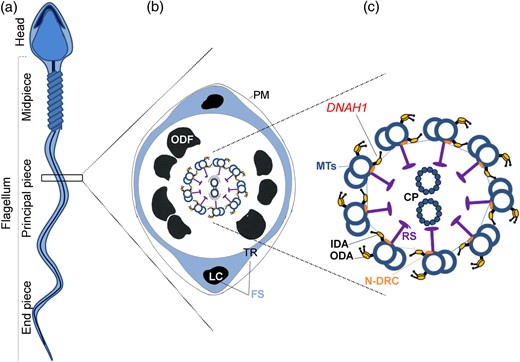
Structure of the sperm flagellum. (a) Mammalian sperm flagellum is structurally divided into three areas: midpiece, principal piece and end piece. (b) Schematic cross section through a representative segment of the principal piece showing the plasma membrane (PM) surrounding seven outer dense fibers (ODF, there are nine ODF in the midpiece). The fibrous sheath (FS) is composed of two longitudinal columns (LC) which are connected by transverse ribs (TR). Within the ODF, are the components of the axoneme. (c) The axoneme is enlarged and the offset shows: the nine outer microtubule doublets of the axoneme (MTs) with associated inner dynein arms (IDA), outer dynein arms (ODA), radial spokes (RS), nexin-dynein regulator complex (N-DRC), nexin links and the central pair of microtubules (CP).
Numerous studies have reported an increased rate of aneuploidy and a poor sperm nuclear quality related to sperm flagellar abnormalities (Coutton et al., 2015), which could impede intracytoplasmic sperm injection (ICSI) outcomes. DNAH1 mutations have been identified in 28% of the MMAF patients analyzed, indicating that many yet unreported genes are likely to be involved in this phenotype. The success rates of ICSI may be influenced by the type of ultrastructural flagellar defects and/or by the gene defects carried by the patients (Mitchell et al., 2006; Fauque et al., 2009) and we can wonder if DNAH1 mutated men have a better or worse prognosis than other MMAF patients or other infertile men. The aim of this study is thus to evaluate the aneuploidy rate in sperm from patients harboring a mutation in the DNAH1 gene and to assess the levels of sperm DNA fragmentation and nuclear condensation. We also present the ICSI outcomes for six couples with spermatozoa from a DNAH1 mutated male and compare them with the outcomes for DNAH1 nonmutated patients and an age-matched control group. This study provides novel information related to the predicted ICSI success rates for MMAF patients with a DNAH1 mutation.
Materials and Methods
Patients and controls
We compared the ICSI outcomes obtained with DNAH1 mutated and nonmutated men and with an age-matched control group of non-MMAF couples treated during the same period. Couples were matched for the women's age at the time of treatment. All were treated for infertility at the CPSR les Jasmins in Tunis (Tunisia) between 2000 and 2012. Results only refer to fresh embryo transfers.
All MMAF patients were described previously (Ben Khelifa et al., 2014). ICSI outcomes were obtained for six couples after regrouping all men with homozygous deleterious DNAH1 mutations, including five truncating variants (P1, P2, P3, P9, P17) and a stop loss variant (P8), and who were fully described in our previous study (Ben Khelifa et al., 2014). Three of these patients were brothers (P1, P2 and P3) born from a consanguineous union. They harbored the same homozygous DNAH1 mutation that was also identified in a fourth unrelated subject (P17). The ages of the men ranged from 28.4 to 41.5 and the ages of the women ranged from 21.7 to 43.3 (Table I). At the time of ICSI, no spermatozoa were found in the ejaculate of patient P2 (Table I) therefore testicular sperm extraction (TESE) was performed. We did not include patient P6 from the original study as he carried a false-sense, nontruncating variant of unknown significance and with 35% motility, he did not present a pure MMAF phenotype (Ben Khelifa et al., 2014).
Main characteristics of patients and ICSI outcomes in the six couples with the infertile males harboring DNAH1 mutations.
| Patients . | Sperm parameters . | Outcomes . | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Couples . | Infertile malea . | DNAH1 mutation . | Female age . | Male age . | Volume (ml) . | Numeration (M/ml) . | % Motility (a + b) . | % Motility (c) . | % Typical forms . | Collected oocytes . | Injected oocytes . | 2PN . | 3PN . | % 2PN Fertilization rate . | Embryos . | No of transferred embryos . | No of frozen embryos . | Clinical pregnancy . | Delivery . |
| 1 | P1 | c.11788-1G>A | 30.0 | 29.9 | 2.8 | <20 | 0 | 2 | 0 | 21 | 21 | 13 | 1 | 62 | 13 | 3 | 6 | 1 (twin) | 1 |
| 2 | P2 | c.11788-1G>A | 28.4 | 28.4 | Testicular biopsy | 25 | 18 | 8 | 44 | 8 | 2 | 6 | 2 | 2 | |||||
| 3 | P3 | c.11788-1G>A | 30.5 | 36.8 | 4 | 42 | 1 | 1 | 0 | 8 | 4 | 2 | 1 | 50 | 2 | 2 | 2 | 0 | 0 |
| 30.8 | 37.1 | 3 | 52 | 0 | 2 | 0 | 15 | 10 | 9 | 90 | 9 | 2 | 5 | 1 | 1 | ||||
| 4 | P8 | c.12796T>C | 21.7 | 40.5 | 4.5 | 9.2 | 0 | 5 | 0 | 40 | 23 | 17 | 74 | 17 | 2 | 9 | 0 | 0 | |
| 5 | P9 | c.5094+1G>A | 42.8 | 40.9 | 3 | 29 | 0 | <1 | 0 | 1 | 1 | 1 | 100 | 1 | 1 | 1 | 0 | ||
| 43.3 | 41.5 | 4 | 35 | 0 | 2 | 0 | 3 | 1 | 0 | 1 | 0 | ||||||||
| 6 | P17 | c.11788-1G>A | 26.4 | 32.3 | 3.0 | >20 | 0 | <1 | 0 | 17 | 13 | 10 | 77 | 10 | 2 | 6 | 1 | 1 | |
| 30.9 | 36.8 | 2.5 | 31 | 0 | <1 | 0 | 15 | 15 | 15 | 100 | 15 | 2 | 10 | 0 | 0 | ||||
| Patients . | Sperm parameters . | Outcomes . | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Couples . | Infertile malea . | DNAH1 mutation . | Female age . | Male age . | Volume (ml) . | Numeration (M/ml) . | % Motility (a + b) . | % Motility (c) . | % Typical forms . | Collected oocytes . | Injected oocytes . | 2PN . | 3PN . | % 2PN Fertilization rate . | Embryos . | No of transferred embryos . | No of frozen embryos . | Clinical pregnancy . | Delivery . |
| 1 | P1 | c.11788-1G>A | 30.0 | 29.9 | 2.8 | <20 | 0 | 2 | 0 | 21 | 21 | 13 | 1 | 62 | 13 | 3 | 6 | 1 (twin) | 1 |
| 2 | P2 | c.11788-1G>A | 28.4 | 28.4 | Testicular biopsy | 25 | 18 | 8 | 44 | 8 | 2 | 6 | 2 | 2 | |||||
| 3 | P3 | c.11788-1G>A | 30.5 | 36.8 | 4 | 42 | 1 | 1 | 0 | 8 | 4 | 2 | 1 | 50 | 2 | 2 | 2 | 0 | 0 |
| 30.8 | 37.1 | 3 | 52 | 0 | 2 | 0 | 15 | 10 | 9 | 90 | 9 | 2 | 5 | 1 | 1 | ||||
| 4 | P8 | c.12796T>C | 21.7 | 40.5 | 4.5 | 9.2 | 0 | 5 | 0 | 40 | 23 | 17 | 74 | 17 | 2 | 9 | 0 | 0 | |
| 5 | P9 | c.5094+1G>A | 42.8 | 40.9 | 3 | 29 | 0 | <1 | 0 | 1 | 1 | 1 | 100 | 1 | 1 | 1 | 0 | ||
| 43.3 | 41.5 | 4 | 35 | 0 | 2 | 0 | 3 | 1 | 0 | 1 | 0 | ||||||||
| 6 | P17 | c.11788-1G>A | 26.4 | 32.3 | 3.0 | >20 | 0 | <1 | 0 | 17 | 13 | 10 | 77 | 10 | 2 | 6 | 1 | 1 | |
| 30.9 | 36.8 | 2.5 | 31 | 0 | <1 | 0 | 15 | 15 | 15 | 100 | 15 | 2 | 10 | 0 | 0 | ||||
PN, pronuclear; SD, standard deviation; ICSI, intracytoplasmic sperm injection.
aInfertile patients previously described in Ben Khelifa et al. (2014).
Main characteristics of patients and ICSI outcomes in the six couples with the infertile males harboring DNAH1 mutations.
| Patients . | Sperm parameters . | Outcomes . | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Couples . | Infertile malea . | DNAH1 mutation . | Female age . | Male age . | Volume (ml) . | Numeration (M/ml) . | % Motility (a + b) . | % Motility (c) . | % Typical forms . | Collected oocytes . | Injected oocytes . | 2PN . | 3PN . | % 2PN Fertilization rate . | Embryos . | No of transferred embryos . | No of frozen embryos . | Clinical pregnancy . | Delivery . |
| 1 | P1 | c.11788-1G>A | 30.0 | 29.9 | 2.8 | <20 | 0 | 2 | 0 | 21 | 21 | 13 | 1 | 62 | 13 | 3 | 6 | 1 (twin) | 1 |
| 2 | P2 | c.11788-1G>A | 28.4 | 28.4 | Testicular biopsy | 25 | 18 | 8 | 44 | 8 | 2 | 6 | 2 | 2 | |||||
| 3 | P3 | c.11788-1G>A | 30.5 | 36.8 | 4 | 42 | 1 | 1 | 0 | 8 | 4 | 2 | 1 | 50 | 2 | 2 | 2 | 0 | 0 |
| 30.8 | 37.1 | 3 | 52 | 0 | 2 | 0 | 15 | 10 | 9 | 90 | 9 | 2 | 5 | 1 | 1 | ||||
| 4 | P8 | c.12796T>C | 21.7 | 40.5 | 4.5 | 9.2 | 0 | 5 | 0 | 40 | 23 | 17 | 74 | 17 | 2 | 9 | 0 | 0 | |
| 5 | P9 | c.5094+1G>A | 42.8 | 40.9 | 3 | 29 | 0 | <1 | 0 | 1 | 1 | 1 | 100 | 1 | 1 | 1 | 0 | ||
| 43.3 | 41.5 | 4 | 35 | 0 | 2 | 0 | 3 | 1 | 0 | 1 | 0 | ||||||||
| 6 | P17 | c.11788-1G>A | 26.4 | 32.3 | 3.0 | >20 | 0 | <1 | 0 | 17 | 13 | 10 | 77 | 10 | 2 | 6 | 1 | 1 | |
| 30.9 | 36.8 | 2.5 | 31 | 0 | <1 | 0 | 15 | 15 | 15 | 100 | 15 | 2 | 10 | 0 | 0 | ||||
| Patients . | Sperm parameters . | Outcomes . | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Couples . | Infertile malea . | DNAH1 mutation . | Female age . | Male age . | Volume (ml) . | Numeration (M/ml) . | % Motility (a + b) . | % Motility (c) . | % Typical forms . | Collected oocytes . | Injected oocytes . | 2PN . | 3PN . | % 2PN Fertilization rate . | Embryos . | No of transferred embryos . | No of frozen embryos . | Clinical pregnancy . | Delivery . |
| 1 | P1 | c.11788-1G>A | 30.0 | 29.9 | 2.8 | <20 | 0 | 2 | 0 | 21 | 21 | 13 | 1 | 62 | 13 | 3 | 6 | 1 (twin) | 1 |
| 2 | P2 | c.11788-1G>A | 28.4 | 28.4 | Testicular biopsy | 25 | 18 | 8 | 44 | 8 | 2 | 6 | 2 | 2 | |||||
| 3 | P3 | c.11788-1G>A | 30.5 | 36.8 | 4 | 42 | 1 | 1 | 0 | 8 | 4 | 2 | 1 | 50 | 2 | 2 | 2 | 0 | 0 |
| 30.8 | 37.1 | 3 | 52 | 0 | 2 | 0 | 15 | 10 | 9 | 90 | 9 | 2 | 5 | 1 | 1 | ||||
| 4 | P8 | c.12796T>C | 21.7 | 40.5 | 4.5 | 9.2 | 0 | 5 | 0 | 40 | 23 | 17 | 74 | 17 | 2 | 9 | 0 | 0 | |
| 5 | P9 | c.5094+1G>A | 42.8 | 40.9 | 3 | 29 | 0 | <1 | 0 | 1 | 1 | 1 | 100 | 1 | 1 | 1 | 0 | ||
| 43.3 | 41.5 | 4 | 35 | 0 | 2 | 0 | 3 | 1 | 0 | 1 | 0 | ||||||||
| 6 | P17 | c.11788-1G>A | 26.4 | 32.3 | 3.0 | >20 | 0 | <1 | 0 | 17 | 13 | 10 | 77 | 10 | 2 | 6 | 1 | 1 | |
| 30.9 | 36.8 | 2.5 | 31 | 0 | <1 | 0 | 15 | 15 | 15 | 100 | 15 | 2 | 10 | 0 | 0 | ||||
PN, pronuclear; SD, standard deviation; ICSI, intracytoplasmic sperm injection.
aInfertile patients previously described in Ben Khelifa et al. (2014).
The nonmutated group was composed of 13 couples with men presenting with the MMAF phenotype but without DNAH1 mutations. All presented a pure phenotype with <5% motility and >96% morphological abnormalities of the flagella. The DNAH1 whole coding regions from these men were sequenced previously and no pathological mutations were identified (Ben Khelifa et al., 2014).
Except for patient P2, semen samples were collected by masturbation after 3–5 days of sexual abstinence and examined after liquefaction for 30 min at 37°C. For patient P2 a microdissection approach was employed in order to enhance sperm retrieval while minimizing damage to the testicle. The utilization of optical magnification allowed targeted identification of seminiferous tubules containing active foci of spermatogenesis for selective removal of seminiferous tubules while sparing damage to vascularization.
All the patients were informed about the investigations and gave their written informed consent before this study. Nonroutine characterization of sperm parameters by FISH, chromatin condensation and DNA fragmentation could only be carried out for three of the DNAH1 mutated patients (P1, P17, P9). These analyses could not be performed for the other patients due to either insufficient biological material or the absence of specific consent. Sperm samples from the 13 nonmutated men were not available for further analyses.
A total of 29 fertile men (all of whom had fathered at least 1 child spontaneously) who were addressed for sperm donation or cryopreservation before vasectomy, served as control subjects for the FISH analysis. All controls were recruited in France, 19 were of European origin and the other 10 were of North African origin (Algeria, Morocco or Tunisia). From these 29 patients, 6 were used as controls to assess the sperm chromatin condensation and DNA fragmentation (3 originated from Europe and 3 from North Africa). Mean sperm parameters of these fertile controls are presented in Supplementary data, Table SI.
Sperm concentration, motility and morphology were evaluated according to the WHO recommendations (WHO, 2010).
The study was approved by the Ethics Committee of the University Hospital of Toulouse (Comité de Protection des Personnes Sud-Ouest et Outre Mer). All patients signed an informed consent to participate in the study.
FISH analysis
Fluorescence in situ hybridization (FISH) analyses were performed on sperm cells from 3 patients (P1, P17 and P9) and 29 fertile control subjects. Sperm samples were analyzed using dual FISH with two locus specific probes located at 13q14.2 (Green spectrum) and 21q22.13 (Orange spectrum) and triple FISH (chromosomes X, Y and 18) with specific centromere probes of the X chromosome (DXZ1X, p11.1-q11.1, spectrum green), Y chromosome (DYZ3, Yp11.1-q11.1 spectrum orange) and chromosome 18 (D18Z1 18p11.1-q11.1 spectrum Blue) (Fast FISH Cytocell Aquarius). Sperm samples were washed in phosphate buffered saline solution and spermatozoa were layered on a slide with methanol and acetic acid. The sperm nuclei were partially decondensed using a solution of NaOH (1 M) and then washed twice in saline sodium citrate (SSC) solution (Thomas et al., 2004). Before hybridization, the sperm DNA slides were immersed in a jar of 2× SSC/0.4% NP40 solution and passed through an ethanol series of increasing concentrations (70/90/100) before being allowed to air-dry. Denaturation was performed on sperm nuclei and probes for 1 min at 70°C. After overnight incubation at 37°C, the slides were washed in 0.4× SSC/0.3% NP40 then twice in SSC/0.1% NP40. The slides were counterstained with DAPI (4,6-diamino-2-phenylindole) and observed using an Axioplan microscope (Zeiss). Slides were kept frozen at −20°C until use.
Subsequent image acquisition was performed using a charge-coupled device camera and in situ imaging system (MetaSystem). The analysis was realized using strict selection criteria: only intact spermatozoa bearing a similar degree of decondensation and clear hybridization signals were scored; disrupted or overlapping spermatozoa were excluded from analysis (Devillard et al., 2002). For each patient, a minimum of 1000 spermatozoa were counted (Tempest et al., 2010). Only disomic or diploid cells were considered since the distinction between nullosomic cells or hybridization defects cannot be made (Martinez et al., 2013). Moreover, no autosomic monosomic conceptions are viable.
Sperm chromatin condensation
Sperm chromatin condensation was determined by aniline blue staining on sperm cells from three patients (P1, P17 and P9) and from six fertile controls. Sperm parameters of the three patients are presented in Table I and those of the six fertile controls are in Supplementary data, Table SI. Semen samples were washed twice with 5 ml of PBS 1×, and 10 μl were spread on a slide, allowed to air dry and then fixed with a 3% glutaraldehyde solution in PBS 1× for 30 min at room temperature. Slides were then incubated for 5 min in water, 10 min in 5% aniline blue diluted in 4% acetic acid solution, 2 min in water (two times), 2 min in 70, 90 and 100% ethanol solutions and finally for 2 min in toluene. Slides were then analyzed using a microscope with a transmitted light microscope 100× objective with oil (Yassine et al., 2015).
Semen DNA fragmentation
Semen DNA fragmentation was evaluated using TUNEL assay on sperm cells from three patients (P1, P17 and P9) and from six fertile controls. Sperm parameters of the three studied patients are presented in Table I and those of the six fertile controls are in Supplementary data, Table SI. Semen samples were washed twice with 5 ml of PBS and fixed in a methanol/acetic acid (3:1, v/v) solution at 4°C for at least 30 min. Cells were spread on Superfrost® slides and air dried at room temperature overnight. Cells were permeabilized using 0.1% (v/v) Triton X-100, 0.1% (w/v) sodium citrate in PBS for 2 min and labeled by terminal deoxynucleotidyl transferase mediated deoxy-UTP nick end labeling (TUNEL) according to the Roche protocol of the In Situ Cell Detection Kit (Roche Diagnostic). Sperm nuclei were counterstained in a 0.5 mg/ml Hoechst solution for 3 min, washed in PBS for 3 min and mounted with DAKO mounting media. Slides were analyzed using a fluorescent microscope (Nikon Eclipse 80i) (Yassine et al., 2015).
Ovarian stimulation and oocyte retrieval
Patients were submitted to standard ovarian stimulation by administration of gonadotrophins using either a long (n = 8) or short (n = 1) protocol. Women were given between 150 and 225 IU recombinant follicle-stimulating hormone (Gonal-F; Merck-Serono) with sometimes human menopausal gonadotrophin (Menopur; Ferring Pharmaceuticals), depending on the age of the patient and previous ovarian response to gonadotrophins. Subsequently, ultrasound was performed, and the follicular response was recorded from Day 5 of gonadotrophin stimulation. When at least two follicles were ≥18 mm in diameter, 6500 or 10 000 IU human chorionic gonadotrophin were administered. Oocytes were retrieved 34–36 h later by vaginal ultrasound-guided follicular puncture.
After retrieval, oocytes were washed with G-MOPS medium (Vitrolife) and incubated in culture medium (GIVF-plus; Vitrolife) for 2–3 h at 37°C, 6% CO2 and 5% O2. The cumulus cells were then removed mechanically by a 30-s exposure to Hyase medium containing 60 IU/ml of hyaluronidase (Vitrolife). Denuded oocytes were then evaluated for nuclear status. Oocytes showing the release of the first polar body were considered mature and were selected for ICSI.
ICSI and embryo culture
Assisted fertilization by ICSI was performed 3–4 h following oocyte collection on mature oocytes. Fertilization was assessed 18–19 h later for the presence of two pronuclei and two polar bodies. The fertilized oocytes were individually cultured in G1 medium (Vitrolife) until the day of transfer that was performed 48–72 h after oocyte retrieval.
Cleaving embryos were evaluated for cell number, blastomere appearance (the presence of vacuoles, multinucleation, cytoplasmic darkness or inclusions, unevenness size of blastomeres) and fragmentation rate. A top quality embryo was defined as follows: 4–6 cells on Day 2, ≥8 cells on Day 3, <10% fragmentation and equally sized mononucleated blastomeres. The best embryos were selected for transfer. Supernumerary good-quality embryos were frozen.
Embryo transfer and pregnancy follow-up
For each couple, one to three embryos were transferred by ultrasound guidance, depending on embryo quality and the age of the woman. All patients received luteal support, including 600 mg of vaginally administered micronized progesterone (Utrogestan; Besins Laboratories). Serum hCG levels were measured 14 days after embryo transfer. Clinical pregnancy was defined as at least a visible sac with fetal heart beat 7 weeks after embryo transfer.
Statistical analyses
For FISH analyses, the statistical comparison between patients and the control group was found using the Student t-test. To compare proportions between two groups (fertilization, implantation, pregnancy and delivery rates), the χ2 test or Fisher's Exact test was used. Student's t-test was used to compare means between two groups. Statistical tests with P values ≤0.05 were considered significant. All statistical analyses were performed with GraphPad Software™.
Results
ICSI outcomes
For couples where the men had MMAF and DNAH1 mutations, the mean male age at treatment was 36 ± 4.8 years (ranging from 28.4 to 41.5 years), and the mean female age was 31.6 ± 7.1 years (ranging from 21.7 to 43.3 years). The 6 couples underwent a total of 9 ovarian stimulation cycles and the mean number of oocytes retrieved per cycle was 16.1 ± 12 (ranging from 1 to 40). There were 106 metaphase II oocytes injected, and the fertilization rate ((2PN/injected oocytes) was 70.8%. A total of 8 transfers were performed. The mean number of transferred embryos was 2.0 ± 0.5 (ranging 1 from to 3). Clinical pregnancy rate per transfer was 50% and delivery rate per transfer was 37.5%. Only one miscarriage at 6 weeks of gestation was observed for couple 5.
Good quality supernumerary embryos were frozen. Of the six couples, four had one to three replacements of frozen embryos, leading to two clinical pregnancies with two deliveries. Taking into account the frozen embryo transfers four out of six couples (67%) had at least one healthy child. Major or minor malformations were not reported in the six children who were born. There was only one twin pregnancy (for couple 1).
To compare these results, we also evaluated the ICSI outcomes of 13 MMAF patients without DNAH1 mutations reported in our previous study (Ben Khelifa et al., 2014). The overall fertilization, pregnancy and delivery rates were of 76.5, 46.5 and 39.3%, respectively (Table II). We also assessed the overall outcomes obtained in our fertility center (Polyclinique les Jasmins, Tunis) using the same ICSI procedure between 2000 and 2012 in aged-matched patients (n = 1431): the fertilization rate was 69.8%, implantation rate was 25.9%, the clinical pregnancy rate per transfer was 39.5% and the delivery rate per transfer was 32.3% (Table II).
Outcomes of ICSI cycles in the three different groups (not including frozen embryo transfers).
| Variable . | MMAF DNAH1+ . | MMAF DNAH1− . | Overall ICSI outcomes in age-matched patients . |
|---|---|---|---|
| No. of patients | 6 | 13 | 1431 |
| Mean male age (years) | 36.1 ± 4.8 | 40.6 ± 7.3 | 40.5 ± 5.6 |
| Mean female age (years) | 31.6 ± 7.1 | 33.4 ± 4.4 | 34.4 ± 3.5 |
| No. of cycles | 9 | 29 | 1574 |
| No. of oocytes collected | 145 | 315 | 15 135 |
| No. of oocytes injected | 106 | 243 | 10 447 |
| No. of oocytes fertilized | 75 | 186 | 7292 |
| Fertilization rate (%) | 70.8 | 76.5* | 69.8 |
| Mean number of embryos replaced | 2.0 ± 0.5 | 2.2 ± 0.5 | 2.0 ± 0.6 |
| No. of transfers | 8 | 28 | 1478 |
| Implantation rate (%) | 31.3 | 24.6 | 25.9 |
| Clinical pregnancy (% per embryo transfer) | 4 (50) | 13 (46.5) | 584 (39.5) |
| No. of miscarriage (n) | 1 | 2 | 106 |
| No. of deliveries (% per embryo transfer) | 3 (37.5) | 11(39.3) | 478 (32.3) |
| Variable . | MMAF DNAH1+ . | MMAF DNAH1− . | Overall ICSI outcomes in age-matched patients . |
|---|---|---|---|
| No. of patients | 6 | 13 | 1431 |
| Mean male age (years) | 36.1 ± 4.8 | 40.6 ± 7.3 | 40.5 ± 5.6 |
| Mean female age (years) | 31.6 ± 7.1 | 33.4 ± 4.4 | 34.4 ± 3.5 |
| No. of cycles | 9 | 29 | 1574 |
| No. of oocytes collected | 145 | 315 | 15 135 |
| No. of oocytes injected | 106 | 243 | 10 447 |
| No. of oocytes fertilized | 75 | 186 | 7292 |
| Fertilization rate (%) | 70.8 | 76.5* | 69.8 |
| Mean number of embryos replaced | 2.0 ± 0.5 | 2.2 ± 0.5 | 2.0 ± 0.6 |
| No. of transfers | 8 | 28 | 1478 |
| Implantation rate (%) | 31.3 | 24.6 | 25.9 |
| Clinical pregnancy (% per embryo transfer) | 4 (50) | 13 (46.5) | 584 (39.5) |
| No. of miscarriage (n) | 1 | 2 | 106 |
| No. of deliveries (% per embryo transfer) | 3 (37.5) | 11(39.3) | 478 (32.3) |
Values are mean ± SD.
embryo transfer, embryo transfer; MMAF, multiple morphological abnormalities of the sperm flagella; ICSI, intracytoplasmic sperm injection.
*P <0.05 (Chi-square test).
Outcomes of ICSI cycles in the three different groups (not including frozen embryo transfers).
| Variable . | MMAF DNAH1+ . | MMAF DNAH1− . | Overall ICSI outcomes in age-matched patients . |
|---|---|---|---|
| No. of patients | 6 | 13 | 1431 |
| Mean male age (years) | 36.1 ± 4.8 | 40.6 ± 7.3 | 40.5 ± 5.6 |
| Mean female age (years) | 31.6 ± 7.1 | 33.4 ± 4.4 | 34.4 ± 3.5 |
| No. of cycles | 9 | 29 | 1574 |
| No. of oocytes collected | 145 | 315 | 15 135 |
| No. of oocytes injected | 106 | 243 | 10 447 |
| No. of oocytes fertilized | 75 | 186 | 7292 |
| Fertilization rate (%) | 70.8 | 76.5* | 69.8 |
| Mean number of embryos replaced | 2.0 ± 0.5 | 2.2 ± 0.5 | 2.0 ± 0.6 |
| No. of transfers | 8 | 28 | 1478 |
| Implantation rate (%) | 31.3 | 24.6 | 25.9 |
| Clinical pregnancy (% per embryo transfer) | 4 (50) | 13 (46.5) | 584 (39.5) |
| No. of miscarriage (n) | 1 | 2 | 106 |
| No. of deliveries (% per embryo transfer) | 3 (37.5) | 11(39.3) | 478 (32.3) |
| Variable . | MMAF DNAH1+ . | MMAF DNAH1− . | Overall ICSI outcomes in age-matched patients . |
|---|---|---|---|
| No. of patients | 6 | 13 | 1431 |
| Mean male age (years) | 36.1 ± 4.8 | 40.6 ± 7.3 | 40.5 ± 5.6 |
| Mean female age (years) | 31.6 ± 7.1 | 33.4 ± 4.4 | 34.4 ± 3.5 |
| No. of cycles | 9 | 29 | 1574 |
| No. of oocytes collected | 145 | 315 | 15 135 |
| No. of oocytes injected | 106 | 243 | 10 447 |
| No. of oocytes fertilized | 75 | 186 | 7292 |
| Fertilization rate (%) | 70.8 | 76.5* | 69.8 |
| Mean number of embryos replaced | 2.0 ± 0.5 | 2.2 ± 0.5 | 2.0 ± 0.6 |
| No. of transfers | 8 | 28 | 1478 |
| Implantation rate (%) | 31.3 | 24.6 | 25.9 |
| Clinical pregnancy (% per embryo transfer) | 4 (50) | 13 (46.5) | 584 (39.5) |
| No. of miscarriage (n) | 1 | 2 | 106 |
| No. of deliveries (% per embryo transfer) | 3 (37.5) | 11(39.3) | 478 (32.3) |
Values are mean ± SD.
embryo transfer, embryo transfer; MMAF, multiple morphological abnormalities of the sperm flagella; ICSI, intracytoplasmic sperm injection.
*P <0.05 (Chi-square test).
There were no statistically significant differences between the DNAH1-mutated MMAF patients (age, fertilization, implantation, pregnancy and delivery rates) and the two control groups (Table II). However, the fertilization rate in the DNAH1 nonmutated MMAF patients was significantly higher compared with non-MMAF control patients (P = 0.0281) (Table II).
Aneuploidy analysis
Results from three color FISH X-Y-18 and dual color FISH 13–21 for the analysis of the chromosomes status in the sperm of the three patients with DNAH1 mutations are given in Table III. For triple-FISH, segregation analysis of the chromosomes X-Y-18 was ascertained for 5807 spermatozoa from the 3 patients (ranging from 1907 to 1952 sperm cells per patients) and for a total of 144 138 spermatozoa from 29 normal fertile controls (Table III). For dual-FISH (chromosomes 13 and 21) 6241 spermatozoa from the patients were studied (ranging from 1896 to 2448 sperm cells per patients) as well as a total of 38 749 spermatozoa from normal fertile controls (Table III). There was a significantly increased proportion of disomy XY and 18 in sperm of DNAH1-mutated patients compared with sperm (n= 144 138) from fertile controls (1.52 versus 0.28%, P = 0.0001 and 0.64 versus 0.09%, P = 0.0001) (Table III). However, there were no statistically significant differences among the two groups in the frequencies of chromosome 13, 21, XX or YY disomy and diploidy (Table III).
| . | . | Disomies (%) . | . | Disomies (%) . | Diploidy (%) . | ||||
|---|---|---|---|---|---|---|---|---|---|
| Analyzed gametes (n) . | 13, 13, 21 . | 13, 21, 21 . | Analyzed gametes (n) . | X, X, 18 . | Y, Y, 18 . | X, Y, 18 . | X or Y, 18, 18 . | . | |
| Patient P1 | 1896 | 0.11 | 0.31 | 1948 | 0.21 | 0.15 | 1.28 | 0.51 | 1.48 |
| Patient P17 | 1897 | 0.36 | 0.36 | 1907 | 0 | 0 | 2.87 | 0.9 | 0.39 |
| Patient P9 | 2448 | 0.28 | 0.28 | 1952 | 0.16 | 0.1 | 0.41 | 0.5 | 0 |
| Mean patients | 0.25 | 0.317 | 0.123 | 0.083 | 1.52 | 0.637 | 0.623 | ||
| SD patients | 0.128 | 0.040 | 0.109 | 0.076 | 1.25 | 0.228 | 0.77 | ||
| Controls | 38 749 | 144 138 | |||||||
| Mean controls | 0.40 | 0.30 | 0.06 | 0.05 | 0.28 | 0.09 | 0.48 | ||
| SD controls | 0.117 | 0.132 | 0.05 | 0.04 | 0.13 | 0.05 | 0.17 | ||
| P value | 0.072 | 0.833 | 0.074 | 0.219 | 0.0001 | 0.0001 | 0.368 | ||
| . | . | Disomies (%) . | . | Disomies (%) . | Diploidy (%) . | ||||
|---|---|---|---|---|---|---|---|---|---|
| Analyzed gametes (n) . | 13, 13, 21 . | 13, 21, 21 . | Analyzed gametes (n) . | X, X, 18 . | Y, Y, 18 . | X, Y, 18 . | X or Y, 18, 18 . | . | |
| Patient P1 | 1896 | 0.11 | 0.31 | 1948 | 0.21 | 0.15 | 1.28 | 0.51 | 1.48 |
| Patient P17 | 1897 | 0.36 | 0.36 | 1907 | 0 | 0 | 2.87 | 0.9 | 0.39 |
| Patient P9 | 2448 | 0.28 | 0.28 | 1952 | 0.16 | 0.1 | 0.41 | 0.5 | 0 |
| Mean patients | 0.25 | 0.317 | 0.123 | 0.083 | 1.52 | 0.637 | 0.623 | ||
| SD patients | 0.128 | 0.040 | 0.109 | 0.076 | 1.25 | 0.228 | 0.77 | ||
| Controls | 38 749 | 144 138 | |||||||
| Mean controls | 0.40 | 0.30 | 0.06 | 0.05 | 0.28 | 0.09 | 0.48 | ||
| SD controls | 0.117 | 0.132 | 0.05 | 0.04 | 0.13 | 0.05 | 0.17 | ||
| P value | 0.072 | 0.833 | 0.074 | 0.219 | 0.0001 | 0.0001 | 0.368 | ||
FISH, fluorescence in-situ hybridization; MMAF, multiple morphological abnormalities of the sperm flagella, n, number; SD, standard deviation.
| . | . | Disomies (%) . | . | Disomies (%) . | Diploidy (%) . | ||||
|---|---|---|---|---|---|---|---|---|---|
| Analyzed gametes (n) . | 13, 13, 21 . | 13, 21, 21 . | Analyzed gametes (n) . | X, X, 18 . | Y, Y, 18 . | X, Y, 18 . | X or Y, 18, 18 . | . | |
| Patient P1 | 1896 | 0.11 | 0.31 | 1948 | 0.21 | 0.15 | 1.28 | 0.51 | 1.48 |
| Patient P17 | 1897 | 0.36 | 0.36 | 1907 | 0 | 0 | 2.87 | 0.9 | 0.39 |
| Patient P9 | 2448 | 0.28 | 0.28 | 1952 | 0.16 | 0.1 | 0.41 | 0.5 | 0 |
| Mean patients | 0.25 | 0.317 | 0.123 | 0.083 | 1.52 | 0.637 | 0.623 | ||
| SD patients | 0.128 | 0.040 | 0.109 | 0.076 | 1.25 | 0.228 | 0.77 | ||
| Controls | 38 749 | 144 138 | |||||||
| Mean controls | 0.40 | 0.30 | 0.06 | 0.05 | 0.28 | 0.09 | 0.48 | ||
| SD controls | 0.117 | 0.132 | 0.05 | 0.04 | 0.13 | 0.05 | 0.17 | ||
| P value | 0.072 | 0.833 | 0.074 | 0.219 | 0.0001 | 0.0001 | 0.368 | ||
| . | . | Disomies (%) . | . | Disomies (%) . | Diploidy (%) . | ||||
|---|---|---|---|---|---|---|---|---|---|
| Analyzed gametes (n) . | 13, 13, 21 . | 13, 21, 21 . | Analyzed gametes (n) . | X, X, 18 . | Y, Y, 18 . | X, Y, 18 . | X or Y, 18, 18 . | . | |
| Patient P1 | 1896 | 0.11 | 0.31 | 1948 | 0.21 | 0.15 | 1.28 | 0.51 | 1.48 |
| Patient P17 | 1897 | 0.36 | 0.36 | 1907 | 0 | 0 | 2.87 | 0.9 | 0.39 |
| Patient P9 | 2448 | 0.28 | 0.28 | 1952 | 0.16 | 0.1 | 0.41 | 0.5 | 0 |
| Mean patients | 0.25 | 0.317 | 0.123 | 0.083 | 1.52 | 0.637 | 0.623 | ||
| SD patients | 0.128 | 0.040 | 0.109 | 0.076 | 1.25 | 0.228 | 0.77 | ||
| Controls | 38 749 | 144 138 | |||||||
| Mean controls | 0.40 | 0.30 | 0.06 | 0.05 | 0.28 | 0.09 | 0.48 | ||
| SD controls | 0.117 | 0.132 | 0.05 | 0.04 | 0.13 | 0.05 | 0.17 | ||
| P value | 0.072 | 0.833 | 0.074 | 0.219 | 0.0001 | 0.0001 | 0.368 | ||
FISH, fluorescence in-situ hybridization; MMAF, multiple morphological abnormalities of the sperm flagella, n, number; SD, standard deviation.
Analysis of sperm chromatin condensation and DNA fragmentation
Sperm chromatin was scored as uncompacted if the sperm head was stained with aniline blue (Fig. 2a). The percentage of sperm with uncompacted chromatin varied from 6 to 16% in the three DNAH1 mutated patients, with a mean of 9.6%. Sperm from normospermic controls had a rate of 6.6% uncompacted DNA (n = 6). The proportion of sperm with abnormal chromatin condensation was not statistically different (P = 0.45) between patients and controls (Fig. 2a).
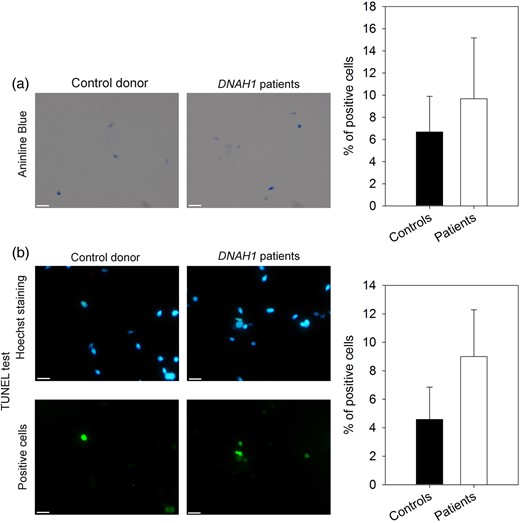
Measure of DNA condensation and fragmentation. (a) DNA condensation was measured from sperm cells stained with aniline blue (AB). Control sperm (left) were not significantly different (the student t-test, P = 0.45) from patients' sperm (right). The histogram shows the percentage of sperm cells stained for fertile controls (n = 6) and patients (n = 3). Bars represent mean + standard deviation (SD). (b) DNA fragmentation analysis with terminal deoxynucleotidyltransferase-mediated dUTP nick-end labeling (TUNEL test) assay. Hoechst and TUNEL staining for control sperm (left) were not significantly different (the student t-test, P = 0.12) from staining for patients' sperm (right). The histogram shows the percentage of TUNEL positive cells for fertile controls (n = 6) and patients (n = 3). Bars represent mean + SD.
The proportion of spermatozoa with a green fluorescence (TUNEL positive) indicating DNA fragmentation varied from 6 to 12.5% in the three patients, with a mean of 9%, compared with a mean of 4.5% for controls (n = 6) (Fig. 2b). These values were not statistically different between the two populations (P = 0.12).
Discussion
The relationship between chromosomal abnormalities and teratozoospermia has been widely investigated. A link has been clearly established for some phenotypes, such as large-headed multiflagellar spermatozoa (Dieterich et al., 2009), but remains disputable for many others (Lewis-Jones et al., 2003). Several studies have demonstrated previously that sperm flagellar abnormalities are often associated with an elevated frequency of gonosomal disomies and diploidies (Baccetti et al., 2005; Rives et al., 2005; Collodel and Moretti 2006; Ghedir et al., 2014). Theses chromosomal abnormalities observed in MMAF patients might be linked to the common components shared between the sperm centrosome, the mitotic spindle and the flagella (Coutton et al., 2015). Therefore, it could be hypothesized that defects in some centrosome-associated proteins may disturb both flagellum formation and spindle assembly during sperm meiosis, resulting in nondisjunction errors and aneuploidy in spermatozoa (Rives et al., 2005). Chromosomal abnormalities have however not been detected in all reports describing the sperm chromosomal constitution of MMAF patients (Viville et al., 2000), indicating that flagellar defects do not systematically lead to sperm aneuploidy.
In the light of the above, the aim of this study was to evaluate the aneuploidy rate and the sperm DNA quality in MMAF patients with a mutation in the DNAH1 gene. Sperm FISH analyses showed that patients with DNAH1 mutations have few abnormal sperm although we observed a significant increase in XY and 18 disomy. These low aneuploidy rates suggest that ICSI with spermatozoa from infertile patients mutated in the DNAH1 gene will likely be of good prognosis for future pregnancies. Pending complementary studies on larger cohorts of DNAH1 mutated men, we propose that sperm FISH analyses should be envisaged in MMAF patients harboring a DNAH1 mutation to verify if the rate of XY and 18 disomy is indeed increased in these patients. Such analysis however is not necessary in a routine setting. Sperm nuclear quality is also an important parameter to predict the chances of success for ICSI treatment, as demonstrated in globozoospermia. Indeed, the lower live birth rate per transfer in globozoospermic patients may be due to sperm DNA damage related to defective chromatin condensation and DNA fragmentation (Dam et al., 2007; Brahem et al., 2011; Perrin et al., 2013; Yassine et al., 2015). For DNAH1 patients, the rate of DNA compaction and fragmentation was not statistically different compared with controls, thus demonstrating a good nuclear sperm quality.
In this study, MMAF patients with DNAH1 mutations had overall fertilization, implantation, pregnancy and live birth rates of 70.8, 31.3, 50.0 and 37.5%, respectively. These results are statistically comparable with the overall outcomes obtained in our fertility center (polyclinique les Jasmins, Tunis) in the same time period. They are also similar to those obtained for MMAF patients without mutations in the DNAH1 gene (Table II). Successful application of ICSI to treat male infertility due to MMAF has also been previously described (Chemes and Rawe, 2003; Chemes and Alvarez Sedo, 2012). A recent meta-analysis estimated the mean overall fertilization, pregnancy and live birth rates in MMAF patients to be 63, 57 and 43%, respectively (Dávila Garza and Patrizio, 2013). These results obtained from nongenotyped MMAF patients are quite similar to those observed here with DNAH1 mutated men. Interestingly, it was reported that the success rates of ICSI may be correlated to the type of ultrastructural flagellar defect carried by the patients, as Mitchell et al. (2006) reported lower implantation (8%) and clinical pregnancy rates (15%) in patients without axonemal central pair. Fauque et al. (2009) also reported a slower kinetics of early embryo cleavage and a low implantation rate when the central microtubule pair was missing. We have described the absence of the central doublet as a hallmark of DNAH1 associated MMAF (Ben Khelifa et al., 2014) but have here observed good ICSI outcomes. The difference may be explained by the molecular defects causing the 9 + 0 phenotype. In DNAH1 patients, it is the lack of the inner arm heavy chain which results in the abrogation of radial spokes anchoring (Ben Khelifa et al., 2014). We can suppose that the absence of the inner arm could be less damaging for embryonic development than other defects like those induced by the absence or defects of centrosomal or pericentrosomal proteins (Chemes, 2012; Coutton et al., 2015). Here, all patients studied had a truncating mutation expected to abrogate the protein functionality. Patients P1, 2, 3 and 17 have the same homozygous splicing mutation in exon 75 (c.11788-1G>A) that was demonstrated to lead to nonsense mediated mRNA decay leading to the complete absence of the protein (Ben Khelifa et al., 2014). We can therefore safely assume that patients bearing other DNAH1 truncating mutations will be similarly affected with a typical MMAF phenotype and a good ICSI prognosis. This expected good prognosis can logically extend to the other patients affected with less severe DNAH1 mutations, such as missense variants (modification of a single amino acid). We also observed that four out the six studied couples subsequently had one to three replacements of frozen embryos leading to two clinical pregnancies with two deliveries. These data also suggest that axonemal defects due to DNAH1 homozygous truncating mutations do not hamper embryo survival and development following freezing and thawing.
To conclude, although we observed an increased rate of XY and 18 disomy, MMAF patients with DNAH1 gene alterations showed a relatively low aneuploidy rate and a good nuclear sperm quality leading to a good embryonic development following ICSI. These parameters explain the high pregnancy rate achieved by these patients. This study demonstrates that MMAF patients present an overall a good prognosis following ICSI. DNAH1 mutations have been found in 28% of the MMAF patients analyzed, suggesting that several other genes also induce MMAF. Some of these genes affecting a small proportion of patients may however be more deleterious and cause a poor prognosis following ICSI. Although we observed that the fertilization rate of MMAF patients without DNAH1 mutations was higher than that in the control group, the clinical pregnancy and delivery rates were comparable between the three groups. We therefore cannot exclude the possibility that some of the yet unidentified gene defects in non-DNAH1 mutated MMAF patients could impair subsequent embryonic development and decrease the success rates of ICSI. Further genetic studies are therefore warranted to identify other MMAF inducing genes to better characterize the genetic etiology of the MMAF phenotype and to improve the management of patients diagnosed with flagellar defects.
Acknowledgements
The authors thank the patients for their interest and cooperation.
Authors' roles
C.W., R.Z., P.F.R, C.A. and C.C. analyzed the data and wrote the manuscript; C.W., F.D., V.S. and F.A. performed sperm FISH analyses; G.M. and S.B. performed DNA fragmentation and chromatin compaction analyses. R.Z., S.F.B.M., S.H., L.H., O.M., M.M., H.L. and M.K. provided clinical samples and data; P.F.R. and C.C. designed the study, supervised all molecular laboratory work, had full access to all of the data in the study and take responsibility for the integrity of the data and its accuracy. All authors contributed to the report.
Funding
This work was supported by following grants: ANR Genopat 2009 for the project ICG2I and the MAS-Flagella project financed by the French Agence Nationale de la Recherche (ANR) and the Direction générale de l'offre de soins (DGOS).
Conflict of interest
None declared.
References
Author notes
These authors should be regarded as joint first and last authors respectively.



