-
PDF
- Split View
-
Views
-
Cite
Cite
A.P. Ferraretti, A. La Marca, B.C.J.M. Fauser, B. Tarlatzis, G. Nargund, L. Gianaroli, on behalf of the ESHRE working group on Poor Ovarian Response Definition, ESHRE consensus on the definition of ‘poor response' to ovarian stimulation for in vitro fertilization: the Bologna criteria, Human Reproduction, Volume 26, Issue 7, 1 July 2011, Pages 1616–1624, https://doi.org/10.1093/humrep/der092
Close - Share Icon Share
Abstract
The definition presented here represents the first realistic attempt by the scientific community to standardize the definition of poor ovarian response (POR) in a simple and reproducible manner. POR to ovarian stimulation usually indicates a reduction in follicular response, resulting in a reduced number of retrieved oocytes. It has been recognized that, in order to define the poor response in IVF, at least two of the following three features must be present: (i) advanced maternal age or any other risk factor for POR; (ii) a previous POR; and (iii) an abnormal ovarian reserve test (ORT). Two episodes of POR after maximal stimulation are sufficient to define a patient as poor responder in the absence of advanced maternal age or abnormal ORT. By definition, the term POR refers to the ovarian response, and therefore, one stimulated cycle is considered essential for the diagnosis of POR. However, patients of advanced age with an abnormal ORT may be classified as poor responders since both advanced age and an abnormal ORT may indicate reduced ovarian reserve and act as a surrogate of ovarian stimulation cycle outcome. In this case, the patients should be more properly defined as ‘expected poor responder’. If this definition of POR is uniformly adapted as the ‘minimal' criteria needed to select patients for future clinical trials, more homogeneous populations will be tested for any new protocols. Finally, by reducing bias caused by spurious POR definitions, it will be possible to compare results and to draw reliable conclusions.
Introduction
The first description of a patient who was a poor responder occurred 28 years ago (Garcia et al., 1983). A patient responding with a decreased follicular response and low oestradiol (E2) levels to ovarian stimulation by FSH/HMG was reported, resulting in few oocytes being retrieved and few transferred embryos. Since then, there are several hundred publications on poor ovarian response (POR), its pathogenesis, clinical characterization and possible treatment (reviewed in Surrey and Schoolcraft, 2000; Tarlatzis et al., 2003; Loutradis et al., 2008; Kyrou et al., 2009; Pandian et al., 2010). The authors’ conclusions in all these reviews are always the same; there is insufficient evidence to identify the use of any particular intervention to improve treatment outcomes in poor responders because of the small numbers of participants and the heterogeneity between the trials on the definition of POR. An internationally accepted definition is needed, which should be universally used in future trials so as to compare results and relevant interventions in IVF.
Despite the growing awareness that a uniform definition is important, no consensus has yet been reached. Since POR represents several controversial issues in the clinical, scientific and psychological sense, an ESHRE Campus Workshop was organized in Bologna, 19–20 March 2010 involving all the ESHRE Special Interest Groups (SIGs) and the majority of research groups who have significantly contributed to the field. The main objective of the workshop was to reach a consensus on the definition and diagnosis of POR. An agreement was reached after a discussion with the representatives from all the SIGs. In the present article, the scientific background of the issue is summarized, the criteria proposed for the definition of POR are presented and the rationale for universally adapting this definition is discussed. As a first step, a consensus was reached on the nomenclature, since different terms are still utilized in the literature.
Bologna consensus on nomenclature
The goal of ovarian stimulation in IVF is the recruitment of multiple follicles in an effort to compensate for the inefficiencies of embryology culture, embryo selection for transfer and subsequent implantation (Macklon et al., 2006). Hence, poor response to ovarian stimulation usually indicates a reduction in follicular response resulting in a reduced number of retrieved oocytes.
Many terms are used in the literature for the type of ovarian response on which the discussion was focused. The most frequent are ‘poor’ and ‘low’, but terms such as ‘bad’, ‘slow’, ‘inadequate’ and ‘suboptimal’ are also present in literature. In addition, the term is combined with ‘response', ‘responder' or ‘ovarian reserve'. The participants agreed that the term should imply an intrinsic inability of a woman's ovaries to react accordingly to the stimulation chosen for her. ‘Low' refers only to the number of oocytes. In the light of the new trend of using mild stimulation, the collection of few oocytes can be an expected optimal result of this strategy. Therefore, the choice focused between the terms ‘poor' and ‘inadequate', which are more consonant with the meaning of a negative condition that could affect the outcome. The term ‘poor' should be considered the best, since it is the most used in literature. The acronyms POR and PORs could enter into conventional assisted reproduction treatment terminology to define POR and poor ovarian responders, respectively.
The need for a consensus on the definition of POR
As mentioned earlier (Surrey and Schoolcraft, 2000; Tarlatzis et al., 2003; Loutradis et al., 2008; Kyrou et al., 2009; Pandian et al., 2010), the lack of a uniform definition of a poor response is the most relevant factor that makes it impossible to compare studies, and very difficult to develop or assess any protocol to improve the outcome.
Since studies began, different authors have used different criteria to define POR. A peak E2 level of <300 pg/ml to <500 pg/ml has been proposed as being crucial for defining a poor response (Garcia et al., 1983; Brzyski et al., 1988; Raga et al., 1999), although a level of <100 pg/ml on Day 5 of stimulation has also been suggested (Schoolcraft et al., 1997). The number of developed follicles and/or number of oocytes retrieved after a standard-dose ovarian stimulation protocol are two of the most frequent criteria used, but the proposed number varies among different authors from less than 3 to less than 6 dominant follicles on the day of hCG administration (Land et al., 1996; Fridstrom et al., 1997; Raga et al., 1999) and/or from less than 3 to less than 5 retrieved oocytes (Chong et al., 1986; Rombauts et al., 1998; Surrey et al., 1998). An elevated day 3 FSH level ranging from ≥7 mIU/ml to ≥15 mIU/ml has been proposed as an additional criterion (Droesch et al., 1989; Feldberg et al., 1994; Faber et al., 1998; Karande and Gleicher, 1999) as well as an advanced patient age ≥40 years (Karande and Gleicher, 1999), disappointing, or no response to, the clomiphene challenge test (Navot et al., 1987), and a failed GnRH analogue stimulation test (Katayama et al., 1988). Other criteria such as at least one cancelled IVF cycle (Manzi et al., 1994), increased total dose of FSH used (Shaker et al., 1992), increased daily (>300 IU/day) gonadotrophin dose used (Faber et al., 1998) and prolonged duration of gonadotrophin stimulation (Toth et al., 1996) have been used to define POR. A review up-dated to 1999 (Surrey and Schoolcraft, 2000) registered more than 35 different definitions.
Despite the clear evidence of a lack of an universal definition reported since then, and the recognized need for standardization, the studies published on the topic in the past 10 years still adopt different criteria to select women for clinical trials (Table I). Predictive criteria such as advanced age or abnormal ovarian reserve tests (ORTs; potential PORs) and previous POR (past PORs) are the most frequently used today, but different thresholds are still often chosen for each criteria. In addition, a variety of studies indifferently select both populations (potential and past PORs), thereby highlighting the complexity in evaluating the results, since the interventions proposed may apply to different patient groups in terms of ovarian response potential. A clear demonstration of this is the high number of oocytes collected in many of the trials listed in Table I—not only when the new intervention is used, but also in the control group (Table II).
| Reference . | Criteria . |
|---|---|
| Garcia-Velasco et al. (2000) | At least one previous cycle cancelled because of ≤3 follicles ≥18 mm |
| Ferraretti et al. (2000) | At least two previous cycles concelled or with ≤3 oocytes |
| Akman et al. (2001) | Two failed IVF attempts for one of the following reasons:
|
| Weissman et al. (2003) |
|
| Marci et al. (2003) | One previous POR in a standard treatment |
| Goswami et al. (2004) | One to three failed IVF attempts due to POR to conventional long-agonist protocol |
| Kolibianakis et al. (2004) | One or more failed IVF cycles in which ≤5 oocytes were retrieved and Day-3 FHS level >12 mIU/ml |
| Morgia et al. (2004) | One previous IVF cycle with ≤3 oocytes |
| Detti et al. (2005) |
|
| Cheung et al. (2005) | One previous POR with ≤3 oocytes on a long-agonist protocol or repeated Day-3 FSH >10 IU/l |
| Garcia-Velasco et al. (2005) | At least one previous cancelled cycle due to ≤4 follicles >16 mm and/or E2 level ≤500 pg/ml |
| Massin et al. (2006) |
|
| Aletebi (2007) | POR in previous cycle(s): ≤4 oocytes following stimulation for ≥15 days involving 300 IU of gonadotrphins daily |
| Schoolcraft et al. (2008) |
|
| Frattarelli et al. (2008a) |
|
| Frattarelli et al. (2008b) | Two previous POR (criteria not defined) |
| Barrenetxea et al. (2008) | Age ≥40 years and Day-3 FSH ≥10 mIU/ml |
| Tazegul et al. (2008) | Previous POR: E2 <500 pg/ml or ≤3 mature follicles or <3 oocytes |
| Fábregues et al. (2009) | First IVF cycle cancelled because of POR (criteria not defined) |
| Kahraman et al. (2009) |
|
| Yarali et al. (2009) | Abnormal ORTs (FSH >10 mIU/ml or AFC <6) or previous POR (cycle cancelled or E2 >500 pg/ml or ≤3 oocytes) |
| Weitzman et al. (2009) |
|
| Demirol and Gurgan (2009) | At least two previous POR (E2 <500 pg/ml or ≤3 oocytes) and Day-3 FSH >15 IU/l |
| Tehraninejad et al. (2009) | At least one previous cycle cancelled because of <3 mature follicles |
| Reference . | Criteria . |
|---|---|
| Garcia-Velasco et al. (2000) | At least one previous cycle cancelled because of ≤3 follicles ≥18 mm |
| Ferraretti et al. (2000) | At least two previous cycles concelled or with ≤3 oocytes |
| Akman et al. (2001) | Two failed IVF attempts for one of the following reasons:
|
| Weissman et al. (2003) |
|
| Marci et al. (2003) | One previous POR in a standard treatment |
| Goswami et al. (2004) | One to three failed IVF attempts due to POR to conventional long-agonist protocol |
| Kolibianakis et al. (2004) | One or more failed IVF cycles in which ≤5 oocytes were retrieved and Day-3 FHS level >12 mIU/ml |
| Morgia et al. (2004) | One previous IVF cycle with ≤3 oocytes |
| Detti et al. (2005) |
|
| Cheung et al. (2005) | One previous POR with ≤3 oocytes on a long-agonist protocol or repeated Day-3 FSH >10 IU/l |
| Garcia-Velasco et al. (2005) | At least one previous cancelled cycle due to ≤4 follicles >16 mm and/or E2 level ≤500 pg/ml |
| Massin et al. (2006) |
|
| Aletebi (2007) | POR in previous cycle(s): ≤4 oocytes following stimulation for ≥15 days involving 300 IU of gonadotrphins daily |
| Schoolcraft et al. (2008) |
|
| Frattarelli et al. (2008a) |
|
| Frattarelli et al. (2008b) | Two previous POR (criteria not defined) |
| Barrenetxea et al. (2008) | Age ≥40 years and Day-3 FSH ≥10 mIU/ml |
| Tazegul et al. (2008) | Previous POR: E2 <500 pg/ml or ≤3 mature follicles or <3 oocytes |
| Fábregues et al. (2009) | First IVF cycle cancelled because of POR (criteria not defined) |
| Kahraman et al. (2009) |
|
| Yarali et al. (2009) | Abnormal ORTs (FSH >10 mIU/ml or AFC <6) or previous POR (cycle cancelled or E2 >500 pg/ml or ≤3 oocytes) |
| Weitzman et al. (2009) |
|
| Demirol and Gurgan (2009) | At least two previous POR (E2 <500 pg/ml or ≤3 oocytes) and Day-3 FSH >15 IU/l |
| Tehraninejad et al. (2009) | At least one previous cycle cancelled because of <3 mature follicles |
AFC, antral follicle count; E2, estradiol; FSH, follicle stimulating hormone; HCG, human chorionic gonadotrophin; IVF, in vitro fertilization; ORTs, ovarian reserve tests.
| Reference . | Criteria . |
|---|---|
| Garcia-Velasco et al. (2000) | At least one previous cycle cancelled because of ≤3 follicles ≥18 mm |
| Ferraretti et al. (2000) | At least two previous cycles concelled or with ≤3 oocytes |
| Akman et al. (2001) | Two failed IVF attempts for one of the following reasons:
|
| Weissman et al. (2003) |
|
| Marci et al. (2003) | One previous POR in a standard treatment |
| Goswami et al. (2004) | One to three failed IVF attempts due to POR to conventional long-agonist protocol |
| Kolibianakis et al. (2004) | One or more failed IVF cycles in which ≤5 oocytes were retrieved and Day-3 FHS level >12 mIU/ml |
| Morgia et al. (2004) | One previous IVF cycle with ≤3 oocytes |
| Detti et al. (2005) |
|
| Cheung et al. (2005) | One previous POR with ≤3 oocytes on a long-agonist protocol or repeated Day-3 FSH >10 IU/l |
| Garcia-Velasco et al. (2005) | At least one previous cancelled cycle due to ≤4 follicles >16 mm and/or E2 level ≤500 pg/ml |
| Massin et al. (2006) |
|
| Aletebi (2007) | POR in previous cycle(s): ≤4 oocytes following stimulation for ≥15 days involving 300 IU of gonadotrphins daily |
| Schoolcraft et al. (2008) |
|
| Frattarelli et al. (2008a) |
|
| Frattarelli et al. (2008b) | Two previous POR (criteria not defined) |
| Barrenetxea et al. (2008) | Age ≥40 years and Day-3 FSH ≥10 mIU/ml |
| Tazegul et al. (2008) | Previous POR: E2 <500 pg/ml or ≤3 mature follicles or <3 oocytes |
| Fábregues et al. (2009) | First IVF cycle cancelled because of POR (criteria not defined) |
| Kahraman et al. (2009) |
|
| Yarali et al. (2009) | Abnormal ORTs (FSH >10 mIU/ml or AFC <6) or previous POR (cycle cancelled or E2 >500 pg/ml or ≤3 oocytes) |
| Weitzman et al. (2009) |
|
| Demirol and Gurgan (2009) | At least two previous POR (E2 <500 pg/ml or ≤3 oocytes) and Day-3 FSH >15 IU/l |
| Tehraninejad et al. (2009) | At least one previous cycle cancelled because of <3 mature follicles |
| Reference . | Criteria . |
|---|---|
| Garcia-Velasco et al. (2000) | At least one previous cycle cancelled because of ≤3 follicles ≥18 mm |
| Ferraretti et al. (2000) | At least two previous cycles concelled or with ≤3 oocytes |
| Akman et al. (2001) | Two failed IVF attempts for one of the following reasons:
|
| Weissman et al. (2003) |
|
| Marci et al. (2003) | One previous POR in a standard treatment |
| Goswami et al. (2004) | One to three failed IVF attempts due to POR to conventional long-agonist protocol |
| Kolibianakis et al. (2004) | One or more failed IVF cycles in which ≤5 oocytes were retrieved and Day-3 FHS level >12 mIU/ml |
| Morgia et al. (2004) | One previous IVF cycle with ≤3 oocytes |
| Detti et al. (2005) |
|
| Cheung et al. (2005) | One previous POR with ≤3 oocytes on a long-agonist protocol or repeated Day-3 FSH >10 IU/l |
| Garcia-Velasco et al. (2005) | At least one previous cancelled cycle due to ≤4 follicles >16 mm and/or E2 level ≤500 pg/ml |
| Massin et al. (2006) |
|
| Aletebi (2007) | POR in previous cycle(s): ≤4 oocytes following stimulation for ≥15 days involving 300 IU of gonadotrphins daily |
| Schoolcraft et al. (2008) |
|
| Frattarelli et al. (2008a) |
|
| Frattarelli et al. (2008b) | Two previous POR (criteria not defined) |
| Barrenetxea et al. (2008) | Age ≥40 years and Day-3 FSH ≥10 mIU/ml |
| Tazegul et al. (2008) | Previous POR: E2 <500 pg/ml or ≤3 mature follicles or <3 oocytes |
| Fábregues et al. (2009) | First IVF cycle cancelled because of POR (criteria not defined) |
| Kahraman et al. (2009) |
|
| Yarali et al. (2009) | Abnormal ORTs (FSH >10 mIU/ml or AFC <6) or previous POR (cycle cancelled or E2 >500 pg/ml or ≤3 oocytes) |
| Weitzman et al. (2009) |
|
| Demirol and Gurgan (2009) | At least two previous POR (E2 <500 pg/ml or ≤3 oocytes) and Day-3 FSH >15 IU/l |
| Tehraninejad et al. (2009) | At least one previous cycle cancelled because of <3 mature follicles |
AFC, antral follicle count; E2, estradiol; FSH, follicle stimulating hormone; HCG, human chorionic gonadotrophin; IVF, in vitro fertilization; ORTs, ovarian reserve tests.
| Reference . | Participants . | Interventions . | Oocytes collected in the trial . |
|---|---|---|---|
| Garcia-Velasco et al. (2000) | 70 | Stop versus non-stop protocol of GnRH analogue (prospective, randomized, controlled trial) | 8.7 versus 6.2 |
| Garcia-Velasco et al. (2005) | 147 | Antagonist GnRH protocol with or without letrozole (observational pilot study) | 6.1 versus 4.3 |
| Cheung et al. (2005) | 66 | GnRH antagonist versus long GnRH agonist (randomized controlled trial) | 5.9 versus 5.6 |
| Detti et al. (2005) | 61 | Three down-regulation approaches (retrospective cohort study) | 10.8 versus 7.8 versus 7.4 |
| Frattarelli et al. (2008a) | 1230 | Low-dose aspirin versus no aspirin in GnRH agonist protocol (retrospective cohort analysis) | 9.1 versus 9.0 |
| Frattarelli et al. (2008b) | 60 | Addition of luteal E2 to the standard IVF protocol (retrospective cohort analysis) | 11.8 versus 9.5 |
| Schoolcraft et al. (2008) | 534 | Microdose GnRH agonist flare versus GnRH antagonist/letrozole (prospective controlled trial) | 12.6 versus 13.5 |
| Barrenetxea et al. (2008) | 84 | LH supplementation in GnRH analogues protocol (prospective randomized trial) | 5.4 versus 5.7 |
| Weitzman et al. (2009) | 121 | Luteal phase E2 patch/GnRH antagonist versus microdose GnRH agonist (retrospective analysis) | 9.1 versus 8.9 |
| Yarali et al. (2009) | 1382 | GnRH antagonist/letrozole versus microdose GnRH agonist flare-up (retrospective case–control study) | 6.7 versus 5.1 |
| Weitzman et al. (2009) | 121 | Luteal phase E2 patch/GnRH antagonist versus microdose GnRH agonist (retrospective analysis) | 9.1 versus 8.9 |
| Kahraman et al. (2009) | 42 | Microdose GnRH agonist flare-up versus GnRH antagonist (prospective randomized study) | 5.8 versus 5.6 |
| Reference . | Participants . | Interventions . | Oocytes collected in the trial . |
|---|---|---|---|
| Garcia-Velasco et al. (2000) | 70 | Stop versus non-stop protocol of GnRH analogue (prospective, randomized, controlled trial) | 8.7 versus 6.2 |
| Garcia-Velasco et al. (2005) | 147 | Antagonist GnRH protocol with or without letrozole (observational pilot study) | 6.1 versus 4.3 |
| Cheung et al. (2005) | 66 | GnRH antagonist versus long GnRH agonist (randomized controlled trial) | 5.9 versus 5.6 |
| Detti et al. (2005) | 61 | Three down-regulation approaches (retrospective cohort study) | 10.8 versus 7.8 versus 7.4 |
| Frattarelli et al. (2008a) | 1230 | Low-dose aspirin versus no aspirin in GnRH agonist protocol (retrospective cohort analysis) | 9.1 versus 9.0 |
| Frattarelli et al. (2008b) | 60 | Addition of luteal E2 to the standard IVF protocol (retrospective cohort analysis) | 11.8 versus 9.5 |
| Schoolcraft et al. (2008) | 534 | Microdose GnRH agonist flare versus GnRH antagonist/letrozole (prospective controlled trial) | 12.6 versus 13.5 |
| Barrenetxea et al. (2008) | 84 | LH supplementation in GnRH analogues protocol (prospective randomized trial) | 5.4 versus 5.7 |
| Weitzman et al. (2009) | 121 | Luteal phase E2 patch/GnRH antagonist versus microdose GnRH agonist (retrospective analysis) | 9.1 versus 8.9 |
| Yarali et al. (2009) | 1382 | GnRH antagonist/letrozole versus microdose GnRH agonist flare-up (retrospective case–control study) | 6.7 versus 5.1 |
| Weitzman et al. (2009) | 121 | Luteal phase E2 patch/GnRH antagonist versus microdose GnRH agonist (retrospective analysis) | 9.1 versus 8.9 |
| Kahraman et al. (2009) | 42 | Microdose GnRH agonist flare-up versus GnRH antagonist (prospective randomized study) | 5.8 versus 5.6 |
E2, estradiol; GnHR, gonadotrophin hormone receptor; IVF, in vitro fertilization; LH, luteinizing hormone.
An internationally accepted universal definition of POR is urgently needed for research purposesto design proper trials avoiding selection bias, to meaningfully assess and compare the interventions proposed. Different studies used different criteria to define POR causing complexity in evaluating reported results. A clear demonstration of this is the high number of oocytes collected in many of the trials listed in the table.
| Reference . | Participants . | Interventions . | Oocytes collected in the trial . |
|---|---|---|---|
| Garcia-Velasco et al. (2000) | 70 | Stop versus non-stop protocol of GnRH analogue (prospective, randomized, controlled trial) | 8.7 versus 6.2 |
| Garcia-Velasco et al. (2005) | 147 | Antagonist GnRH protocol with or without letrozole (observational pilot study) | 6.1 versus 4.3 |
| Cheung et al. (2005) | 66 | GnRH antagonist versus long GnRH agonist (randomized controlled trial) | 5.9 versus 5.6 |
| Detti et al. (2005) | 61 | Three down-regulation approaches (retrospective cohort study) | 10.8 versus 7.8 versus 7.4 |
| Frattarelli et al. (2008a) | 1230 | Low-dose aspirin versus no aspirin in GnRH agonist protocol (retrospective cohort analysis) | 9.1 versus 9.0 |
| Frattarelli et al. (2008b) | 60 | Addition of luteal E2 to the standard IVF protocol (retrospective cohort analysis) | 11.8 versus 9.5 |
| Schoolcraft et al. (2008) | 534 | Microdose GnRH agonist flare versus GnRH antagonist/letrozole (prospective controlled trial) | 12.6 versus 13.5 |
| Barrenetxea et al. (2008) | 84 | LH supplementation in GnRH analogues protocol (prospective randomized trial) | 5.4 versus 5.7 |
| Weitzman et al. (2009) | 121 | Luteal phase E2 patch/GnRH antagonist versus microdose GnRH agonist (retrospective analysis) | 9.1 versus 8.9 |
| Yarali et al. (2009) | 1382 | GnRH antagonist/letrozole versus microdose GnRH agonist flare-up (retrospective case–control study) | 6.7 versus 5.1 |
| Weitzman et al. (2009) | 121 | Luteal phase E2 patch/GnRH antagonist versus microdose GnRH agonist (retrospective analysis) | 9.1 versus 8.9 |
| Kahraman et al. (2009) | 42 | Microdose GnRH agonist flare-up versus GnRH antagonist (prospective randomized study) | 5.8 versus 5.6 |
| Reference . | Participants . | Interventions . | Oocytes collected in the trial . |
|---|---|---|---|
| Garcia-Velasco et al. (2000) | 70 | Stop versus non-stop protocol of GnRH analogue (prospective, randomized, controlled trial) | 8.7 versus 6.2 |
| Garcia-Velasco et al. (2005) | 147 | Antagonist GnRH protocol with or without letrozole (observational pilot study) | 6.1 versus 4.3 |
| Cheung et al. (2005) | 66 | GnRH antagonist versus long GnRH agonist (randomized controlled trial) | 5.9 versus 5.6 |
| Detti et al. (2005) | 61 | Three down-regulation approaches (retrospective cohort study) | 10.8 versus 7.8 versus 7.4 |
| Frattarelli et al. (2008a) | 1230 | Low-dose aspirin versus no aspirin in GnRH agonist protocol (retrospective cohort analysis) | 9.1 versus 9.0 |
| Frattarelli et al. (2008b) | 60 | Addition of luteal E2 to the standard IVF protocol (retrospective cohort analysis) | 11.8 versus 9.5 |
| Schoolcraft et al. (2008) | 534 | Microdose GnRH agonist flare versus GnRH antagonist/letrozole (prospective controlled trial) | 12.6 versus 13.5 |
| Barrenetxea et al. (2008) | 84 | LH supplementation in GnRH analogues protocol (prospective randomized trial) | 5.4 versus 5.7 |
| Weitzman et al. (2009) | 121 | Luteal phase E2 patch/GnRH antagonist versus microdose GnRH agonist (retrospective analysis) | 9.1 versus 8.9 |
| Yarali et al. (2009) | 1382 | GnRH antagonist/letrozole versus microdose GnRH agonist flare-up (retrospective case–control study) | 6.7 versus 5.1 |
| Weitzman et al. (2009) | 121 | Luteal phase E2 patch/GnRH antagonist versus microdose GnRH agonist (retrospective analysis) | 9.1 versus 8.9 |
| Kahraman et al. (2009) | 42 | Microdose GnRH agonist flare-up versus GnRH antagonist (prospective randomized study) | 5.8 versus 5.6 |
E2, estradiol; GnHR, gonadotrophin hormone receptor; IVF, in vitro fertilization; LH, luteinizing hormone.
An internationally accepted universal definition of POR is urgently needed for research purposesto design proper trials avoiding selection bias, to meaningfully assess and compare the interventions proposed. Different studies used different criteria to define POR causing complexity in evaluating reported results. A clear demonstration of this is the high number of oocytes collected in many of the trials listed in the table.
If a solid approach to the problem is absent in the scientific literature, the management of POR in clinical practice can be even worse in terms of standardization. A recent survey (IVF-Worldwide, 2010), conducted in 196 centres from 45 countries, clearly shows a huge variation in defining and treating PORs.
Thus, an internationally accepted universal definition of POR is urgently needed for research purpose to design proper trials avoiding selection bias, to meaningfully assess and compare the interventions proposed, and to estimate the incidence. It is time to produce evidence-based medicine in the field for the benefit of these very difficult groups of patients.
Critical evaluation of the criteria used
Predictive criteria
Age and other risk factors associated with POR
It is widely accepted that POR may be an early sign of ovarian ageing and of reduced ovarian reserve (Beckers et al., 2002; De Boer et al., 2002; Lawson et al., 2003). Hence, ovarian stimulation can be viewed as a dynamic test for the resting ovarian follicular pool (Beckers et al., 2002). In fact, the size of the cohort of recruitable follicles may be a reflection of the actual resting follicle pool (Gougeon, 1996). Secondary to the physiological decline in the ovarian follicle pool with ageing, the ovarian response to FSH decreases with advancing age (Goverde et al., 2005). Hence, the occurrence of poor response should similarly increase with age. Figure 1 shows the relationship between age and POR (cycles cancelled because of absent or low ovarian response or pick-ups with ≤3 oocytes) in 3825 women entering the first cycle in the Bologna S.I.S.Me.R unit, Italy and in the IVF unit of University Hospital of Modena, Italy between January 2004 and December 2009. All patients underwent conventional controlled ovarian stimulation (COS) protocols with different FSH/HMG starting doses depending on age. As expected, the prevalence of POR increases with age, and in women over 40 years of age it is >50%. However, several of these women are still able to produce more follicles and oocytes, whereas young age does not completely protect against POR (El-Toukhy et al., 2002). Advanced age (≥40 years) can be considered the most relevant risk factor, but needs to be confirmed with other tests. From this point of view, age may be proposed as a post hoc test, allowing clinicians to classify women aged over 40 years with one previous poor IVF cycle as poor responders. In the same manner, if young women present with a POR during the first cycle, a truly diminished ovarian reserve must be confirmed using a post hoc test, an abnormal ovarian reserve test or a subsequent POR despite maximal stimulation.
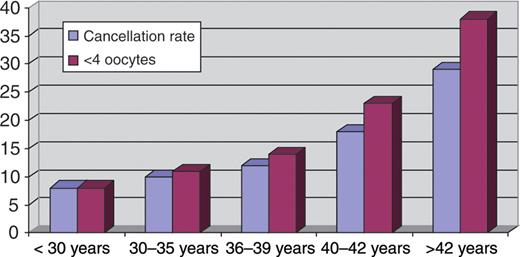
The relationship between age and POR (cycles cancelled because of absent or low ovarian response or pick-ups with ≤3 oocytes) in 3825 women entering the first cycle in the Bologna S.I.S.Me.R unit and in the Modena IVF university unit between January 2004 and December 2009. All patients underwent conventional COS protocols with different FSH/HMG starting doses depending on age. As expected, the prevalence of POR increases with female age.
A similar approach should be used for the other risk factors associated with POR, such as genetic or acquired conditions. Numerical and structural chromosomal aberrations as well as mutations or variability in specific genes in reproductive ageing may be implicated in reduced ovarian reserve. This may lead to an early menopause and to a reduced ovarian response to gonadotrophins when women undergo ovarian stimulation (De Vos et al., 2010). Typical examples may be Turner syndrome and FMR1 premutations (Gleicher et al., 2009; De Vos et al., 2010). Both conditions are clinically associated with both early menopause or primary ovarian insufficiency and reduced ovarian response to gonadotrophins. In conclusion, these conditions demonstrate the close relationship that exists between the pool of resting ovarian follicles, the response to ovarian stimulation and the duration of reproductive life-span.
Pelvic infection, as evidenced by tubal damage and positivity to Chlamydia antibody testing, is associated with poor response (Molloy et al., 1987; Keay et al., 1998). Similarly, women with ovarian endometriomas and patients who have undergone ovarian surgery for ovarian cysts are potential poor responders (Nargund et al., 1996; Garcia-Velasco and Somigliana, 2009). Chemotherapy, especially when it includes an alkylating agent, has been reported to seriously reduce the pool of resting follicles and is associated with a variable degree of risk for primary ovarian insufficiency (Oktem and Oktay, 2007; De Vos et al., 2010). Shortening of the menstrual cycle can represent another condition associated to increased risk for POR (Brodin et al., 2008).
Ovarian reserve tests
Other than age, a large number of clinical parameters might predict the poor response to stimulation with gonadotrophins and are introduced in the clinical practice. These include basal FSH, inhibin B, antral follicle count (AFC), ovarian volume, a number of dynamic tests and more recently anti-Mullerian hormone (AMH) (Navot et al., 1987; Fanchin et al., 1994; Lass et al., 1997; Tomas et al., 1997; Hall et al., 1999; Bancsi et al., 2002; Broekmans et al., 2006, La Marca et al., 2010).
The ideal ORT would accurately measure the extent of the primordial follicle pool (the true ovarian reserve) and reflect oocytes’ reproductive competence. Both quantity and quality of primordial follicles are difficult to establish because the development from primordial into antral follicles takes 6–8 months, during which the gamete's reproductive competence and follicular steroidogenic activity develops (Gougeon, 1998; McGee and Hsueh, 2000). Actually, ovarian reserve tests provide an indirect measure of the cohort of recruitable antral follicles present in the FSH window at the beginning of each menstrual cycle (Fauser and Van Heusden, 1997; McGee and Hsueh, 2000). The relationship between test results and true ovarian reserve is unknown, but is probably moderate or good for the quantitative aspect and low for the qualitative aspect.
The utility of ovarian reserve tests in predicting individual response to COS depends, above all, on the accuracy of the test itself, i.e. the possibility of predicting the outcome of interest correctly. Several reviews analysed the predictive value of single and combined tests performed in basal conditions. Of all the tests, AFC and AMH had the best sensitivity and specificity for predicting ovarian response (Broekmans et al., 2006; Broer et al., 2009; La Marca et al., 2010). However, even the best ovarian reserve marker at the best cut off values is associated with a false positive rate of 10–20% (Broekmans et al., 2006; La Marca et al., 2010). Overall, Broekmans et al. (2006) concluded that ovarian reserve tests had modest clinical utility because of their limited predictive properties, and hypothesized that ovarian response during the first IVF cycle could be used as a surrogate ovarian response test.
A large amount of research has been done to evaluate the use of various combined tests to improve the overall predictive accuracy. A meta-analysis of cohort studies demonstrated that the use of combined tests is not an improvement over single tests in predicting a poor response (Verhagen et al., 2008). This further confirms the hypothesis that most tests represent the same quantitative aspect of ovarian reserve.
In conclusion, both AMH and AFC must be considered as the most reliable and accurate markers of ovarian reserve (Broer et al., 2009; La Marca et al., 2010). Their overall performance in the prediction of poor response is acceptable, although not optimal, implying that they should be used as a post hoc test. Women with a previous poor response to maximal FSH stimulation and a subsequent abnormal result in the ovarian reserve assessment may be classified with high probability as women with reduced ovarian reserve.
Regarding the cut-off values to be used in clinical practice, a complete discussion on every marker of ovarian reserve is beyond the scope of this consensus and readers are referred to other detailed reviews (Broekmans et al., 2006; Broer et al., 2010; La Marca et al., 2010). For AMH, the best cut-off values reported are in the range from 0.5 to 1.1 ng/ml, whereas for AFC the values may range from less than 5 to less than 7 (Fig. 2). Clinicians should be aware that, because the results of an ORT may have relevant consequences for couples, extreme cut-off values are preferred since they are associated with high specificity (low falsepositive rate), even if this implies reduced sensitivity. From a practical point of view, clinicians confirm with high probability that AFC is the most widely used marker of ovarian reserve, in consequence of the almost universal presence of ultrasound equipment in the medical office.
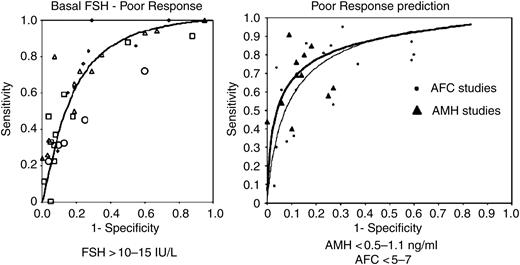
Estimated ROC curve and sensitivity–specificity points for studies reporting on the performance of basal FSH (left), AMH and AFC (right) in the prediction of a poor response. With the use of basal FSH in regularly cycling women, accuracy in the prediction of poor response is adequate only at very high threshold levels. The accuracy of AMH and AFC for predicting a poor response in regularly cycling women is considered adequate and these two markers are considered superior to inhibin B, FSH, ovarian volume and age of women in the prediction of ovarian response to stimulation. Most frequently used cut off values for FSH, AMH and AFC are reported (modified with permission from Broekmans et al., 2006; Broer et al., 2009).
Previous POR
Problems related to this criterion can be presented at two levels; the parameters used to identify a POR during treatment, and the accuracy of a previous POR to identify patients with a reduced ovarian reserve.
By definition, the term POR is related to the number of ultrasound-detectable growing follicles on ultrasound scan during gonadotrophin stimulation. However, the lack of uniformity in ovarian monitoring on the day in which the measurement should be performed and in the diameter of follicles that should be measured has brought the number of oocytes retrieved to be used as a diagnostic criterion for patients with a poor response. But to define POR, as previously reported, the number of oocytes retrieved ranges from <3 to <6. In addition, several authors also consider the peak E2 and the total amount of gonadotrophins used.
However defined, the majority of the studies identify POR after a single cycle. A single episode of POR to ovarian stimulation may be an occasional finding, and could be absent in a subsequent cycle if an increased starting dose of FSH/HMG is used or even if the same stimulation protocol is applied. Clinically, in poor responder patients, the occurrence of a poor response in a second cycle accounts only for 62.4% (Klinkert et al., 2004), implying that at least one-third of previous poor responders will have a normal response in subsequent cycles. The reason for the variability in the number of recruited follicles, and consequently in the number of retrieved oocytes, is that the cohort of recruitable follicles indeed varies from cycle to cycle. A clear demonstration of this statement arises from the recent evidence of a significant and clinically relevant inter-cycle variability of antral follicles detectable on ultrasound scan (the pool of recruitable follicles) (Van Disseldorp et al., 2010). Possible explanations for the varying cohort of recruitable follicles might be cyclic differences in follicular decay or growth rate.
Hence, this high intercycle variability in ovarian response should be taken into account when planning studies to investigate ovarian stimulation protocols in poor responders (Pantos et al., 1990).
Methodology to reach the consensus
According to what was discussed in detail in the previous paragraphs, an agreement was reached on the following issues:
The risk factors for POR are represented by maternal age ≥40 years and by all the known genetic or acquired conditions possibly linked to a reduced amount of resting follicles.
A POR is represented by a cycle cancelled (following the development of less than three growing follicles) or the collection of less than four oocytes in response to an ovarian stimulation protocol of at least 150 IU FSH per day. Parameters of oocyte maturity are not included in this definition. Although based on limited scientific evidence, the cut-off point of four oocytes is the most frequently used in the literature (Table I). The same definition was adopted by the Evian Annual Reproduction Group in 2008 (Devroey et al., 2009). For the purpose of the present paper, it is important to clearly distinguish between conventional and mild stimulations (Nargund et al., 2007). The collection of less than four eggs after a mild IVF programme is not to be considered a poor response.
Any marker may help us to predict POR. AFC and AMH have the best sensitivity and specificity, but even the best marker is associated with a 10–20% falsepositive rate. The choice of marker used may depend on the organization, setting, availability of equipment or patient-related conditions. In some cases, patients are tested for more than one marker. For the purpose of this paper (consensus on definition), a single test is considered sufficient and AFC seems to be the most used into the daily clinical practice. In the future, any other single and simple test that demonstrates a better performance could be preferred.
Each criterion (risk factor, previous cycle and ORT) used alone is insufficiently accurate to identify women with the highest probability of being a real POR, and more than one criterion should be contemporaneously present in each subject.
Results: POR definition
Following the same logical approach utilized for polycystic ovarian syndrome (PCOS) diagnostic criteria (The Rotterdam ESHRE/American Society for Reproductive Medicine (ASRM) Sponsored PCOS Consensus Workshop Group, 2004), a consensus was reached on the minimal criteria needed to define POR.
At least two of the following three features must be present: Two episodes of POR after maximal stimulation are sufficient to define a patient as poor responder in the absence of advanced maternal age or abnormal ORT.
Advanced maternal age (≥40 years) or any other risk factor for POR;
A previous POR (≤3 oocytes with a conventional stimulation protocol);
An abnormal ovarian reserve test (i.e. AFC <5–7 follicles or AMH <0.5–1.1 ng/ml).
By definition, the term POR refers to the ovarian response and, therefore, one stimulated cycle is considered essential for the diagnosis of POR. However, patients over 40 years of age with an abnormal ORT may be classified as poor responders since both advanced age and an abnormal ORT may indicate reduced ovarian reserve and act as a surrogate of ovarian stimulation cycle. In this case, the patients should be more properly defined as expected PORs.
Conclusions
Similar approaches, taking more than one test to define POR into consideration, have been previously attempted. Sun and colleagues (2008) observed that sequential testing for ovarian reserve may improve the likelihood of diagnosis of expected poor response when compared with a single test; however, the clinical performance of the testing may vary on the basis of the prevalence of the ‘disease' we want to diagnose. Indeed, they reported an exhaustive simulation in which the same test, when applied only to young women (patients with a low prevalence of poor response) or to women older than 40 (patients with a high prevalence of poor response), resulted in the positive predictive values increasing from 27 to 89%, respectively.
The definition presented in this article represents the first realistic attempt by the scientific community (ESHRE) to standardize the definition of POR in a simple and reproducible manner. If uniformly adapted as the ‘minimal' criteria needed to select patients for future trials, more homogeneous populations will be tested for any new protocols designed. Finally, by reducing bias caused by spurious POR definitions, it will be possible to compare results and to draw reliable conclusions. A standard definition will also enable a correct estimate of the incidence of POR.
Finally, the authors wish to underline that the aim of this paper is to identify PORs only for research purposes, to include homogeneous populations in future trials testing new strategies and not to exclude poor prognosis patients from IVF programmes. The latter is a different and controversial issue involving economic, ethical and psychological aspects that remain to be evaluated. Each definition adopted has no absolute value in predicting the prognosis. It is widely demonstrated that poor responders can become pregnant and have live births. In particular, young poor responders have a different prognosis from older women (Hanoch et al., 1998; Ulug et al., 2003; Kailasam et al., 2004) and screening them may be questioned.
Acknowledgements
The authors are grateful to the co-ordinators or deputies of the SIGs for their lectures and contribution in the discussion for the Consensus on POR definition at the ESHRE Campus held in Bologna, Italy, the 19–20 March 2010. In particular to: C. Bergh (Safety and Quality in ART), F. Shenfield (Ethics and Law), J.A. Garcia Velasco, (Endocrinology) N. Macklon (Special task Force on Mild Approaches in ART), C. Magli (Embryology), P. Thorn (Psychology and Couselling), R. Farquharson and C. Boomsma (Early Pregnancy), M. Gerogolet (Surgery), S. Lewis (Andrology) , A. Veiga (Stem Cells), T.C. Li (Endometrium) and S. Ziebe as SIGs Coordinator.
References
Author notes
This manuscript has been approved by the Executive Committee of ESHRE. It has not been externally peer-reviewed.
The ESHRE working group on Poor Ovarian Response Definition is endorsed by the coordinators of all the Special Interest Groups (Andrology, Early Pregnancy, Embryology, Endometriosis and Endometrium, Ethics and Law, Psychology and Counselling, Reproductive Endocrinology, Reproductive Genetics, Reproductive Surgery, Safety and Quality in ART, Stem Cell) and by the Special Task Force on Mild Approaches in ART.



