-
PDF
- Split View
-
Views
-
Cite
Cite
Evelyne Vanneste, Thierry Voet, Cindy Melotte, Sophie Debrock, Karen Sermon, Catherine Staessen, Inge Liebaers, Jean-Pierre Fryns, Thomas D'Hooghe, Joris R. Vermeesch, What next for preimplantation genetic screening? High mitotic chromosome instability rate provides the biological basis for the low success rate, Human Reproduction, Volume 24, Issue 11, November 2009, Pages 2679–2682, https://doi.org/10.1093/humrep/dep266
Close - Share Icon Share
Abstract
Preimplantation genetic screening is being scrutinized, as recent randomized clinical trials failed to observe the expected significant increase in live birth rates following fluorescence in situ hybridization (FISH)-based screening. Although these randomized clinical trials are criticized on their design, skills or premature stop, it is generally believed that well-designed and well-executed randomized clinical trials would resolve the debate about the potential benefit of preimplantation genetic screening. Since FISH can analyze only a limited number of chromosomal loci, some of the embryos transferred might be diagnosed as ‘normal’ but in fact be aneuploid for one or more chromosomes not tested. Hence, genome-wide array comparative genome hybridization screening enabling aneuploidy detection of all chromosomes was thought to be a first step toward a better design. We recently showed array screening indeed enables accurate determination of the copy number state of all chromosomes in a single cell. Surprisingly, however, this genome-wide array screening revealed a much higher frequency and complexity of chromosomal aberrations in early embryos than anticipated, with imbalances in a staggering 90% of all embryos. The mitotic error rate in cleavage stage embryos was proven to be higher than the meiotic aneuploidy rate and as a consequence, the genome of a single blastomere is not representative for the genome of the other cells of the embryo. Hence, potentially viable embryos will be discarded upon screening a single blastomere. This observation provides a biological basis for the failure of the randomized clinical trials to increase baby-take-home rates using FISH on cleavage stage embroys.
Preimplantation genetic screening (PGS), also termed preimplantation genetic diagnosis with aneuploidy screening or PGD-AS, is increasingly implemented in fertility centers worldwide (Harper et al., 2008). The decreasing fecundity of older women caused by age-dependent oocytic and embryonic aneuploidy, and the knowledge that the majority of chromosomal errors cause embryonic lethality led to the hypothesis that selection of chromosomally normal embryos for uterine transfer would increase the live birth rate and decrease the spontaneous miscarriage rate per embryo transferred (Verlinsky et al., 1995). In PGS, one or two blastomeres from a cleavage stage embryo are biopsied for genetic analysis. Routinely, 7 up to 12 chromosomal loci are scored using multi-probe fluorescence in situ hybridization (FISH). In addition to advanced maternal age, recurrent implantation failure, sporadic or recurrent miscarriages as well as factors of severe male infertility have been added to the list of indications for PGS (Harper et al., 2008). Some groups even propose performing PGS on all preimplantation in vitro fertilization (IVF) embryos (Verlinsky et al., 2005).
Despite the attractiveness of the scientific and clinical rationale for PGS and promising results from studies with matched controls (Gianaroli et al., 1999; Munne et al., 2003), recent randomized prospective clinical trials failed to observe a significantly increased live birth rate after transfer of chromosomally ‘normal’ embryos selected by FISH following PGS (Staessen et al., 2004, 2008; Mastenbroek et al., 2007; Hardarson et al., 2008; Schoolcraft et al., 2008; Twisk et al., 2006, 2008; Debrock et al., 2009). These results, at odds with the expected outcome based upon the scientific rationale for PGS, triggered a fierce debate about the usefulness of PGS in clinical practice (Goldman, 2007; Fauser, 2008; Harper et al., 2008; Mastenbroek et al., 2008; Simpson, 2008). According to proponents, FISH-based PGS improves IVF outcome on the condition that a specific set of chromosomes is screened in only one blastomere biopsied by an experienced embryologist. Consequently, PGS using full genomic screening for chromosomal imbalances is expected to further improve the outcome of PGS. According to PGS skeptics, FISH-based PGS is not efficient in selecting the best embryo and whole genome analysis of one blastomere might be superior to FISH-based PGS. A second concern is that the presence of chromosomal mosaicism might hamper its success.
We developed a novel array-based approach to reliably screen genome-wide chromosomal imbalances with a high resolution in a single cell and analyzed every blastomere from 23 good-quality cleavage stage embryos (2–5 blastomeres on day 2, 6–10 blastomeres on day 3, <20% fragmentation, equal-sized blastomeres). These embryos were derived from nine fertile young couples (female age <35 years; normal karyotype of both partners; a maternal body mass index of 18–30 kg/m2; initial normal semen parameters according to WHO regulations; no history of recurrent miscarriages) treated with ART and PGD for sex selection due to X-linked disorders and a BRCA mutation or for familial microdeletion syndromes. The mean and median number of biopsied embryos was, respectively, 8.5 and 6 per cycle (13 cycles for nine patients) of which 29% were FISH-diagnosed to be suitable for transfer (least affected sex or non-microdeletion carrier). We used all remaining blastomeres of the fresh and biopsied 3- and 4-day-old good-quality embryos carrying a microdeletion or a sex-selected against. This set-up allowed us to identify accurately the frequency of chromosomal and segmental imbalances and to analyze the meiotic versus mitotic origin of each aberration in embryos that are the best available representation of normal human embryogenesis. Stringent interpretation criteria and exclusion due to quality control (34% of the blastomeres) ensured that the number of chromosomal imbalances represents a lower estimate. Surprisingly, a staggering 21 embryos showed chromosomal mosaicism and only 2 embryos were normal diploid in all blastomeres. In the abnormal embryos, not only mosaicism for whole chromosome aneuploidies (∼83% of the embryos) and uniparental disomies (∼9% of the embryos) were detected, but also frequent terminal segmental deletions, duplications or amplifications (∼70% of the embryos), these being often recurrent or reciprocal in the sister blastomeres of an embryo (Figure 1) (Vanneste et al., 2009). Although this aneuploidy rate in embryos from normal fertile young women might seem excessively high, 48% of the embryos do contain normal diploid blastomeres as well (Vanneste et al., 2009). Moreover, when the genome-wide screening data are extrapolated to the data obtained on human embryos by screening a limited number of chromosomal loci by FISH, the degrees of aneuploidy obtained by both techniques are similar (Vanneste et al., 2009). Finally, complex chromosomal abnormalities that have been reported from the investigation of fetal tissue after spontaneous miscarriages have now been detected in human embryos as well (Vanneste et al., 2009).
These data provide the molecular basis for the failure of randomized controlled PGS trials in improving the baby-take-home rate. First, rather than being confined to groups at risk of a low pregnancy success rate following IVF/ICSI, our study shows that chromosome instability (i.e. the gain or loss of complete chromosomes or segments of chromosomes, resulting in cell-to-cell variability) is common to all human embryos (post-IVF/ICSI). Although the meiotic aneuploidy rate is known to increase with age (Wilton, 2002), this mitotic chromosome instability rate of 91% is higher than any meiotic aneuploidy rate. Therefore, there is no argument to target cleavage stage PGS treatment to specific patients with low pregnancy success rates following IVF/ICSI. Second, PGS assumes that one or two blastomeres are representative of the chromosome status of the remaining blastomeres of the embryo (Wilton, 2002). The astonishingly high degree of chromosomal mosaicism in cleavage stage embryos undermines this assumption. Third, the high frequency of potentially detrimental segmental chromosomal aberrations (∼70% of the embryos) shows that locus-specific FISH screening can only detect a minor fraction of all chromosomal imbalances. Consequently, all studies that use FISH screening have a serious bias toward diagnosing genetically ‘normal’ embryos for transfer. Genome-wide screening would increase the accuracy of the genetic diagnosis but would significantly reduce the number of embryos suitable for transfer. Finally, the <10% fully normal diploid cleavage stage embryos is less than the pregnancy success rate following IVF/ICSI with PGD in our as well as other centers (ESHRE PGD consortium Goossens et al., 2008; Vanneste et al., 2009). This adds another argument to the concept that at least some uterine-transferred mosaic diploid/aneuploid embryos can survive to term in a healthy state, suggesting that chromosomally normal blastomeres grow preferentially to abnormal cells during early embryogenesis before, during and after embryo implantation. Determining the genetic content of human embryos based on the analysis of one or two cells before self-correction and/or self-selection has occurred, most often at day 3 after fertilization, will thus inevitably result in discarding potentially viable embryos.
In conclusion, the prevalent chromosome instability in all early human cleavage stage embryos (post-IVF/ICSI) provides a biological basis for the failure of cleavage stage PGS in improving the live birth rate per embryo transferred. Although, in general, the field assumes that genome-wide cleavage stage aneuploidy screening is the way ahead, our new data demonstrate that other strategies will be required. Understanding the mechanisms underlying this chromosome instability in embryos, which is reminiscent of the chromosome instability observed during tumorigenesis, will be a first step toward this aim.
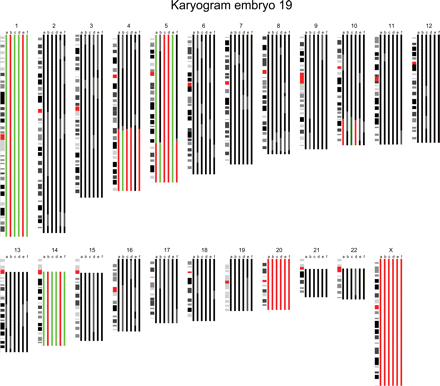
The karyogram of embryo 19 is shown. The karyogram is composed of color bars that each represent the chromosome copy number state based on the results of the BAC (bacterial artificial chromosomes) array and the SNP (single nucleotide polymorphism) copy number and genotyping data in a specific blastomere. Black represents a normal region, red a hemizygous deletion, green a duplication, dark green an amplification and gray discordance between the analyses or unreliable aberrations. In this male embryo, whole chromosome imbalances of mitotic origin were detected in chromosomes 1 and 14, whereas chromosome 20 showed a monosomy in all sister blastomeres suggesting a meiotic non-disjunction. Moreover, 4q and 10q terminal deletions with a reciprocal 4q duplication and 10q amplification respectively were detected in a proportion of its sister blastomeres. Finally, a 5q terminal deletion and reciprocal 5q duplication were detected in two blastomeres, whereas the remaining part of the chromosome proximal to the 5q deletion was trisomic. Two sister blastomeres contained a monosomy for chromosome 5. In addition, a remaining sister blastomere contained three copies of chromosome 5, whereas a fifth lacked a 5q terminal part of which the size was equal to the partial deletion and duplication in its sister blastomeres.
References
Author notes
These authors contributed equally to this work.



