-
PDF
- Split View
-
Views
-
Cite
Cite
M F Costello, M L Misso, A Balen, J Boyle, L Devoto, R M Garad, R Hart, L Johnson, C Jordan, R S Legro, R J Norman, E Mocanu, J Qiao, R J Rodgers, L Rombauts, E C Tassone, S Thangaratinam, E Vanky, H J Teede, International PCOS Network, Evidence summaries and recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome: assessment and treatment of infertility, Human Reproduction Open, Volume 2019, Issue 1, 2019, hoy021, https://doi.org/10.1093/hropen/hoy021
Close - Share Icon Share
Abstract
What is the recommended assessment and management of infertile women with polycystic ovary syndrome (PCOS), based on the best available evidence, clinical expertize and consumer preference?
International evidence-based guidelines, including 44 recommendations and practice points, addressed prioritized questions to promote consistent, evidence-based care and improve the experience and health outcomes of infertile women with PCOS.
Previous guidelines on PCOS lacked rigorous evidence-based processes, failed to engage consumer and multidisciplinary perspectives or were outdated. The assessment and management of infertile women with PCOS are inconsistent. The needs of women with PCOS are not being adequately met and evidence practice gaps persist.
Governance included a six continent international advisory and a project board, a multidisciplinary international guideline development group (GDG), consumer and translation committees. Extensive health professional and consumer engagement informed the guideline scope and priorities. The engaged international society-nominated panel included endocrinology, gynaecology, reproductive endocrinology, obstetrics, public health and other experts, alongside consumers, project management, evidence synthesis and translation experts. Thirty-seven societies and organizations covering 71 countries engaged in the process. Extensive online communication and two face-to-face meetings over 15 months addressed 19 prioritized clinical questions involving nine evidence-based reviews and 10 narrative reviews. Evidence-based recommendations (EBRs) were formulated prior to consensus voting within the guideline panel.
International evidence-based guideline development engaged professional societies and consumer organizations with multidisciplinary experts and women with PCOS directly involved at all stages. A (AGREE) II-compliant processes were followed, with extensive evidence synthesis. The Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) framework was applied across evidence quality, desirable and undesirable consequences, feasibility, acceptability, cost, implementation and ultimately recommendation strength. The guideline was peer-reviewed by special interest groups across our partner and collaborating societies and consumer organizations, was independently assessed against AGREE II criteria and underwent methodological review. This guideline was approved by all members of the GDG and has been approved by the NHMRC.
The quality of evidence (QOE) for the EBRs in the assessment and management of infertility in PCOS included very low (n = 1), low (n = 9) and moderate (n = 4) quality with no EBRs based on high-quality evidence. The guideline provides 14 EBRs, 10 clinical consensus recommendations (CCRs) and 20 clinical practice points on the assessment and management of infertility in PCOS. Key changes in this guideline include emphasizing evidence-based fertility therapy, including cheaper and safer fertility management.
Overall evidence is generally of low to moderate quality, requiring significantly greater research in this neglected, yet common condition. Regional health systems vary and a process for adaptation of this guideline is provided.
The international guideline for the assessment and management of infertility in PCOS provides clinicians with clear advice on best practice based on the best available evidence, expert multidisciplinary input and consumer preferences. Research recommendations have been generated and a comprehensive multifaceted dissemination and translation program supports the guideline with an integrated evaluation program.
The guideline was primarily funded by the Australian National Health and Medical Research Council of Australia (NHMRC) supported by a partnership with ESHRE and the American Society for Reproductive Medicine (ASRM). GDG members did not receive payment. Travel expenses were covered by the sponsoring organizations. Disclosures of conflicts of interest were declared at the outset and updated throughout the guideline process, aligned with NHMRC guideline processes. Dr Costello has declared shares in Virtus Health and past sponsorship from Merck Serono for conference presentations. Prof. Norman has declared a minor shareholder interest in the IVF unit Fertility SA, travel support from Merck and grants from Ferring. Prof. Norman also has scientific advisory board duties for Ferring. The remaining authors have no conflicts of interest to declare.
This article was not externally peer-reviewed by Human Reproduction Open.
Introduction
Polycystic ovary syndrome (PCOS) is the most common endocrinopathy affecting reproductive aged women, with a prevalence of between 8% and 13% depending on the population studied and definitions used. PCOS is complex with reproductive, metabolic and psychological features (Teede et al., 2010). Infertility is a prevalent presenting feature of PCOS with ~75% of these women suffering infertility due to anovulation, making PCOS by far the most common cause of anovulatory infertility (Homburg, 2004).
This guideline aims to optimize evidence-based, consistent care that meets the needs and improves the quality of life of infertile women with polycystic ovary syndrome (PCOS). The guideline and translation program were developed with full consumer participation at all stages, targeting areas and outcomes of priority for women with PCOS. The overall aim is to support women and their healthcare providers to optimize the assessment and management of infertility in PCOS. There is an emphasis on partnership in care and empowerment of women with PCOS. Personal characteristics, preferences, culture and values are considered. With effective translation, the guideline and suite of health professional and consumer resources will address the gaps and priorities identified by women with PCOS.
The treatment of infertility in PCOS includes lifestyle changes (diet and exercise), pharmacological therapies (oral agents such as clomiphene citrate, letrozole or metformin or injectable agents such as gonadotrophins), surgical therapy (laparoscopic ovarian surgery) or IVF (Costello et al., 2012). IVM has been proposed to offer a promising alternative to conventional IVF (Chian, 2004).
Current guidelines on PCOS either are limited in breadth, do not follow rigorous best practice in development, have not involved consumers or are outdated (Teede et al., 2011; Legro et al., 2013; Conway et al., 2014; Goodman et al., 2015; Balen et al., 2016) resulting in inconsistent guidance for clinicians and women alike. To address these identified gaps, the first ever international evidence-based guideline for the assessment and management of PCOS was recently published and it integrates the best available evidence with international, multidisciplinary clinical expertize and consumer preferences in order to provide health professionals, consumers and policy makers with guidance (International Evidence-Based Guidelines for the Assessment and Management of Polycystic Ovary Syndrome, 2018; Teede et al., 2018a,b,c). This current paper is restricted to the section of the guideline addressing the assessment and management of infertility in PCOS. The clinical context and evidence informing the infertility section of the guideline are published in the full guideline (International Evidence-Based Guidelines for the Assessment and Management of Polycystic Ovary Syndrome, 2018).
Materials and Methods
Best practice evidence-based guideline development methodology was applied and are detailed in the full guideline (International Evidence-Based Guidelines for the Assessment and Management of Polycystic Ovary Syndrome, 2018). In short, the international evidence-based guideline development engaged health professional societies and consumer organizations, with multidisciplinary experts and women with PCOS directly involved at all stages.
A six continent international advisory and a project board, a multidisciplinary international guideline development group (GDG), plus consumer and translation committees provided governance. The engaged international society-nominated panel provided experts in endocrinology, gynaecology, reproductive endocrinology, obstetrics and public health, alongside consumers, project management, evidence synthesis and translation experts. Thirty-seven societies and organizations representing 71 countries were engaged in a 15-month process that addressed 19 prioritized clinical questions encompassing 9 evidence-based reviews and 10 narrative reviews. Appraisal of Guidelines for Research and Evaluation (AGREE) II-compliant processes were followed, with extensive evidence synthesis. The Grading of Recommendations, Assessment, Development and Evaluation (GRADE) framework was applied across evidence quality, desirable and undesirable consequences, feasibility, acceptability, cost, implementation and, ultimately, recommendation strength. Evidence-based recommendations (EBRs) were formulated prior to consensus voting within the guideline panel.
Interpreting the recommendations
The category of the recommendations includes evidence-based or consensus recommendations and has accompanying relevant clinical practice points (CPP), as described in Table I. When sufficient evidence was available in PCOS, an EBR was made, where there was insufficient evidence in PCOS, evidence in general or other relevant populations was also considered and if appropriate and there was consensus, the GDG made clinical consensus recommendations (CCRs). CPP highlighted important clinical and implementation issues arising from GDG consideration of evidence-based or CCR and from peer review.
| EBR | Evidence-based recommendations: evidence sufficient to inform a recommendation made by GDG. |
| CCR | Clinical consensus recommendations: in the absence of evidence, a clinical consensus recommendation has been made by the GDG. |
| CPP | Clinical practice points: evidence not sought. A practice point has been made by the GDG where important issues arose from discussion of evidence-based or clinical consensus recommendations. |
| EBR | Evidence-based recommendations: evidence sufficient to inform a recommendation made by GDG. |
| CCR | Clinical consensus recommendations: in the absence of evidence, a clinical consensus recommendation has been made by the GDG. |
| CPP | Clinical practice points: evidence not sought. A practice point has been made by the GDG where important issues arose from discussion of evidence-based or clinical consensus recommendations. |
GDG: the guideline development group (Teede et al., 2018a,b,c).
| EBR | Evidence-based recommendations: evidence sufficient to inform a recommendation made by GDG. |
| CCR | Clinical consensus recommendations: in the absence of evidence, a clinical consensus recommendation has been made by the GDG. |
| CPP | Clinical practice points: evidence not sought. A practice point has been made by the GDG where important issues arose from discussion of evidence-based or clinical consensus recommendations. |
| EBR | Evidence-based recommendations: evidence sufficient to inform a recommendation made by GDG. |
| CCR | Clinical consensus recommendations: in the absence of evidence, a clinical consensus recommendation has been made by the GDG. |
| CPP | Clinical practice points: evidence not sought. A practice point has been made by the GDG where important issues arose from discussion of evidence-based or clinical consensus recommendations. |
GDG: the guideline development group (Teede et al., 2018a,b,c).
The recommendation terms include ‘should’, ‘could’ and ‘should not’. These terms are informed by the nature of the recommendation (evidence or consensus), the GRADE framework and evidence quality and are independent descriptors reflecting the judgement of the multidisciplinary GDG including consumers. They refer to overall interpretation and practical application of the recommendation, balancing benefits and harms. ‘Should’ is used where benefits of the recommendation exceed harms, and where the recommendation can be trusted to guide practice. ‘Could’ is used where either the QOE was limited or the available studies demonstrate little clear advantage of one approach over another, or the balance of benefits to harm was unclear. ‘Should not’ is used where there is either a lack of appropriate evidence, or the harms may outweigh the benefits.
The GRADE of the recommendation is determined by the GDG from structured consideration of the GRADE framework (National Health and Medical Research Council, 2009) including desirable effects, undesirable effects, balance of effects, resource requirements and cost effectiveness, equity, acceptability and feasibility, and includes: *Conditional recommendation against the option; **Conditional recommendation for either the option or the comparison; ***Conditional recommendation for the option; **** Strong recommendation for the option.
QOE is categorized (see Table II) according to:
- information about the number and design of studies addressing the outcome;
- judgments about the quality of the studies and/or synthesized evidence, such as risk of bias, inconsistency, indirectness, imprecision and any other considerations that may influence the QOE: key statistical data;
- and classification of the importance of the outcomes.
Quality (certainty) of evidence categories adapted from the grading of recommendations, assessment, development and evaluation frameworka.
| High | ⊕⊕⊕⊕ | Very confident that the true effect lies close to that of the estimate of the effect |
| Moderate | ⊕⊕⊕o | Moderate confidence in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different |
| Low | ⊕⊕oo | Limited confidence in the effect estimate: the true effect may be substantially different from the estimate of the effect |
| Very Low | ⊕ooo | Very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect |
| High | ⊕⊕⊕⊕ | Very confident that the true effect lies close to that of the estimate of the effect |
| Moderate | ⊕⊕⊕o | Moderate confidence in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different |
| Low | ⊕⊕oo | Limited confidence in the effect estimate: the true effect may be substantially different from the estimate of the effect |
| Very Low | ⊕ooo | Very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect |
aAdapted from the grading of recommendations, assessment, development, and evaluation framework (GRADE) (National Health and Medical Research Council 2009)
Quality (certainty) of evidence categories adapted from the grading of recommendations, assessment, development and evaluation frameworka.
| High | ⊕⊕⊕⊕ | Very confident that the true effect lies close to that of the estimate of the effect |
| Moderate | ⊕⊕⊕o | Moderate confidence in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different |
| Low | ⊕⊕oo | Limited confidence in the effect estimate: the true effect may be substantially different from the estimate of the effect |
| Very Low | ⊕ooo | Very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect |
| High | ⊕⊕⊕⊕ | Very confident that the true effect lies close to that of the estimate of the effect |
| Moderate | ⊕⊕⊕o | Moderate confidence in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different |
| Low | ⊕⊕oo | Limited confidence in the effect estimate: the true effect may be substantially different from the estimate of the effect |
| Very Low | ⊕ooo | Very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect |
aAdapted from the grading of recommendations, assessment, development, and evaluation framework (GRADE) (National Health and Medical Research Council 2009)
The QOE reflects the extent to which our confidence in an estimate of the effect is adequate to support a particular recommendation (National Health and Medical Research Council, 2009) and was largely determined by the expert evidence synthesis team.
GRADE note that QOE is a continuum; any discrete categorization involves a degree of arbitrariness. Nevertheless, advantages of simplicity, transparency, and vividness outweigh these limitations (National Health and Medical Research Council, 2009). CCR are not rated in QOE (as no evidence was found) (see below) and CCP do not have a ‘GRADE’ rating as CPPs arose from discussion of evidence-based or CCR (Table I).
The meaning or interpretation of the GRADE of recommendations according to strength (strong or conditional [or weak]) can be seen in Table III.
Interpretation of the strength of the GRADE of recommendations according to consumers, health professionals and policy makers.
| Target group . | Strong recommendationsa . | Conditional (weak) recommendations for the option (test or treatment) . | Conditional (weak) recommendation for either the option or the comparison . | CPPb . |
|---|---|---|---|---|
| Consumers | Most people in your situation would want the recommended course of action and only a small proportion would not. | The majority of people in your situation would want the recommended course of action, but some would not. | There is considerable lack of clarity over whether the majority of people in your situation would want the recommended course of action or not. | Clinicians, patients and policy makers are informed on the clinical implications relevant to implementation of recommendations. |
| Health Professionals | Most patients should receive the recommended course of action. | Recognize that different choices will be appropriate for different patients and that greater effort is needed with individuals to arrive at management decisions consistent with values and preferences. Decision aids and shared decision making are important here. | ||
| Policy makers | The recommendation can be adopted as policy in most situations. | Policy making needs to consider perspectives and involvement of diverse stakeholders. | Policy decisions remain unclear. |
| Target group . | Strong recommendationsa . | Conditional (weak) recommendations for the option (test or treatment) . | Conditional (weak) recommendation for either the option or the comparison . | CPPb . |
|---|---|---|---|---|
| Consumers | Most people in your situation would want the recommended course of action and only a small proportion would not. | The majority of people in your situation would want the recommended course of action, but some would not. | There is considerable lack of clarity over whether the majority of people in your situation would want the recommended course of action or not. | Clinicians, patients and policy makers are informed on the clinical implications relevant to implementation of recommendations. |
| Health Professionals | Most patients should receive the recommended course of action. | Recognize that different choices will be appropriate for different patients and that greater effort is needed with individuals to arrive at management decisions consistent with values and preferences. Decision aids and shared decision making are important here. | ||
| Policy makers | The recommendation can be adopted as policy in most situations. | Policy making needs to consider perspectives and involvement of diverse stakeholders. | Policy decisions remain unclear. |
aStrong recommendations based on high-quality evidence will apply to most patients for whom these recommendations are made, but they may not apply to all patients in all conditions; no recommendation can take into account all of the often-compelling unique features of individual patients and clinical circumstances.
bA CPP is developed by the GDG to support recommendations. Advice can be provided to enhance shared decision making, and on factors to be considered in implementing a specific test or intervention.
Interpretation of the strength of the GRADE of recommendations according to consumers, health professionals and policy makers.
| Target group . | Strong recommendationsa . | Conditional (weak) recommendations for the option (test or treatment) . | Conditional (weak) recommendation for either the option or the comparison . | CPPb . |
|---|---|---|---|---|
| Consumers | Most people in your situation would want the recommended course of action and only a small proportion would not. | The majority of people in your situation would want the recommended course of action, but some would not. | There is considerable lack of clarity over whether the majority of people in your situation would want the recommended course of action or not. | Clinicians, patients and policy makers are informed on the clinical implications relevant to implementation of recommendations. |
| Health Professionals | Most patients should receive the recommended course of action. | Recognize that different choices will be appropriate for different patients and that greater effort is needed with individuals to arrive at management decisions consistent with values and preferences. Decision aids and shared decision making are important here. | ||
| Policy makers | The recommendation can be adopted as policy in most situations. | Policy making needs to consider perspectives and involvement of diverse stakeholders. | Policy decisions remain unclear. |
| Target group . | Strong recommendationsa . | Conditional (weak) recommendations for the option (test or treatment) . | Conditional (weak) recommendation for either the option or the comparison . | CPPb . |
|---|---|---|---|---|
| Consumers | Most people in your situation would want the recommended course of action and only a small proportion would not. | The majority of people in your situation would want the recommended course of action, but some would not. | There is considerable lack of clarity over whether the majority of people in your situation would want the recommended course of action or not. | Clinicians, patients and policy makers are informed on the clinical implications relevant to implementation of recommendations. |
| Health Professionals | Most patients should receive the recommended course of action. | Recognize that different choices will be appropriate for different patients and that greater effort is needed with individuals to arrive at management decisions consistent with values and preferences. Decision aids and shared decision making are important here. | ||
| Policy makers | The recommendation can be adopted as policy in most situations. | Policy making needs to consider perspectives and involvement of diverse stakeholders. | Policy decisions remain unclear. |
aStrong recommendations based on high-quality evidence will apply to most patients for whom these recommendations are made, but they may not apply to all patients in all conditions; no recommendation can take into account all of the often-compelling unique features of individual patients and clinical circumstances.
bA CPP is developed by the GDG to support recommendations. Advice can be provided to enhance shared decision making, and on factors to be considered in implementing a specific test or intervention.
Results
The guideline section addressing the assessment and management of infertile women with PCOS includes 14 EBRs, 10 CCR and 20 CPP. The QOE for the EBRs included very low (n = 1), low (n = 9), and moderate (n = 4) quality, with no EBRs based on high-quality evidence. The GRADE of recommendations included the following: *Conditional recommendation against the option (n = 2, all CCRs); **Conditional recommendation for either the option or the comparison (n = 1, a CCR); ***Conditional recommendation for the option (n = 15, 11 EBRs and four CCRs); and **** Strong recommendation for the option (n = 3, all EBRs).
The recommendations for the assessment and treatment of infertility can be found in the full guideline (International Evidence-Based Guidelines for the Assessment and Management of Polycystic Ovary Syndrome, 2018). The comprehensive evidence reviews, profiles and GRADE frameworks supporting each recommendation, can be found in the supplementary Technical report (2018) at https://www.monash.edu/__data/assets/pdf_file/0020/1412282/PCOS-Guideline_Technical-report.pdf. A summary of the recommendations for the assessment and treatment of infertility can be seen in Fig. 1
Practitioner support tool Algorithm 1: Screening, diagnostic assessment risk assessment and lifestage. ©International Evidence-based Guideline for the Assessment and Management of Polycystic Ovary Syndrome 2018, Helena Teede et al. Monash University (monash.edu/medicine/sphpm/mchri/pcos), 2018, by permission of Monash University, on behalf of the NHMRC Centre for Research Excellence in PCOS. This image/content is not covered by the terms of the Creative Commons licence of this publication. For permission to reuse, please contact the rights holder.
Tubal patency testing
Clinical question
Should women with PCOS and infertility due to anovulation alone with normal semen analysis have tubal patency testing prior to starting ovulation induction with timed intercourse or IUI treatment or delayed tubal patency testing?
Clinical need for the question
One of the leading causes of female infertility is tubal pathology, potentially affecting around 30% of infertile women (Al Subhi et al., 2013). The diagnostic assessment of infertility often includes tubal testing by hysterosalpingography or laparoscopy as outlined in the World Health Organization (WHO) evidence report on infertility management in PCOS. PCOS is the most frequent cause of anovulation in infertile women and ovulation induction is the most common treatment, however, there is little information about the prevalence of tubal pathology or the need for IUI with normal semen analysis in infertile women with PCOS.
Summary of narrative evidence
A systematic review was not conducted to answer this question and this was reviewed narratively based on clinical expertize. There is no evidence to support that hydrosalpinges or other Fallopian tube disorders are more frequent in PCOS women (Broeze et al., 2011). Yet the assessment of tubal patency is considered in infertility workup, as outlined in the WHO evidence report on infertility treatment in PCOS. Whilst adverse effects from this intervention are not common, false positives have been described and tubal patency testing may be more appropriate when targeted to those at increased risk of tubal infertility (Schankath et al., 2012). In this context, consideration of risks for tubal pathology are clinically appropriate including: previous abdominal or pelvic sepsis, previous pelvic and/or abdominal surgery, cases of recurrent acute pelvic pain (Luttjeboer et al., 2009), history of sexual transmitted diseases or pelvic inflammatory disease, or endometriosis.
Justification
If the patient has a clinical history of factors associated with tubal infertility, it was deemed that hysterosalpingography could be considered, consistent with routine assessment of infertility. Hysterosalpingography requires dilation of the cervix that generally produces some discomfort, false positives are described and other related complications are uncommon. A lack of evidence to guide practice was noted in PCOS when considering these recommendations, however general population approaches were judged as applicable in this population, where other risk factors are present.
Ovulation induction principles
In reviewing the literature on pharmacological treatment for ovulation induction, general principles emerged that apply across all recommendations. These have been extracted into a set of CPP to inform women and guide health professionals when considering or recommending pharmacological therapy for ovulation induction in PCOS. These practice points apply to all pharmacological treatments prioritized and addressed in the guidelines. In addition, duration of ovulation induction was considered under general principles.
Letrozole
Clinical question
In women with PCOS, are aromatase inhibitors (AI) effective for improving fertility outcomes?
Clinical need for the question
AI are effective as ovulation-inducing agents, including letrozole and anastrozole, with letrozole being the most widely used (Mitwally and Casper, 2001; Elizur and Tulandi, 2008). These agents prevent the aromatase-induced conversion of androgens to oestrogens, including in the ovary. Yet their mechanisms of ovulation induction are unknown, however they increase the secretion of FSH thereby stimulating ovarian follicle development and maturation (Adashi, 1984). The efficacy, adverse effects and overall role of letrozole in oral ovulation induction have remained controversial.
Summary of systematic review evidence
AI versus placebo
One small RCT (Kamath et al., 2010) with a low risk of bias compared letrozole to placebo in women with clomiphene citrate-resistant PCOS and found that letrozole was better than placebo for ovulation rate per patient (Letrozole: 6 patients/18 patients (33.33%), Placebo: 0 patients/18 patients (0%), P = 0.006) but there was no difference between letrozole and placebo for pregnancy rate per patient or live birth rate per patient. It is important to note that the findings from this study are of low certainty due to serious risk of imprecision. This study was included in a meta-analysis by Franik et al. (2014) and Misso et al.(2012), however since there is only one study, the meta-analyses do not provide additional evidence.
AI versus clomiphene citrate
Thirteen RCTs compared letrozole with clomiphene citrate. Seven of these RCTs had a high risk of bias (Atay et al., 2006; Zeinalzadeh et al., 2010; Sheikh-El-Arab Elsedeek and Elmaghraby, 2011; Banerjee Ray et al., 2012; Kar, 2012; Nazik and Kumtepe, 2012; Selim and Borg, 2012), two had a moderate risk of bias (Begum et al., 2009; Dehbashi et al., 2009) and four had a low risk of bias (Bayar et al., 2006; Badawy et al., 2009; Roy et al., 2012; Legro et al., 2014a,b). Upon meta-analysis, we found that letrozole was better than clomiphene citrate for ovulation rate per patient, pregnancy rate per patient and live birth rate per patient. There was no difference between letrozole and clomiphene citrate for multiple pregnancy rate per patient and miscarriage rate per patient. When subgroup analysis was conducted for studies that included women with PCOS who were therapy naïve, there was no difference between the two interventions for any outcome although we note that for pregnancy rate per patient the odds ratio (OR) 1.68 [95% CI 0.96, 2.94] had an I2 of 0% and a P value of 0.07.
AI versus clomiphene citrate + metformin
One RCT with moderate risk of bias found that there is no statistical difference between letrozole and clomiphene citrate plus metformin for ovulation rate per cycle, pregnancy rate per cycle, miscarriage rate per pregnancy and multiple pregnancy rate per pregnancy in clomiphene citrate-resistant women with PCOS (Abu Hashim et al., 2010b). This study was included in a meta-analysis by Franik et al. (2014) and Misso et al. (2012), however since there is only one study, the meta-analysis does not provide additional evidence.
AI versus laparoscopic ovarian surgery
Three RCTs with low risk of bias (Abu Hashim et al., 2010a, Abdellah, 2011; Ibrahim et al., 2017) compared letrozole to laparoscopic ovarian surgery and found that there was insufficient evidence of a difference between letrozole and laparoscopic ovarian surgery. One of the RCTs in 147 women with clomiphene citrate resistance found that letrozole was better than laparoscopic ovarian surgery for ovulation rate per cycle (Abdellah, 2011), however the evidence is of low certainty. The systematic review by Farquhar et al. (2012) combined these studies in meta-analysis for pregnancy rate per patient, multiple pregnancy rate per pregnancy and miscarriage rate per pregnancy, and there was no statistical difference between the two interventions.
Summary of narrative review evidence
Aromatase catalyses the conversion of androgens to oestrogens, including in the ovary, and increase FSH secretion (Adashi, 1984), stimulating ovarian follicle development and maturation. AIs prevent this conversion. These agents were originally used to improve pregnancy rates and limit adverse effects (Casper, 2003; Healey et al., 2003), especially with clomiphene resistance and failure (Imani et al., 1999; Imani et al., 2002 January, Casper, 2003; Legro et al., 2007). Letrozole has side-effects include gastrointestinal disturbances, hot flushes, headache and back pain (Holzer et al., 2006; Legro et al., 2014a,b) and concerns have been raised in an abstract on potential teratogenic effects (Biljan et al., 2005), which are as yet unconfirmed in peer-reviewed publications, yet this has sparked a series of warnings to avoid use of AI in infertility. Multiple subsequent case series (Tulandi et al., 2006; Forman et al., 2007; Dehbashi et al., 2009; Sharma et al., 2014; Tatsumi et al., 2017), multi-centre RCTs (Legro et al., 2014a,b; Diamond et al., 2015) and a recent systematic review and meta-analysis (Wang et al., 2017), all failed to note an increased congenital anomaly rate, with prevalence of anomalies with letrozole or clomiphene under 5% (the expected anomaly rate in this population is 5–8%) (Davies et al., 2012). Whilst use of letrozole is evidence-based, patient explanation and consent is appropriate as letrozole therapy for infertility is off label.
Justification
Women with PCOS are significantly more likely both to ovulate and to have a live birth after use of letrozole compared to clomiphene, the previous first line agent. The likelihood of live birth is increased 40–60% with letrozole compared to clomiphene. Similarly, failure to ovulate (letrozole resistance) is lower with letrozole versus clomiphene. Multiple pregnancy rates appear lower with letrozole than clomiphene. Hot flushes, generally the least desired side effect of any anti-oestrogen, are less common with letrozole than clomiphene, but still present, while fatigue and dizziness are more common with letrozole. The balance of benefits in terms of improved live births with letrozole and less hot flushes was considered to currently outweigh the adverse effects of relatively increased fatigue and dizziness, multiple pregnancy, and unconfirmed concerns about higher congenital anomalies.
Clomiphene citrate and/or metformin
Clinical questions
In women with PCOS, is clomiphene citrate effective for improving fertility outcomes?
In women with PCOS, is metformin effective for improving fertility outcomes?
In women with PCOS and a BMI > 30–32 kg/m2, what is the effectiveness of metformin compared to clomiphene citrate for improving fertility outcomes?
Clinical need for the questions
Clomiphene citrate is a selective oestrogen receptor modulator with both oestrogenic and anti-oestrogenic properties (Shelly et al., 2008). It was first approved for use in women with anovulation in 1967 (Pritts, 2010) and acts as an anti-oestrogen (Adashi, 1984). Clomiphene citrate resistance and failure is well documented (Palomba et al., 2009a) and a discrepancy is noted between good ovulation rates and lower pregnancy rates due to the anti-oestrogenic effects of clomiphene citrate on the endometrium and cervical mucus. Rates of twin pregnancy and triplets with clomiphene citrate are 5–7% and 0.3%, respectively, and ovarian hyperstimulation syndrome (OHSS) is less than 1% (Kafy and Tulandi, 2007). The potential for borderline increased risk of ovarian tumours with 12 cycles or more has been noted (Rossing et al., 1994).
Insulin resistance is common in PCOS (Dunaif et al., 1989; DeUgarte et al., 2005), driving ovarian androgen biosynthesis and increased bioavailability of free androgens. Excess local ovarian androgen production augmented by hyperinsulinaemia causes premature follicular atresia and anovulation (Costello and Eden, 2003). This has led to insulin-sensitizing drugs use in ovulation induction. Metformin has been most widely studied in PCOS and has the most reassuring safety profile (Palomba et al., 2009b). Efficacy has been controversial and therapeutic regimens are not well standardized in clinical practice, with variable doses in use (Hoeger et al., 2008).
Summary of systematic review evidence
Metformin versus placebo
One systematic review (Morley et al., 2017) with up to 14 studies and one RCT (Kjotrod et al., 2011) were identified to address this comparison. Metformin was better than placebo for live birth rate per participant, pregnancy rate per participant and ovulation rate per participant. Pregnancy rate and ovulation rate remained statistically significantly better than placebo when subgrouped by BMI (BMI < 30 kg/m2 and BMI > 30 or 32 kg/m2 subgroups); however live birth rate lost statistical significance when subgrouped by BMI. There was no statistically significant difference between metformin and placebo for miscarriage rate per pregnancy (including when subgrouped). Gastrointestinal upsets were statistically significantly lower with placebo than metformin (including when subgrouped). Multiple pregnancy and OHSS were not reported in the systematic review. It is important to note that the findings for live birth rate and miscarriage rate are of low certainty due to serious risk of bias and serious risk of imprecision in the body of evidence; and findings for pregnancy rate, ovulation rate and adverse events are of moderate certainty due to serious risk of bias. Risk of bias appraisals and GRADE assessments have been adopted from previous versions of this guideline (Balen et al., 2016).
In an RCT of 149 participants, with moderate certainty, there were no statistically significant differences between metformin and placebo for pregnancy rate per participant, multiple pregnancy rate per pregnancy or miscarriage rate per pregnancy. The majority of the trials stopped metformin at diagnosis of pregnancy or at week 12. Note: insufficient evidence of a differential effect of metformin on BMI.
Clomiphene citrate versus placebo
One high-quality systematic review with low risk of bias found that clomiphene citrate was better than placebo for pregnancy rate per participant and ovulation rate per participant, however the evidence was of very low certainty due to very serious risk of bias and imprecision (Brown and Farquhar, 2016).
Metformin versus clomiphene citrate
One systematic review (Morley et al., 2017) with up to seven studies was identified to address this comparison. There were no statistically significant differences between metformin and clomiphene for live birth rate per pregnancy, multiple pregnancy per pregnancy, miscarriage rate per pregnancy, pregnancy rate or ovulation rate. When subgrouped by BMI, clomiphene citrate was better than metformin for live birth rate, pregnancy rate and ovulation rate in BMI > 30 kg/m2; and metformin was better than clomiphene citrate for pregnancy rate in BMI < 30 kg/m2. Adverse events and OHSS were not reported in the systematic review. It is important to note that the findings for live birth rate, multiple pregnancy rate and pregnancy rate are of very low certainty due to very serious risk of bias, serious risk of imprecision and for live birth rate, also serious risk of inconsistency; findings for miscarriage rate and ovulation rate are of low certainty due to serious risk of bias and serious risk of imprecision in the body of evidence.
Metformin versus metformin + clomiphene citrate
One high-quality systematic review with low risk of bias evaluating two RCTs with a mean BMI ≥ 30 kg/m2 (Palomba et al., 2009c) and two RCTs [one medium quality RCT with moderate risk of bias (Johnson et al., 2010) and one low-quality RCT with high risk of bias (Karimzadeh and Javedani, 2010)] were identified by the search. Metformin plus clomiphene citrate was better than metformin alone for ovulation rate, pregnancy rate and live birth rate. There was no statistically significant difference between metformin plus clomiphene citrate and metformin alone for miscarriage rate or adverse events.
Clomiphene citrate versus metformin + clomiphene citrate
One systematic review (Morley et al., 2017) with up to 21 studies, and one RCT (Maged et al., 2015) were identified to address this comparison. Metformin plus clomiphene citrate was statistically significantly better than clomiphene citrate alone for pregnancy rate per participant and ovulation rate per participant, including when subgrouped by BMI (BMI < 30 kg/m2 and BMI > 30 kg/m2 subgroups). Adverse events were statistically significantly fewer with clomiphene citrate alone than with metformin plus clomiphene citrate. There was no statistically significant difference between metformin plus clomiphene citrate and clomiphene citrate alone for live birth rate per pregnancy, multiple pregnancy rate per pregnancy or miscarriage rate per pregnancy. OHSS was not reported in the systematic review. It is important to note that the findings for live birth rate, multiple pregnancy and miscarriage rate are of low certainty due to serious risk of bias and serious risk of imprecision in the body of evidence; and findings for pregnancy rate, ovulation rate and adverse events are of moderate certainty due to serious risk of bias. The additional RCT (Maged et al., 2015) was insufficient evidence to supplement the findings of Morley et al. (2017).
Clomiphene citrate versus AI (letrozole)
Thirteen RCTs (level II) compared letrozole with clomiphene citrate. Seven of these RCTs had a high risk of bias (Atay et al., 2006; Zeinalzadeh et al., 2010; Sheikh-El-Arab Elsedeek and Elmaghraby, 2011; Banerjee Ray et al., 2012; Kar, 2012; Nazik and Kumtepe, 2012; Selim and Borg, 2012), two had a moderate risk of bias (Begum et al., 2009; Dehbashi et al., 2009) and four had a low risk of bias (Bayar et al., 2006; Badawy et al., 2009; Roy et al., 2012; Legro et al., 2014a,b). Upon meta-analysis, we found that letrozole was better than clomiphene citrate for ovulation rate per patient (Atay et al., 2006; Begum et al., 2009; Dehbashi et al., 2009; Sheikh-El-Arab Elsedeek and Elmaghraby, 2011; Banerjee Ray et al., 2012; Nazik and Kumtepe, 2012; Roy et al., 2012; Legro et al., 2014a,b); pregnancy rate per patient (Atay et al., 2006; Bayar et al., 2006; Badawy et al., 2009; Begum et al., 2009; Dehbashi et al., 2009; Zeinalzadeh et al., 2010; Sheikh-El-Arab Elsedeek and Elmaghraby, 2011; Banerjee Ray et al., 2012; Kar, 2012; Nazik and Kumtepe, 2012; Roy et al., 2012; Selim and Borg, 2012; Legro et al., 2014a,b); and per cycle (Nazik and Kumtepe, 2012; Roy et al., 2012; Selim and Borg, 2012); and live birth rate per patient (Bayar et al., 2006; Dehbashi et al., 2009; Banerjee Ray et al., 2012; Roy et al., 2012; Legro et al., 2014a,b). There was no difference between letrozole and clomiphene citrate for ovulation rate per cycle (Bayar et al., 2006; Badawy et al., 2009; Nazik and Kumtepe, 2012; Roy et al., 2012; Selim and Borg, 2012); multiple pregnancy rate per patient (Atay et al., 2006; Badawy et al., 2009; Dehbashi et al., 2009; Zeinalzadeh et al., 2010; Kar, 2012; Nazik and Kumtepe, 2012; Roy et al., 2012; Legro et al., 2014a,b) and miscarriage rate per patient (Bayar et al., 2006; Badawy et al., 2009; Begum et al., 2009; Dehbashi et al., 2009; Banerjee Ray et al., 2012; Kar, 2012; Nazik and Kumtepe, 2012; Roy et al., 2012; Legro et al., 2014a,b). When subgroup analysis was conducted for studies that included women with PCOS who were therapy naïve, there was no difference between the two interventions for any outcome, though we note that for pregnancy rate per patient the OR 1.68 [95% CI 0.96, 2.94] had an I2 of 0% and a P value of 0.07.
Clomiphene citrate versus gonadotrophin (FSH)
Two RCTs were identified by the search to address this comparison. One RCT with low-quality and high risk of bias (Lopez et al., 2004) compared recombinant FSH with clomiphene citrate in women with PCOS who were therapy naïve and found that there was no difference between the two interventions for all fertility outcomes. The second was a multi-centre RCT with moderate risk of bias (Homburg et al., 2012) comparing clomiphene citrate with low-dose gonadotrophins, as the first line therapy for ovulation induction in anovulatory women with PCOS who were therapy naïve. They reported with per protocol analysis that the clinical pregnancy rate was significantly higher in the gonadotrophin treated group. Furthermore, the chance of pregnancy was almost double in the first treatment cycle when compared to clomiphene citrate. Brown and Farquhar (2016) meta-analysed these same two RCTs combining data for live birth rate and ongoing pregnancy rate and found that gonadotrophins were better than clomiphene citrate (OR 0.64 [0.41, 0.98] P = 0.041, I2=0%). Meta-analysis of the two studies for clinical pregnancy rate found that clomiphene citrate was better than gonadotrophins (OR 0.61 [0.40, 0.93] P = 0.021, I2=0%). It is important to be cautious of these results (using per protocol event rates), as the number of participants randomized has been used as the denominator when the denominator should have been the number of participants per protocol.
Clomiphene citrate versus clomiphene citrate + gonadotrophin (FSH)
Two RCTs were identified to address this comparison, however there was insufficient evidence to determine whether one intervention was better than the other (Mukherjee et al., 2010; Abu Hashim et al., 2012).
Justification
Clomiphene citrate therapy requires specialist care. Costs to the patient of monitoring (tests and specialist visits) and accessibility to specialist care may present barriers, however increased costs will be offset by reduced multiple pregnancies. Metformin is low cost, accessible and can be used alone and/or in combination with clomiphene citrate, given efficacy on systematic review. Usual doses of metformin range from 1500 mg (most commonly) to 1700 mg per day for non-fertility studies. A change in usual care may result as clinicians may now be more likely to prescribe metformin. Metformin may be associated with mild gastrointestinal related adverse events (see Chapter 4 of full guideline document). Whilst use is evidence-based, patient explanation and consent is appropriate as metformin therapy for infertility is off label.
Gonadotrophins
Clinical question
In women with PCOS, are gonadotrophins effective for improving fertility outcomes?
Clinical need for the question
Gonadotrophin therapy is used clinically in women with anovulatory PCOS who have been treated with other first line ovulation induction agents if they have failed to ovulate or if the responses reduce chances of conception (e.g. persistent hypersecretion of LH, or an anti-oestrogenic endometrial effects). To prevent overstimulation and multiple pregnancy, the traditional standard step-up regimens (Lunenfeld and Insler, 1974) were replaced by either low-dose step-up regimens (Hamilton-Fairley et al., 1991; White et al., 1996) or step-down regimens (van Santbrink et al., 1995) with gonadotrophins used alone and different gonadotrophin preparations appearing to work equally well (Nugent et al., 2000). It can be difficult to predict stimulation responses in PCOS and to achieve a single dominant follicle to reduce multiple pregnancy and OHSS, and careful monitoring of follicular development by ultrasound is required with triggers only used with two or less follicles over 14 mm. The efficacy, safety and role of gonadotrophins compared to other alternatives, including single or combined oral ovulation induction agents or laparoscopic surgery, remains unclear.
Summary of systematic review evidence
Gonadotrophin (FSH) versus clomiphene citrate
Two RCTs were identified by the search to address this comparison. One RCT with low quality and a high risk of bias (Lopez et al., 2004) compared recombinant FSH with clomiphene citrate in women with PCOS who were therapy naïve and found that there was no difference between the two interventions for all fertility outcomes. The second was a multi-centre RCT with moderate risk of bias (Homburg et al., 2012) comparing clomiphene citrate with low-dose gonadotrophins as the first line therapy for ovulation induction in anovulatory women with PCOS who were therapy naïve. They reported with per protocol analysis that the clinical pregnancy rate was significantly higher in the gonadotrophin treated group. Furthermore, the chance of pregnancy was almost double in the first treatment cycle when compared to clomiphene citrate. Brown and Farquhar (2016) meta-analysed these same two RCTs combining data for live birth rate and ongoing pregnancy rate and found that gonadotrophins were better than clomiphene citrate (OR 0.64 [0.41, 0.98] P = 0.041, I2=0%). Meta-analysis of the two studies for clinical pregnancy rate found that clomiphene citrate was better than gonadotrophins (OR 0.61 [0.40, 0.93] P = 0.021, I2=0%). It is important to be cautious of these results (using per protocol event rates), as the number of participants randomized has been used as the denominator when the denominator should have been the number of participants per protocol.
Gonadotrophins versus clomiphene citrate + metformin
Two RCTs compared FSH with clomiphene citrate plus metformin (Abu Hashim, et al. 2011a,b, Begum et al., 2013). The RCTs found that FSH was better than clomiphene citrate plus metformin for ovulation rate per participant and pregnancy rate per participant. There was no statistical difference between the two interventions for live birth rate per participant, multiple pregnancy rate per pregnancy, OHSS, miscarriage rate per pregnancy or gastrointestinal side-effects or adverse events. A systematic review by Abu Hashim et al. (2015) conducted a meta-analysis including RCTs comparing gonadotrophins versus clomiphene citrate combined with metformin in clomiphene resistant PCOS women that do not meet our PICO (P—patient, problem or population; I—intervention; C—comparison, control or comparator; O—outcome), however some sensitivity analysis was conducted with the two RCTs listed below. A sensitivity analysis for ovulation rate in 263 patients demonstrated that gonadotrophins are better for ovulation rate (OR 0.13; 95% CI 0.07–0.25; P < 0.00001, I2 = 7%); but there was no statistically significant difference between the two interventions for multiple pregnancy rate (n = 263, OR 0.33; 95% CI 0.06–1.68; P = 0.18, heterogeneity not reported). This meta-analysis, which the GDG considered to be clinically relevant, demonstrated a significantly higher ovulation, clinical pregnancy and ongoing pregnancy/live birth rate with gonadotrophins compared to clomiphene citrate plus metformin in clomiphene resistant PCOS women.
Gonadotrophins versus gonadotrophins + metformin
One RCT with moderate risk of bias found that FSH plus metformin was better than FSH alone for live birth rate per participant, ovulation rate per participant and pregnancy rate per participant (Begum et al., 2013). There was no statistical difference between the two interventions for multiple pregnancy rate per pregnancy, miscarriage rate per pregnancy or adverse events.
A Cochrane review evaluating the use of metformin as an adjunct to gonadotrophin ovulation induction in PCOS (Bordewijk et al., 2017) was identified by the search, however it included studies that did not meet the selection criteria for this question. The GDG considered the meta-analyses in the Cochrane review as clinically relevant. The meta-analysis demonstrated a statistically significantly higher ongoing pregnancy, clinical pregnancy and live birth rate with no statistically significant difference in multiple pregnancy, miscarriage or OHSS rates when metformin is combined with gonadotrophins in clomiphene citrate-resistant PCOS women.
Gonadotrophins versus laparoscopic ovarian surgery
One high-quality systematic review of RCTs (Level I) with low risk of bias compared laparoscopic ovarian surgery to gonadotrophins and found that there was no difference between the interventions for live birth rate per patient and pregnancy rate per patient, ovulation rate per patient and miscarriage rate per pregnancy, but laparoscopic ovarian surgery had a lower multiple pregnancy rate (OR 0.13 [0.03–0.59] I2 = 0%, four studies, 303 participants) (Farquhar et al., 2012) compared to gondaotrophins.
Gonadotrophins versus gonadotrophins + clomiphene citrate
One RCT (Ghanem et al., 2013) with moderate risk of bias found that FSH plus clomiphene citrate was better than FSH alone for ovulation rate per woman randomized and per protocol, total FSH dose used per woman randomized and per protocol, and duration of stimulation per woman randomized and per protocol. There was no statistical difference between the two interventions for pregnancy rate and live birth rate per woman randomized and per protocol.
Justification
Gonadotrophin therapy is suitable for improving infertility in women with PCOS in specialist care, with close monitoring. Gonadotrophin therapy provides better per cycle and cumulative pregnancy and live birth rates compared with the use of oral anti-oestrogens and/or no therapy in anovulatory women with PCOS; and there is no evidence of teratogenicity. It is important to note that gonadotrophin therapy requires daily injections and the need for intensive monitoring with ultrasound; with a risk of multiple pregnancy, OHSS and increased cost of medication compared with oral agents.
Anti-obesity agents
Clinical question
In women with PCOS, are anti-obesity pharmacological agents effective for improving fertility outcomes?
Clinical need for the question
A 2017-systematic review and meta-analysis (Domecq et al., 2013) found that lifestyle interventions benefitted weight loss and natural pregnancy rate, with limited evidence for live birth rate or birthweight, yet natural birth rate did increase (Kiddy et al., 1992; Clark et al., 1995). Hence, the impact of non-pharmacological lifestyle interventions on live birth rates remains controversial. Engagement and adherence in lifestyle interventions are challenging. There is a need to assess other weight loss methods, such as pharmacological agents commenced in the pre-conception period, with some evidence they can induce weight loss and improve fertility outcomes in PCOS.
Summary of systematic review evidence
We did not identify any evidence in women with PCOS to answer the question and therefore the literature has been reviewed narratively.
Summary of narrative evidence
A randomized trial (that did not meet the inclusion criteria for this systematic review due to a change in interventions and combination of treatments) evaluated pre-conception treatment in women with PCOS with: lifestyle weight loss intervention incorporating caloric restriction, increased physical activity and pharmacological agent (initially sibutramine, and then orlistat); oral contraceptive pill; combined lifestyle and contraceptive pill on fertility outcomes (Legro et al., 2015). The trial randomized 149 women, and was stopped prematurely due to supposed futility with a low likelihood of showing a clinically meaningful difference. Given the small sample size in a three-arm trial, with no control group, no meaningful conclusions can be inferred. Within the lifestyle arm, including anti-obesity agents, there was a significant reduction in weight from baseline (−6.2Kg, 95% CI −07.1 to −5.3), and compared to the women on a combined oral contraception pill pre-conception, those on lifestyle with anti-obesity agents showed no differences in pregnancy outcomes. Evidence for these agents in other relevant population groups is lacking.
Justification
With inadequate evidence in both PCOS and infertility generally, the risk/benefit ratio is currently too uncertain to advocate anti-obesity pharmacological agents as a fertility treatment and it was deemed that it should remain an experimental treatment for this indication.
Laparoscopic ovarian surgery
Clinical question
In women with PCOS, is ovarian surgery effective for improving fertility outcomes?
Clinical need for the question
Observations that women with PCOS resumed regular ovulation following ovarian biopsies led to the development of surgical wedge resection via laparotomy (Stein, 1964). Observational data looked promising, but surgery was surpassed by ovulation induction agents until less invasive laparoscopic surgery was developed (Gjønnæss, 1984), with potential for fewer adhesions and lower cost. Minor methodological variations are reported (electrocautery, laser vaporization, multiple ovarian biopsies and others), all seemingly with effects on the endocrine profile. OHSS and multiple pregnancy risks are lower than with other options, but other risks potentially are higher, and clarification of the role of laparoscopic ovarian surgery, particularly in comparison to other treatments, is needed in infertile women with PCOS.
Summary of systematic review evidence
Laparoscopic ovarian surgery versus metformin
Two medium quality RCTs (Level II) (published across three papers) with a moderate risk of bias compared laparoscopic ovarian surgery to metformin and found that there was insufficient evidence to make a recommendation about laparoscopic ovarian surgery compared to metformin for live birth rate per patient, ovulation rate per cycle, pregnancy rate per cycle, pregnancy rate per patient, multiple pregnancies, miscarriage rate per pregnancy, adverse effects and quality of life (QOL; Palomba et al., 2004, 2005; Hamed et al., 2010) largely because the evidence was conflicting. One RCT reported that laparoscopic ovarian surgery was better than metformin for ovulation (OR 2.05; [1.4–2.9] P = 0.001) and pregnancy rate (per cycle: OR 2.19 [1.03–4.63] P = 0.03; per patient: OR 2.47 [1.05–5.81] P = 0.03) (Hamed et al., 2010) and the other study reported that metformin was better than laparoscopic ovarian surgery for live birth rate (metformin: 82.1%, LOS: 64.5%, P < 0.05), pregnancy rate per cycle (metformin: 18.6%, LOS: 13.4%, P < 0.05), and miscarriage rate (metformin: 15.4%, LOS:29.0%, P < 0.05) (Palomba et al., 2004, 2005). Both medium quality single centre studies had a small sample size and moderate risk of bias and therefore need to be interpreted with caution.
Laparoscopic ovarian surgery versus clomiphene citrate
Two high-quality RCTs (Level II) with a low risk of bias compared laparoscopic ovarian surgery to clomiphene citrate (Amer et al., 2009, Abu Hashim, et al. 2011a,b) and found that there was no difference between laparoscopic ovarian surgery and clomiphene citrate for live birth rate per patient and pregnancy rate per patient, ovulation rate per patient and miscarriage rate per pregnancy (Amer et al., 2009, Abu Hashim, et al. 2011a). There was insufficient evidence to support or refute the use of laparoscopic ovarian surgery over clomiphene citrate for multiple pregnancies (Amer et al., 2009, Abu Hashim, et al. 2011a,b).
Laparoscopic ovarian surgery versus clomiphene citrate + metformin
Three low to moderate-quality RCTs with low to moderate risk of bias compared laparoscopic ovarian surgery to clomiphene citrate plus metformin [all three studies reported in Farquhar 2012 systematic review (Farquhar et al., 2012)]. Meta-analyses found that clomiphene citrate plus metformin was better than laparoscopic ovarian surgery for live birth rate, but there was no difference for pregnancy rate per patient, multiple pregnancy rate, or miscarriage rate per pregnancy (Farquhar et al., 2012). There was insufficient evidence to support or refute the use of laparoscopic ovarian surgery over clomiphene citrate plus metformin for ovulation rate per patient, and OHSS (Farquhar et al., 2012).
Laparoscopic ovarian surgery versus AIs
Three RCTs with low risk of bias (Abu Hashim et al., 2010a, Abdellah, 2011; Ibrahim et al., 2017) compared letrozole to laparoscopic ovarian surgery and found that there was insufficient evidence of a difference between letrozole and laparoscopic ovarian surgery. One of the RCTs in 147 women with clomiphene citrate resistance found that letrozole was better than laparoscopic ovarian surgery for ovulation rate per cycle (Abdellah, 2011), however the evidence is of low certainty. The systematic review by Farquhar et al. (2012) combined these studies in meta-analysis for pregnancy rate per patient, multiple pregnancy rate per pregnancy and miscarriage rate per pregnancy and there was no statistical difference between the two interventions.
Laparoscopic ovarian surgery versus aromatase inhibitors + metformin
One low-quality RCT with moderate risk of bias compared laparoscopic ovarian surgery with letrozole plus metformin and found that there was insufficient evidence of a difference between the two interventions for ovulation, pregnancy and miscarriage rate per pregnancy (Abd Elgafor, 2013).
Laparoscopic ovarian surgery versus gonadotrophins
One high-quality systematic review of RCTs (Level I) with low risk of bias compared laparoscopic ovarian surgery to gonadotrophins and found that there was no difference between the interventions for live birth rate per patient and pregnancy rate per patient, ovulation rate per patient and miscarriage rate per pregnancy, but laparoscopic ovarian surgery was better than gonadotrophins for multiple pregnancy rate (OR 0.13 [0.03–0.59] I2 = 0%, four studies, 303 participants) (Farquhar et al., 2012).
Summary of narrative review evidence
Observational data was sourced to evaluate long-term impacts. A 15–25-year follow-up of nearly 150 women after ovarian wedge resection shows that regular menstrual patterns lasting up to 25 years after surgery were restored in 88% of patients with a cumulative pregnancy/live birth rate of 78% (Lunde et al., 2001). This was considered along with the RCT data.
Justification
Laparoscopic ovarian surgery is an intervention that can lead to a singleton birth in women with PCOS. There is no convincing evidence of inferiority over other common ovulation induction agents, there is no need for monitoring (because of mono-ovulation) and only a background risk of multiple pregnancy. However, it is important to note that laparoscopic ovarian surgery is an invasive surgical intervention; there is a small risk of reduced ovarian reserve or loss of ovarian function, and adhesion formation should be considered. Issues covered in the CPP should be carefully considered.
Bariatric surgery
Clinical question
In women with PCOS, what is the effectiveness of lifestyle interventions compared to bariatric surgery for improving fertility and adverse outcomes?
Clinical need for the question
Obesity is increasing in prevalence throughout the world, as is morbid obesity (BMI≥ 40 kg/m2) (Sturm and Hattori, 2013). Women with PCOS have higher rates of weight gain and of obesity, adversely affecting fertility. Weight loss improves outcomes, as previously outlined. In severe obesity, lifestyle interventions have limited efficacy. Substantial efficacy of bariatric surgery on weight loss has been demonstrated in severely obese women. Potential benefits need to be balanced with the delay in infertility treatment and pregnancy for surgery and stabilization of weight, the risks of bariatric surgery and the potential risks of pregnancy after bariatric surgery. Controversy persists around efficacy for fertility and pregnancy outcomes, optimal timing, adverse effects and comparative efficacy with other treatments, as well as on adverse effects on subsequent pregnancies.
Summary of systematic review evidence
We did not identify any evidence in women with PCOS to answer the question and therefore the literature has been reviewed narratively.
Summary of narrative review evidence
UK clinical guidelines for obesity management in the general population (Scottish Intercollegiate Guidelines Network, 2010) recommend considering bariatric surgery with a BMI ≥35 kg/m2 with one or more severe complications expected to improve with weight loss and failure of structured lifestyle intervention (Scottish Intercollegiate Guidelines Network, 2010). Obesity surgery can be considered after non-surgical treatment has failed with a BMI ≥40 kg/m2 and obesity surgery can be first line treatment with a BMI ≥50 kg/m2 (National Institute for Health and Clinical Excellence, 2006). Other guidelines recommend lower barriers to surgery (Jensen et al., 2014). For type of surgery, vertical sleeve gastrectomy has overtaken the roux-en-Y gastric bypass and gastric band surgery as the most commonly performed bariatric surgery, with lower operative morbidity (Lager et al., 2017). Adjustable gastric banding, once the choice for women planning pregnancy, is now less common given complications and overall lower long-term weight loss (Lager et al., 2017).
High-quality RCTs of bariatric surgery versus medical management in type 2 diabetes mellitus show persistent benefits and superiority of weight loss and bariatric surgery in curing or ameliorating diabetes (Mingrone et al., 2012; Schauer et al., 2017). Yet these studies are absent in PCOS for fertility and pregnancy outcomes, with current PCOS studies poorly designed (Shah and Ginsburg, 2010), and with failure to report key perinatal outcomes to inform risk to benefit ratio. In PCOS, the balance between delaying infertility treatment and pregnancy whilst undertaking bariatric surgery and attaining stable post-operative weight, is also unclear (Mutsaerts et al., 2016), as is the optimal type of bariatric surgery.
Bariatric surgery can cause malabsorption and psychological issues including disordered eating (Månsson et al., 2008) and may adversely affect maternal and neonatal health. Adequate intake and absorption of iron, folate, iodine and other nutrients are of concern. While supplement use is widely recommended following bariatric surgery, especially for pregnant women, there are reports of poor compliance (Nilsen et al., 2006) and challenges tolerating fortified foods such as bread. National registries (surgery, pregnancy, infants) show that obese women who undergo bariatric surgery and conceive, compared to similarly obese controls, had more small for gestational age babies, shorter gestations, and a trend towards increased neonatal mortality (Johansson et al., 2015), with similar findings in retrospective studies (Gonzalez et al., 2015). Benefits have included less gestational diabetes mellitis and large for gestational age babies.
Justification
Bariatric surgery improves weight loss and can improve comorbidities associated with PCOS. However, evidence in relation to fertility and pregnancy outcomes is limited, with some concerns about potential perinatal adverse effects of bariatric surgery. Overall, the indications, role and comparative effectiveness with other fertility therapies, ideal timing, optimal type of surgery, adverse effects and risk to benefit ratio in PCOS are still to be resolved. Given the concerns about the potential perinatal adverse effects of bariatric surgery and the remaining controversies, no recommendation can be made at this time about the use of bariatric surgery to improve fertility and pregnancy outcomes in women with PCOS.
IVF
Clinical question
In women with PCOS, is stimulated IVF/ICSI effective for improving fertility outcomes?
Clinical need for the question
Ovulation induction therapies are first and second line in infertility management in women with PCOS, anovulation and no other fertility factors. Yet resistance to and failure of ovulation induction therapies and inability to overcome other concomitant causes of infertility means that ART therapies, including IVF and ICSI, used in male factor infertility, have a role in PCOS. IVF has risks and limitations, yet also offers the opportunity for pregnancy and live birth. Challenges exist across the diversity of protocols available for IVF and concerns in PCOS including OHSS, high oestradiol levels, accelerated endometrial maturation and optimally the use of ‘freeze all’ interventions. The clinical practice questions here include indications, timing and comparative efficacy with other treatments, yet RCTs in this area are very limited in women with anovulatory PCOS.
Summary of systematic review evidence
We did not identify any evidence in women with PCOS to answer the question and therefore the literature has been reviewed narratively.
Summary of narrative review evidence
There are no RCTs identified by the guideline development team, comparing stimulated IVF ± ICSI therapy with ovulation induction in women diagnosed with PCOS. The role of IVF in PCOS was explored by the WHO guidance group, and the review and recommendations were considered here by the GDG, in making their recommendations (Balen et al., 2016). Factors that influenced considerations here include access, cost and risks. The patient and societal benefits of ovulation induction compared with IVF treatments in anovulatory PCOS women require RCTs and systematic analysis. Outcomes such as time to conception, cost of therapy, QOL, OHSS risk, multiple pregnancy, miscarriage and live birth rates should be investigated.
Justification
The GDG deemed IVF should be considered after failed ovulation induction treatment with high pregnancy rates per cycle, especially in younger women. Given the risks and the high costs that can be prohibitive for many patients, IVF should be considered a third-line medical therapy. It was noted that conception and delivery are highly valued by health professionals and women with PCOS and even when cost and risks are increased, many may elect to undertake IVF. Health professionals must weigh benefits and risk when advising PCOS patients to enable an informed decision.
GnRH protocol
Clinical question
In women with PCOS undergoing IVF/ICSI treatment, is the GnRH antagonist protocol or GnRH agonist long protocol the most effective for improving fertility outcomes?
Clinical need for the question
Women with PCOS are particularly vulnerable to OHSS with IVF ± ICSI treatment, prompting caution and leading to exploration of different protocols, including with GnRH and other options such as IVM (Walls et al., 2015a). One of the proposed methods to reduce the risk of OHSS is to use a GnRH antagonist (as opposed to an agonist) (Al-Inany et al., 2007; Mancini et al., 2011; Lin et al., 2014; American Society for Reproductive Medicine, 2016). There is acknowledged complexity in interpreting outcomes from IVF treatments in PCOS, with variable protocols and endpoint reporting, requiring close evaluation of the literature. One of the proposed methods to reduce the risk of OHSS is to use a GnRH antagonist (as opposed to an agonist) to suppress pituitary LH secretion.
Summary of systematic review evidence
In the eight included studies of low (Hwang et al., 2004; Kurzawa et al., 2008; Tehraninejad et al., 2010), moderate (Bahceci et al., 2005; Lainas et al., 2007, 2010; Mokhtar et al., 2015), and high risk of bias (Haydardedeoglu et al., 2012) comparing a GnRH antagonist protocol with a long GnRH agonist protocol, there were statistically significant differences in the amount of gonadotrophin required (five studies in favour of the antagonist protocol) (Hwang et al., 2004; Kurzawa et al., 2008; Lainas et al., 2010; Tehraninejad et al., 2010; Haydardedeoglu et al., 2012), in the duration of gonadotrophin use (six studies in favour of the antagonist protocol) (Bahceci et al., 2005; Lainas et al., 2007, 2010; Kurzawa et al., 2008; Tehraninejad et al., 2010; Haydardedeoglu et al., 2012), in OHSS rates (two studies in favour of the antagonist protocol) (Lainas et al., 2007; Tehraninejad et al., 2010). No statistically significant differences were found between groups for clinical pregnancy rates, miscarriage rates, number of oocytes collected, cancellation rates, and multiple pregnancy rates.
A systematic review and meta-analysis comparing the GnRH antagonist protocol versus GnRH agonist long protocol in women with PCOS undergoing an IVF ± ICSI cycle (Pundir et al., 2012) was also identified by the search, however it included studies that did not meet the selection criteria for this question. The GDG considered this meta-analyses as clinically relevant. The meta-analysis demonstrated a statistically significantly reduction in the total dose of gonadotrophins, duration of gonadotrophin stimulation and rate of moderate to severe OHSS with the GnRH antagonist protocol, with no statistically significant difference in clinical pregnancy, miscarriage or number of eggs collected.
Justification
The duration of stimulation with a GnRH antagonist approach is around 1 day shorter than the standard ‘long-down regulation’ approach with a GnRH agonist. The rate of OHSS appears less with a GnRH antagonist approach in comparison to the standard ‘long-down regulation’ approach with a GnRH agonist. The effect size is difficult to quantify, as all most of these studies used a high dose hCG trigger in both arms, whereas this may not reflect clinical practice. There does not appear to be an increase in undesirable side-effects with an antagonist down-regulation approach. The choice to trigger final oocyte maturation with GnRH agonist instead of hCG is important to prevent OHSS.
Trigger type
Clinical question
In women with PCOS undergoing GnRH antagonist IVF/ICSI treatment, is the use of hCG trigger or GnRH agonist trigger the most effective for improving fertility outcomes?
Clinical need for the question
One of the prominent causes of OHSS is the occurrence in women with PCOS undergoing ovarian hyperstimulation for IVF, particularly where hCG is used to trigger ovulation. Early in 1990, an alternative to exogenous hCG triggering emerged with GnRH-agonist use, providing an additional ovulatory option for IVF. A single bolus of GnRH-agonist administration during late follicular development in women with PCOS treated with gonadotrophins results in a surge of endogenous FSH and LH for final oocyte maturation and fertilization. OHSS appears reduced yet lower pregnancy rates with GnRH-agonist triggers are observed and may vary when transferring fresh versus frozen-thawed embryos in cycles from the same cohort, suggesting that the pregnancy rate is dependent of endometrial quality. An alternative option therefore in women with PCOS at high risk of OHSS is to freeze oocytes or embryos after GnRH agonist triggering and transfer the embryos in subsequent cycles. The choice to trigger final oocyte maturation with GnRH-agonist, instead of hCG, and to transfer frozen embryos requires clarification.
Summary of systematic review evidence
We did not identify any evidence in women with PCOS to answer the question and therefore the literature has been reviewed narratively.
Summary of narrative review evidence
This question was addressed in a Cochrane review in 2014 (Youssef et al., 2014). In 17 RCTs (n = 1847), in fresh autologous cycles, GnRH-agonists were associated with a lower live birth rate than hCG (OR 0.47, 95% CI 0.31–0.70; five RCTs, 532 women, I2 = 56%, moderate-quality evidence), yet there was also a lower incidence of mild, moderate or severe OHSS than with hCG (OR 0.15, 95% CI 0.05–0.47; 8 RCTs, 989 women, I2 = 42%, moderate-quality evidence). In fresh autologous cycles, GnRH-agonists were associated with a lower ongoing pregnancy rate than hCG (OR 0.70, 95% CI 0.54–0.91; 11 studies, 1198 women,I2 = 59%, low-quality evidence) and a higher early miscarriage rate (OR 1.74, 95% CI 1.10–2.75; 11 RCTs, 1198 women, I2 = 1%, moderate-quality evidence). However, the effect was dependent on the type of luteal phase support provided. Multiple pregnancy rates were similar. The authors concluded that final oocyte maturation triggering with GnRH-agonist instead of hCG in fresh autologous GnRH-antagonist IVF ± ICSI cycles prevents OHSS to the detriment of the live birth rate. In donor-recipient cycles, use of GnRH agonists instead of hCG resulted in a lower incidence of OHSS, with no evidence of a difference in live birth rate. GnRH agonist as an oocyte maturation trigger could be useful for women who choose to avoid fresh transfers, where donated oocytes are used or in women who wish to freeze their eggs for later use.
Justification
The choice to trigger final oocyte maturation with GnRH-agonist instead of hCG is important in prevention of OHSS as hCG alone induces oocyte maturation but is associated with OHSS. GnRH-agonist triggers are associated with lower pregnancy rates, primarily in fresh embryo transfers, and data on cumulative pregnancy rates after the use of all embryos frozen and replaced in thawed cycles needs specific focus in the future.
Choice of FSH
Clinical question
In women with PCOS undergoing (controlled) ovarian (hyper) stimulation for IVF/ICSI, does the choice of FSH effect fertility outcomes?
Clinical need for the question
FSH can be purified from human urine (uFSH) or synthesized using recombinant DNA techniques (rFSH). Urinary preparations have impurities with LH activity known to stimulate androgen production in theca cells and completing maturation of follicles. However, it is known that less than 1% of follicular LH receptors needs to be occupied in order to elicit maximal steroidogenesis and it is therefore possible that enough endogenous LH is present during controlled ovarian stimulation to promote androgen synthesis and oocyte maturation without the need for extra LH activity in FSH preparations. The perceived clinical benefits of rFSH versus uFSH are the subject of ongoing debate and both types of preparations remain commonly used.
Summary of systematic review evidence
One small study (80 participants) with moderate risk of bias compared rFSH with hMG and found that rFSH was better for the duration of ovarian stimulation required and the number of oocytes retrieved; whereas hMG was better for the maximum serum estradiol level (Figen Turkcapar et al., 2013). No statistically significant differences were found between groups for the total dose of gonadotrophin used, OHSS rate, clinical pregnancy rate per cycle and take home baby rate per cycle.
Summary of narrative review evidence
Given the limited evidence in PCOS, additional information was sought from rFSH and uFSH use in the general population. In a Cochrane systematic review and meta-analysis, 42 trials with a total of 9606 couples compared rFSH against three different uFSH preparations (van Wely et al., 2011). rFSH, irrespective of the down-regulation protocol, did not result in a statistically significant different live birth rate or OHSS rate, concluding that clinical choice of gonadotrophin should depend on availability, convenience and costs, and that further research on these comparisons is unlikely to identify substantive differences in effectiveness or safety.
Justification
Only one small study in PCOS has been identified investigating uFSH versus rFSH in PCOS during ovarian stimulation for IVF/ICSI (Figen Turkcapar et al., 2013). This study shows similar results to a systematic review and meta-analysis in the general IVF population, where extensive research has concluded no significant difference in birth rate or OHSS was detected and no further research in the general population was recommended. Hence clinical choice of gonadotrophin should depend on availability, convenience and costs.
Exogenous LH
Clinical question
In women with PCOS undergoing (controlled) ovarian (hyper) stimulation for IVF/ICSI, is exogenous LH treatment during IVF±ICSI effective for improving fertility outcome?
Clinical need for the question
Options have been explored to reduce OHSS risk in IVF/ICSI in PCOS. The low-dose step-up protocol with exogenous FSH in securing single (fewer) dominant follicle selection is an alternative method to avoid multi-follicular development. During late follicular development, LH is essential to achieve adequate ovarian steroidogenesis and develop the subsequent capacity of the follicle to ovulate and luteinize. Increased LH secretion or elevated LH/FSH ratio in PCOS may influence fertility, with inhibition of oocyte maturation, deleterious effects on granulosa cell steroidogenesis and endometrial receptivity and with potential increased early pregnancy loss (Jacobs and Homburg, 1990; Tarlatzis et al., 1995; Willis et al., 1998). The lack of clarity around the role of exogenous LH in the setting of IVF/ICSI prompted this clinical question.
Summary of systematic review evidence
We did not identify any evidence in women with PCOS to answer the question and therefore the literature has been reviewed narratively.
Summary of narrative review evidence
Obesity adversely impacts on ovulation and on responses to ovulation induction in PCOS (Kiddy et al., 1990). In PCOS, granulosa cells respond to LH at a relatively earlier follicular stage and are significantly more responsive than for ovulatory women with PCOS or women without PCOS (Willis et al., 1998). Granulosa cell differentiation may be prematurely advanced. Controlled ovarian stimulation for multiple follicular development in ART can be performed in a variety of ways to increase efficacy and reduce risks. Systematic reviews and meta-analysis have demonstrated that there is no significant difference between different ovarian stimulation protocols (hMG, purified FSH, rFSH) regarding the fertility outcomes. Therefore, clinical gonadotrophin choice depends on availability, convenience, and cost. In standard IVF/ICSI protocols, the types of controlled ovarian stimulation (FSH alone or addition of LH as a supplement) have little impact on the fertility outcomes (van Wely et al., 2011; Schwarze et al., 2016). Endogenous LH levels may fall too low in older women (>35 years) during ovarian stimulation, especially with GnRH-antagonist use, and LH supplementation has been proposed. However, a multi-centre RCT of exogenous LH during the follicular phase showed no fertility benefit outcomes in women over 35 years of age (Konig et al., 2013). No current study investigates efficacy of exogenous LH supplement for fertility outcomes in PCOS during IVF/ICSI. Careful monitoring of follicular development during ovarian stimulation is critical.
Justification
There is no anticipated effect or benefit to add exogenous LH supplement in women with PCOS undergoing ovarian stimulation for IVF±ICSI. There is insufficient evidence to determine the benefits of using or not using exogenous LH.
Adjunct metformin
Clinical question
In women with PCOS undergoing (controlled) ovarian (hyper) stimulation for IVF±ICSI, is adjunct metformin effective for improving fertility outcomes?
Clinical need for the question
IVF±ICSI treatment in women with PCOS is usually recommended either third-line (after failed ovulation induction) or in those with other infertility factors such as tubal damage, severe endometriosis or male factors (Baskind and Balen, 2016). IVF±ICSI treatment in PCOS poses challenges, including OHSS (Costello et al., 2006). Metformin has been studied to restore ovulation and enhance pregnancy rates in PCOS (Huang et al., 2015) through a range of mechanisms (Kjotrod et al., 2004, 2011; Lin and Coutifaris, 2007). These mechanisms provide a physiological rationale for management of insulin resistance in IVF in PCOS. It has also been suggested that metformin may reduce serum estradiol levels during ovarian stimulation and it has also been hypothesized that metformin may reduce the production of vascular endothelial growth factor, both of which are important factors involved in the pathophysiology of OHSS (Legro, 2016). Therefore, it was deemed important to explore the effectiveness and safety of metformin as a co-treatment in achieving pregnancy or live birth and reducing OHSS in IVF in PCOS.
Summary of systematic review evidence
Six RCTs of low (Kjotrod et al., 2004; Tang et al., 2006; Palomba et al., 2011), moderate (Kjotrod et al., 2011) and high risk of bias (Fedorcsak et al., 2003; Onalan et al., 2005) found that IVF with adjuvant metformin was better for OHSS, clinical pregnancy rate, cancellation rate and live birth rate. No statistically significant differences were found between groups for the amount of gonadotrophins used, the duration of ovarian stimulation, miscarriage rates, number of oocytes collected, and multiple pregnancy rates.
Summary of narrative review evidence
A Cochrane review (Tso et al., 2014) was identified by the search, however it included studies that did not meet the selection criteria for this question. The GDG considered the meta-analyses in the Cochrane review as clinically relevant and noted that there was no evidence of a difference with adjunct metformin for live birth rate, miscarriage rate, number of oocytes collected, days of ovarian stimulation or cycle cancellation rate; and clinical pregnancy rate was increased with adjuvant metformin whilst OHSS was reduced. Mild, generally self-limiting side-effects, were noted with adjunct metformin, as outlined in Chapter 4.
Justification
Women and health professionals would generally value an increased clinical pregnancy rate (with no evidence of a difference in miscarriage rate) and reduced OHSS (with its associated morbidity and rarely mortality). Gastrointestinal side-effects were recognized, but noted as mild and self-limiting, and may be minimized with a lower metformin starting dose and extended release preparations. Metformin was noted to be low cost and readily available, and while off label use was generally allowed, an explanation is required for use.
IVM
Clinical question
In women with PCOS, is IVM effective for improving fertility outcomes?
Clinical need for the question
Where IVF is indicated in PCOS, OHSS risks are increased with gonadotrophin stimulation. IVM of oocytes limits or omits ovarian stimulation prior to oocyte retrieval, with maturation of oocytes post retrieval, avoiding OHSS risk (Walls et al., 2015a). The definition of an IVM cycle requires clarification (De Vos et al., 2016), as cycles employing an hCG trigger injection are generally associated with asynchronous oocyte maturation rates, poor embryo implantation rates and lower pregnancy rates (De Vos et al., 2011; Reavey et al., 2016). There are no RCTs of IVM versus ICSI or ovulation induction in PCOS, however observational studies suggest that offspring from IVM are not adversely affected (Roesner et al., 2017b). Given that IVM is used in practice and has theoretical benefits, this question was prioritized.
Summary of systematic review evidence
We did not identify any evidence in women with PCOS to answer the question and therefore the literature has been reviewed narratively.
Summary of narrative review evidence
With an absence of relevant RCTs (Sauerbrun-Cutler et al., 2015), retrospective studies suggest IVM is similarly successful for live birth with frozen embryos generated with IVM as embryo transfers generated by standard IVF treatment (Walls et al., 2015a). However, pregnancy rates are reduced and miscarriage rates are higher if a fresh embryo transfer is performed with IVM (Walls et al., 2015a). Embryo development appears slower with a greater degree of embryo arrest in IVM (Walls et al., 2015b; Roesner et al., 2017a).
Justification
The GDG deemed that key elements to consider with IVM included a clear definition of the term IVM, use in clinical units with sufficient expertize and the advantages of a reduced risk of OHSS. The group considered the lack of evidence as important. It was considered that IVM could be offered to achieve pregnancy and live birth rates that may approach those of standard IVF ± ICSI treatment, where frozen embryos are used. Given the lack of evidence, the group voted for a conditional consensus recommendation that neither favoured this option or other options (IVF), with strong research recommendations.
Discussion
The recommendations in the international evidence-based guidelines were formulated after engagement with professional societies and consumer organizations with multidisciplinary experts and women with PCOS directly involved at all stages. AGREE II-compliant processes were followed, with extensive evidence synthesis. The GRADE framework was applied across evidence quality, feasibility, acceptability, cost, implementation and ultimately recommendation strength.
The evidence and recommendations for ovulation induction in this guideline are applicable to women with PCOS who have anovulatory infertility and no other infertility factors. The discussion will be limited to what recommendations are considered new as compared to previous guidelines.
Letrozole should now be considered the first line pharmacological treatment for ovulation induction (EBRs, strong recommendation, low QOE although evidence was of moderate quality for the outcomes of ovulation, pregnancy and live-birth rates) although one could use clomiphene citrate alone (EBRs, conditional recommendation, very low QOE) or metformin alone (EBRs, conditional recommendation, moderate QOE). If using metformin alone for ovulation induction, then one should inform the woman that there are more effective ovulation induction agents.
Gonadotrophins could also be considered as first line ovulation induction treatment, in the presence of ultrasound monitoring, and following counselling on cost and potential risk of multiple pregnancy (EBR, conditional recommendation, low QOE). Gonadotrophins should be used in preference to clomiphene citrate combined with metformin therapy for ovulation induction in clomiphene citrate-resistant women with PCOS to improve live birth rates (EBRs, strong recommendation, moderate QOE). Gonadotrophins with the addition of metformin could be used rather than gonadotrophins alone to improve live birth rates in clomiphene citrate-resistant PCOS women (EBRs, conditional recommendation, moderate QOE).
Both pharmacological anti-obesity agents and bariatric surgery should be considered experimental ovulation induction therapies for the purpose of improving fertility and having a baby, with risk to benefit ratios currently too uncertain to advocate these as fertility therapies (CCR, conditional recommendation against the options, QOE not applicable).
In the absence of an absolute indication for IVF ± ICSI, women with PCOS and anovulatory infertility could be offered IVF as third-line therapy where first or second line ovulation induction therapies have failed (CCR, conditional recommendation, QOE not applicable). In terms of the controlled ovarian hyperstimulation for IVF ± ICSI, a GnRH antagonist protocol is preferred to reduce the duration of stimulation, total gonadotrophin dose and incidence of OHSS (EBR, conditional recommendation, low QOE), uFSH or rFSH can be used due to insufficient evidence to recommend specific FSH preparations (CCR, conditional recommendation, QOE not applicable), and exogenous recombinant LH treatment should not be routinely used in combination with FSH therapy (CCR, conditional recommendation, QOE not applicable). Adjunct metformin therapy could be used before and/or during FSH ovarian stimulation for IVF ± ICSI therapy with a GnRH-agonist protocol, to improve the clinical pregnancy rate and reduce the risk of OHSS (EBR, conditional recommendation, low QOE).
In units with sufficient expertize, IVM could be offered to achieve pregnancy and live birth rates approaching those of standard IVF ± ICSI treatment without the risk of OHSS for women with PCOS, where an embryo is generated, then vitrified and thawed and transferred in a subsequent cycle (CCR, conditional recommendation for either the IVM or standard IVF ± ICSI treatment, QOE not applicable).
PCOS guideline translation program
A comprehensive, international translation and dissemination program is currently underway to disseminate, translate and amplify the impact of the International Evidence-based Guideline on the Assessment and Management of Polycystic Ovary Syndrome. The program is inclusive of a range of practice support tools including a GP tool, care plan, a set of five algorithms (available at; https://www.monash.edu/medicine/sphpm/mchri/pcos/resources/practice-tools-for-health-practitioners), and resources to increase the PCOS related health literacy of consumers (Fig. 2 PCOS consumer information graphic—PCOS fertility and pregnancy) (available at; https://www.monash.edu/medicine/sphpm/mchri/pcos/resources/resources-for-women-with-pcos). In addition, the first evidence-based PCOS App (AskPCOS) (Fig. 3 Screens shots of the PCOS App—AskPCOS) has been developed and now available for purchase at iTunes. AskPCOS has a range of innovative features including a self-diagnostic quiz, a comprehensive repository of information on PCOS, a range of info-graphics, videos by experts, and a question prompt list to build health practitioner interaction skills
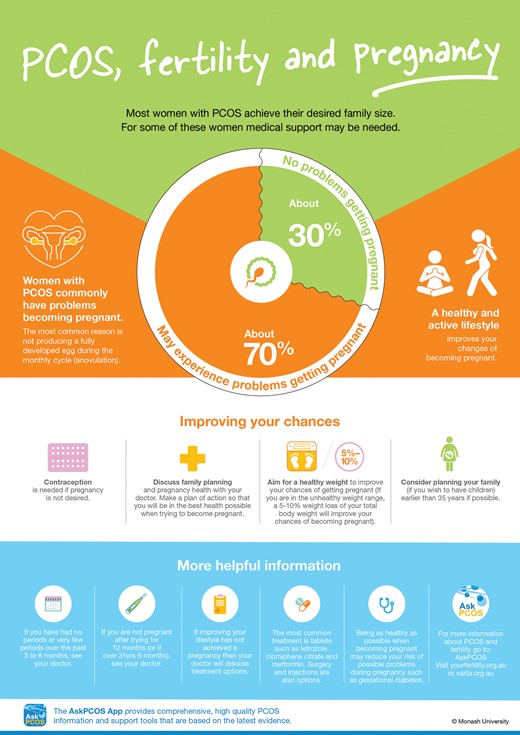
PCOS consumer information graphic—PCOS, fertility and pregnancy. PCOS: polycystic ovary syndrome.
The comprehensive evidence reviews, profiles and GRADE frameworks supporting each recommendation, can be found in the supplementary Technical report (2018) Available at: https://www.monash.edu/__data/assets/pdf_file/0020/1412282/PCOS-Guideline_Technical-report.pdf
Acknowledgements
We gratefully acknowledge the collaborating societies and consumer groups available at: https://www.monash.edu/medicine/sphpm/mchri/pcos/guideline.
Authors’ roles
Helena Teede, Robert Norman, Michael Costello and Marie Misso were members of the project board, and coordinated Guideline Development Group (GDG) activities from prioritizing clinical questions, providing clinical input into evidence synthesis, chairing the GDG process and GRADE framework application, finalizing recommendations, responding to feedback and endorsing the guideline.
Helena Teede was the guideline development and translation lead and engaged with all GDG meetings, overseeing the process. Michael Costello chaired the GDG on the assessment and management of infertile women with PCOS. Marie Misso led the guideline development and evidence synthesis processes. Robert Norman was the PCOS Centre for Research Excellence co-director, the deputy chair of the International advisory board and the deputy chair of the GDG.
All other authors, were actively engaged as GDG members, consumer, translation, or international advisory board members or members of the evidence synthesis and translation team, contributed to the manuscript, prioritizing clinical questions, discussing recommendations until voting and consensus, responses to external peer review and approval of the final recommendations for the GDG.
Funding
The Australian National Health and Medical Research Council (NHMRC) funded guideline development through the NHMRC Centre for Research Excellence in Polycystic Ovary Syndrome (APP1078444), administered through Monash University and University of Adelaide, Australia. Guideline partners, ESHRE and the American Society for Reproductive Medicine (ASRM) provided additional funding and assisted in guideline development activities.
Conflict of interest
Disclosures of conflicts of interest were declared at the outset and updated throughout the guideline development process, aligned with NHMRC guideline processes. Dr Costello has declared shares in Virtus Health and past sponsorship from Merck Serono for conference presentations. Prof. Norman has declared a minor shareholder interest in the IVF unit Fertility SA, travel support from Merck and grants from Ferring. Prof. Norman also has scientific advisory board duties for Ferring. The remaining authors have no conflicts of interest to declare. The guideline was peer-reviewed by special interest groups across our partner and collaborating societies and consumer organizations, was independently assessed against AGREE II criteria, and underwent methodological review. This guideline was approved by all members of the GDG and was submitted for final approval by the NHMRC.
Footnotes
Adapted from the grading of recommendations, assessment, development and evaluation framework (GRADE) (National Health and Medical Research Council, 2009).
References






