-
PDF
- Split View
-
Views
-
Annotate
-
Cite
Cite
Wei Li, Huaitong Wu, Xiaoping Li, Yingnan Chen, Tongming Yin, Fine mapping of the sex locus in Salix triandra confirms a consistent sex determination mechanism in genus Salix, Horticulture Research, Volume 7, 2020, 64, https://doi.org/10.1038/s41438-020-0289-1
Close - Share Icon Share
Abstract
Salix triandra belongs to section Amygdalinae in genus Salix, which is in a different section from the willow species in which sex determination has been well studied. Studying sex determination in distantly related willow species will help to clarify whether the sexes of different willows arise through a common sex determination system. For this purpose, we generated an intraspecific full-sib F1 population for S. triandra and constructed high-density genetic linkage maps for the crossing parents using restriction site-associated DNA sequencing and following a two-way pseudo-testcross strategy. With the established maps, the sex locus was positioned in linkage group XV only in the maternal map, and no sex linkage was detected in the paternal map. Consistent with previous findings in other willow species, our study showed that chromosome XV was the incipient sex chromosome and that females were the heterogametic sex in S. triandra. Therefore, sex in this willow species is also determined through a ZW sex determination system. We further performed fine mapping in the vicinity of the sex locus with SSR markers. By comparing the physical and genetic distances for the target interval encompassing the sex determination gene confined by SSRs, severe recombination repression was revealed in the sex determination region in the female map. The recombination rate in the confined interval encompassing the sex locus was approximately eight-fold lower than the genome-wide average. This study provides critical information relevant to sex determination in S. triandra.
Introduction
Flowering plants have evolved a considerably more complex sex determination system than animals, which have distinct germlines. Sexual diversity can first be observed in the various floral forms, ranging from hermaphroditic to monoecious and dioecious or even trioecious1. Approximately 5–10% of angiosperms are dioecious, with either heteromorphic or homomorphic sex chromosomes2. All sex chromosomes in dioecious plants harbor a sex determination region (SDR), which is characterized by suppressed recombination, leading to haplotype divergence3. Pseudo-autosomal regions (PARs) are also present in plant sex chromosomes, with the physical lengths of PARs varying among different plants4. Different sex determination systems, including male heterogamety (XY system) and female heterogamety (ZW system), have been described in dioecious plants5. The overall interactions among elements such as plant hormones, genetic factors, and epigenetic modifications determine plant sex6.
Populus and Salix are sister genera in the Salicaceae family. These two lineages diverged at least 45 million years ago7,8. The chromosome number of willows and poplars is the same, and they do not exhibit a morphologically differentiated sex chromosome9,10. The Salicaceae family, whose young sex chromosomes have evolved from different autosomes, provides a valuable comparative system for studying sex differentiation in plants11. Multiple studies have reported that chromosome XIX is the incipient sex chromosome in poplars12, and both XX/XY13,14 and ZW/ZZ12,15 sex determination systems have been observed in different poplar species. In poplars, the sex-determining locus has been mapped to either the peritelomeric region12 or the centromeric region of chromosome XIX14,16. However, no sex-determining genes have been identified among the Populus species analyzed so far, and only candidate genes have been reported. For example, the male-specific TOZ19 gene was identified as a sex determination candidate in P. tremula and P. tremuloides17. The sex determination region in poplar was found to contain a large number of R genes12,18, and thus, it was hypothesized that the emergence of the sex determination region might have been due to selective pressure arising from sex-specific floral pathogens19. In P. balsamifera, the PbRR9 gene exhibits male-biased methylation, indicating a role of epigenetic regulation in poplar sex determination20.
In willows, the sex determination locus has been consistently mapped to chromosome XV, and only the ZW sex determination system has been observed21–24. The reconstruction of alternate haplotypes in the SDR revealed sequence divergence between the Z and W chromatids22, and no homologous genes in the SDR have been found between the willow and poplar22,23. Pucholt et al.23 localized the sex determination locus to a 2.5-Mb genomic region in S. viminalis that harbors 48 protein-coding genes. Further study showed that the SDR in S. viminalis is of limited size (~804 kb) and exhibits a higher SNP density in females25. Pseudogenization and the accumulation of repetitive elements in the SDR suggest that the fundamental process of sex chromosome formation occurred very swiftly after recombination ceased11. In a recent study, the SDR of S. purpurea was found to contain large palindromic repeats, and the SpRR9 gene was considered a putative candidate for controlling sex determination through the modulation of the cytokinin signaling pathway26. Whether willow exhibits a relatively conserved sex determination system needs to be explored in more willow species.
S. triandra is a shrub willow belonging to section Amygdalinae in genus Salix. It is distributed widely from Japan to western Europe27. More recently, S. triandra has received attention because of its potential implications in insect resistance28,29. Due to the reproductive efficiency, easy cultivation, and small genome, S. triandra is suitable for obtaining additional information to better understand sex determination in dioecious plants. In this study, S. triandra is used to provide new evidence of the sex determination mechanism in willow. Our purpose is to clarify whether the previously reported willow sex determination system also functions in a willow species belonging to a different section of genus Salix.
Materials and methods
Plant materials and DNA extraction
The mapping population, which consisted of 152 full-sib F1 progenies, was established in 2013 by crossing the S. triandra female clone “DB447” with the male clone “DB134”. “DB447” and “DB134” were sampled from the Maoer Mountain in Heilongjiang Province of China (permissions were granted by the local administration). The parental clones and progeny were maintained at the Baima Forest Farm in Lishui in Jiangsu Province, China. Genomic DNA was extracted from the young leaves of each individual by using an E.Z.N.A. Plant DNA Kit (Omega Bio-tek, Norcross, GA, USA). DNA quality was assessed by 1% agarose gel electrophoresis, and the DNA concentration was measured with a Nanodrop 2000 (Thermo Scientific, MA, USA).
Library construction and sequencing
The whole-genome sequencing (WGS) was conducted with the two crossing parents, and restriction site-associated DNA (RAD) sequencing was performed for 152 progenies of the mapping population. For the crossing parents, two paired-end libraries with 300–500 bp insert sizes were constructed separately according to the standard protocol of Illumina (Illumina). For each progeny, the RAD library was prepared following the method described by Baird et al.30 with minor modifications. Briefly, 300 ng of genomic DNA from each progeny was digested separately by using 5 U of Tap I (Takara Bio, Japan) at 37 °C for 60 min, and then the P1 adapter, which contained a forward primer site, an Illumina sequencing primer site and a barcode (4–8 bp), was ligated to the fragments. Subsequently, the P1-ligated fragments of all samples (1 µL each) were pooled and then randomly sheared (Bioruptor) to an average size of 500 bp.
The entire sample was separated using 1% agarose gel electrophoresis, and the DNA fraction corresponding to 300–700 bp was isolated using an E.Z.N.A. Gel Extraction Kit (Omega Bio-tek, USA). The purified fragments were subjected to end repair and the 3′-end addition of dATP overhangs, followed by the ligation of a P2 adapter containing a reverse primer site and an Illumina sequencing primer site. Finally, the RAD library was selectively enriched by PCR amplification with the P1-forward primer and P2-reverse primer, and the 300–700 bp amplicons were purified again with the Gel Extraction Kit (Omega Bio-tech, USA).
Both WGS and RAD sequencing were performed on the Illumina HiSeq X Ten platform (Illumina, USA) at Shanghai Major Biological Medicine Technology following the manufacturer's protocol (Illumina).
Sequence analysis and nucleotide variant identification
Raw reads were assigned to each individual based on the unique barcodes and then subjected to quality control, adapter trimming and read filtering by using FASTP (version 0.6.0, https://github.com/OpenGene/fastp). Reads that contained >40% low-quality bases (base quality value <15) or >10% N bases were discarded. Sequences shorter than 30 bp after trimming were also removed.
The resulting high-quality reads were mapped to the reference genome of S. purpurea v1.0 (DOE-JGI, http://phytozome.jgi.doe.gov/pz/portal.html#!info?alias= Org_Spurpurea) by using BWA (version 0.7.16, http://bio-bwa.sourceforge.net/) software31 with the default parameters. GATK Haplotype Caller32 was used to call nucleotide variants, including SNPs and InDels, which were further filtered according to GATK Best Practices33.
Linkage map construction
To obtain high-quality linkage maps, all the filtered genetic markers were further screened based on the following criteria: (1) average sequence depth >5× in the parents and >4× in the progeny; (2) heterozygous as least in one parent; (3) present in ≥70% progeny; and (4) following a Mendelian segregation ratio. Markers with significant segregation distortion (χ2 test, P < 0.05) were excluded from linkage map construction.
The integrated linkage analysis was performed by using JoinMap 5.0 (https://www.kyazma.nl/index.php/JoinMap/), and a logarithm of odds (LOD) score threshold of 4.0 was employed to establish linkage groups (LGs). The female and male maps were constructed with a two-way pseudo-testcross strategy. The LGs were nominated according to the alignment of the mapped markers with the S. purpurea v1.0. genome assembly. The genetic distance between markers was estimated using the Kosambi mapping function34. The marker distribution in each LG was analyzed using the sliding window (10 cM) approach35. The quality of the genetic map was assessed using a haplotype map and a heat map36.
Linkage analysis of the sex locus
The sex of the plants was visually recorded for the 152 progenies. Among these progenies, 77 were female and 75 were male. The phenotypic data were included in the data matrix of each parent and scored as a testcross marker. Based on the established genetic maps, the sex locus was mapped as a segregating morphological marker with MapMaker software (version 3.0). To verify the accuracy of the positioning interval, we designed SSR markers with a physical distance of 4 Mb upstream and downstream from two SNP markers that were completely linked with sex.
Results
DNA sequencing data
For each parent, WGS yielded 9.65 Gb of clean sequencing data on average, and the sequencing depth was ~20× genome coverage (Fig. 1). After quality control, a total of 136.96 M clean reads were obtained from the two parents, with an average Q30 ratio of 90.03% and an average guanine-cytosine (GC) content of 37.25% (Table 1). For the 152 F1 offspring, a total of 504.21 Gb of clean data were generated, including 3622.24 M of high-quality clean reads with a length of 150 bp (Table 1). The number of the clean reads ranged from 11.03 to 53.06 M among different offspring, with an average of 23.83 M. The average sequencing depth for each progeny was approximately 7.53×, varying from 4.06× to 16.78× (Fig. 1). The average Q30 ratio was 88.88%, and the GC content was 41.58% (Table 1).
Sequencing depth of each sample

| Sample . | Raw data (Gb) . | Clean data (Gb) . | Clean reads (M) . | Clean data percentage (%) . | Q30 (%) . | GC (%) . |
|---|---|---|---|---|---|---|
| DB134 | 10.94 | 9.90 | 70.07 | 90.49 | 90.43 | 36.40 |
| DB447 | 10.49 | 9.41 | 66.89 | 89.70 | 89.63 | 38.10 |
| Progeny | 603.69 | 504.21 | 3622.24 | 83.52 | 88.88 | 41.58 |
| Total | 625.12 | 523.52 | 3759.20 | — | — | — |
| Sample . | Raw data (Gb) . | Clean data (Gb) . | Clean reads (M) . | Clean data percentage (%) . | Q30 (%) . | GC (%) . |
|---|---|---|---|---|---|---|
| DB134 | 10.94 | 9.90 | 70.07 | 90.49 | 90.43 | 36.40 |
| DB447 | 10.49 | 9.41 | 66.89 | 89.70 | 89.63 | 38.10 |
| Progeny | 603.69 | 504.21 | 3622.24 | 83.52 | 88.88 | 41.58 |
| Total | 625.12 | 523.52 | 3759.20 | — | — | — |
| Sample . | Raw data (Gb) . | Clean data (Gb) . | Clean reads (M) . | Clean data percentage (%) . | Q30 (%) . | GC (%) . |
|---|---|---|---|---|---|---|
| DB134 | 10.94 | 9.90 | 70.07 | 90.49 | 90.43 | 36.40 |
| DB447 | 10.49 | 9.41 | 66.89 | 89.70 | 89.63 | 38.10 |
| Progeny | 603.69 | 504.21 | 3622.24 | 83.52 | 88.88 | 41.58 |
| Total | 625.12 | 523.52 | 3759.20 | — | — | — |
| Sample . | Raw data (Gb) . | Clean data (Gb) . | Clean reads (M) . | Clean data percentage (%) . | Q30 (%) . | GC (%) . |
|---|---|---|---|---|---|---|
| DB134 | 10.94 | 9.90 | 70.07 | 90.49 | 90.43 | 36.40 |
| DB447 | 10.49 | 9.41 | 66.89 | 89.70 | 89.63 | 38.10 |
| Progeny | 603.69 | 504.21 | 3622.24 | 83.52 | 88.88 | 41.58 |
| Total | 625.12 | 523.52 | 3759.20 | — | — | — |
Nucleotide variant discovery and genotyping
The high-quality reads obtained from all samples were separately mapped to the reference genome of S. purpurea v1.0, and the mapped ratio ranged from 46.89% to 88.04%, with an average of 80.34% (Supplementary Table S1). All mapped reads were used for SNP calling, and a total of 1,150,885 putative nucleotide variant loci were detected in both parents. Based on genotyping information and the stringent filtering criteria described in the ‘Materials and methods' section, 22,830 high-quality markers were retained from the whole F1 population, including 20,695 SNPs and 2135 InDels (Table 2). Among these markers, 9188 (8301 SNPs and 887 InDels) were only maternally informative (nn×np), 9089 (8297 SNPs and 792 InDels) were only paternally informative (lm×ll), and the remaining 4553 (4097 SNPs and 456 InDels) were intercrossing markers in both parents (Table 2).
Summary of the marker types and numbers of markers used for genetic map construction
| Marker type . | SNP number . | InDel number . |
|---|---|---|
| ef×eg (1:1:1:1) | 6 | 2 |
| hk×hk (1:2:1) | 4091 | 454 |
| lm×ll (1:1) | 8297 | 792 |
| nn×np (1:1) | 8301 | 887 |
| Total | 20,695 | 2135 |
| Marker type . | SNP number . | InDel number . |
|---|---|---|
| ef×eg (1:1:1:1) | 6 | 2 |
| hk×hk (1:2:1) | 4091 | 454 |
| lm×ll (1:1) | 8297 | 792 |
| nn×np (1:1) | 8301 | 887 |
| Total | 20,695 | 2135 |
Note: The ‘lm×ll’ and ‘nn×np’ segregation types represent markers that are heterozygous only in the paternal or maternal parent, respectively.
Summary of the marker types and numbers of markers used for genetic map construction
| Marker type . | SNP number . | InDel number . |
|---|---|---|
| ef×eg (1:1:1:1) | 6 | 2 |
| hk×hk (1:2:1) | 4091 | 454 |
| lm×ll (1:1) | 8297 | 792 |
| nn×np (1:1) | 8301 | 887 |
| Total | 20,695 | 2135 |
| Marker type . | SNP number . | InDel number . |
|---|---|---|
| ef×eg (1:1:1:1) | 6 | 2 |
| hk×hk (1:2:1) | 4091 | 454 |
| lm×ll (1:1) | 8297 | 792 |
| nn×np (1:1) | 8301 | 887 |
| Total | 20,695 | 2135 |
Note: The ‘lm×ll’ and ‘nn×np’ segregation types represent markers that are heterozygous only in the paternal or maternal parent, respectively.
Construction and evaluation of the high-density linkage map
We constructed the paternal, maternal and consensus maps for S. triandra separately. The maternal map was 2193.78 cM in length, with LG sizes ranging from 91.25 cM (LG13) to 141.64 cM (LG14) (Table 3). The paternal map was 2381.93 cM in length, with LG sizes varying from 90.34 cM (LG17) to 148.70 cM (LG1) (Table 3).
| Maternal map . | Paternal map . | Consensus map . | ||||||
|---|---|---|---|---|---|---|---|---|
| Group . | Total marker . | Total distance (cM) . | Group . | Total marker . | Total distance (cM) . | Group . | Total marker . | Total distance (cM) . |
| FLG1 | 837 | 118.00 | MLG1 | 805 | 148.70 | LG1 | 1355 | 143.26 |
| FLG2 | 788 | 132.74 | MLG2 | 882 | 124.02 | LG2 | 1402 | 142.75 |
| FLG3 | 725 | 136.62 | MLG3 | 713 | 137.28 | LG3 | 1183 | 103.54 |
| FLG4 | 481 | 101.33 | MLG4 | 549 | 136.98 | LG4 | 869 | 142.71 |
| FLG5 | 782 | 128.83 | MLG5 | 793 | 104.40 | LG5 | 1296 | 110.57 |
| FLG6 | 872 | 129.78 | MLG6 | 794 | 125.72 | LG6 | 1387 | 96.87 |
| FLG7 | 602 | 138.12 | MLG7 | 618 | 140.52 | LG7 | 998 | 102.74 |
| FLG8 | 729 | 113.96 | MLG8 | 667 | 136.27 | LG8 | 1164 | 140.66 |
| FLG9 | 524 | 91.60 | MLG9 | 576 | 125.62 | LG9 | 912 | 98.54 |
| FLG10 | 775 | 91.70 | MLG10 | 827 | 107.81 | LG10 | 1377 | 101.10 |
| FLG11 | 800 | 129.95 | MLG11 | 827 | 128.47 | LG11 | 1353 | 116.95 |
| FLG12 | 512 | 127.17 | MLG12 | 612 | 103.98 | LG12 | 967 | 97.31 |
| FLG13 | 770 | 91.25 | MLG13 | 719 | 117.28 | LG13 | 1231 | 129.91 |
| FLG14 | 491 | 141.64 | MLG14 | 527 | 123.03 | LG14 | 874 | 145.29 |
| FLG15 | 810 | 105.68 | MLG15 | 713 | 133.58 | LG15 | 1269 | 125.36 |
| FLG16 | 1401 | 110.86 | MLG16 | 1432 | 123.74 | LG16 | 2314 | 144.95 |
| FLG17 | 702 | 105.81 | MLG17 | 725 | 90.34 | LG17 | 1170 | 139.85 |
| FLG18 | 667 | 96.65 | MLG18 | 580 | 132.16 | LG18 | 1094 | 120.19 |
| FLG19 | 374 | 102.09 | MLG19 | 382 | 142.02 | LG19 | 615 | 137.16 |
| Total | 13,642 | 2193.78 | Total | 13,741 | 2381.93 | Total | 22,830 | 2239.71 |
| Maternal map . | Paternal map . | Consensus map . | ||||||
|---|---|---|---|---|---|---|---|---|
| Group . | Total marker . | Total distance (cM) . | Group . | Total marker . | Total distance (cM) . | Group . | Total marker . | Total distance (cM) . |
| FLG1 | 837 | 118.00 | MLG1 | 805 | 148.70 | LG1 | 1355 | 143.26 |
| FLG2 | 788 | 132.74 | MLG2 | 882 | 124.02 | LG2 | 1402 | 142.75 |
| FLG3 | 725 | 136.62 | MLG3 | 713 | 137.28 | LG3 | 1183 | 103.54 |
| FLG4 | 481 | 101.33 | MLG4 | 549 | 136.98 | LG4 | 869 | 142.71 |
| FLG5 | 782 | 128.83 | MLG5 | 793 | 104.40 | LG5 | 1296 | 110.57 |
| FLG6 | 872 | 129.78 | MLG6 | 794 | 125.72 | LG6 | 1387 | 96.87 |
| FLG7 | 602 | 138.12 | MLG7 | 618 | 140.52 | LG7 | 998 | 102.74 |
| FLG8 | 729 | 113.96 | MLG8 | 667 | 136.27 | LG8 | 1164 | 140.66 |
| FLG9 | 524 | 91.60 | MLG9 | 576 | 125.62 | LG9 | 912 | 98.54 |
| FLG10 | 775 | 91.70 | MLG10 | 827 | 107.81 | LG10 | 1377 | 101.10 |
| FLG11 | 800 | 129.95 | MLG11 | 827 | 128.47 | LG11 | 1353 | 116.95 |
| FLG12 | 512 | 127.17 | MLG12 | 612 | 103.98 | LG12 | 967 | 97.31 |
| FLG13 | 770 | 91.25 | MLG13 | 719 | 117.28 | LG13 | 1231 | 129.91 |
| FLG14 | 491 | 141.64 | MLG14 | 527 | 123.03 | LG14 | 874 | 145.29 |
| FLG15 | 810 | 105.68 | MLG15 | 713 | 133.58 | LG15 | 1269 | 125.36 |
| FLG16 | 1401 | 110.86 | MLG16 | 1432 | 123.74 | LG16 | 2314 | 144.95 |
| FLG17 | 702 | 105.81 | MLG17 | 725 | 90.34 | LG17 | 1170 | 139.85 |
| FLG18 | 667 | 96.65 | MLG18 | 580 | 132.16 | LG18 | 1094 | 120.19 |
| FLG19 | 374 | 102.09 | MLG19 | 382 | 142.02 | LG19 | 615 | 137.16 |
| Total | 13,642 | 2193.78 | Total | 13,741 | 2381.93 | Total | 22,830 | 2239.71 |
| Maternal map . | Paternal map . | Consensus map . | ||||||
|---|---|---|---|---|---|---|---|---|
| Group . | Total marker . | Total distance (cM) . | Group . | Total marker . | Total distance (cM) . | Group . | Total marker . | Total distance (cM) . |
| FLG1 | 837 | 118.00 | MLG1 | 805 | 148.70 | LG1 | 1355 | 143.26 |
| FLG2 | 788 | 132.74 | MLG2 | 882 | 124.02 | LG2 | 1402 | 142.75 |
| FLG3 | 725 | 136.62 | MLG3 | 713 | 137.28 | LG3 | 1183 | 103.54 |
| FLG4 | 481 | 101.33 | MLG4 | 549 | 136.98 | LG4 | 869 | 142.71 |
| FLG5 | 782 | 128.83 | MLG5 | 793 | 104.40 | LG5 | 1296 | 110.57 |
| FLG6 | 872 | 129.78 | MLG6 | 794 | 125.72 | LG6 | 1387 | 96.87 |
| FLG7 | 602 | 138.12 | MLG7 | 618 | 140.52 | LG7 | 998 | 102.74 |
| FLG8 | 729 | 113.96 | MLG8 | 667 | 136.27 | LG8 | 1164 | 140.66 |
| FLG9 | 524 | 91.60 | MLG9 | 576 | 125.62 | LG9 | 912 | 98.54 |
| FLG10 | 775 | 91.70 | MLG10 | 827 | 107.81 | LG10 | 1377 | 101.10 |
| FLG11 | 800 | 129.95 | MLG11 | 827 | 128.47 | LG11 | 1353 | 116.95 |
| FLG12 | 512 | 127.17 | MLG12 | 612 | 103.98 | LG12 | 967 | 97.31 |
| FLG13 | 770 | 91.25 | MLG13 | 719 | 117.28 | LG13 | 1231 | 129.91 |
| FLG14 | 491 | 141.64 | MLG14 | 527 | 123.03 | LG14 | 874 | 145.29 |
| FLG15 | 810 | 105.68 | MLG15 | 713 | 133.58 | LG15 | 1269 | 125.36 |
| FLG16 | 1401 | 110.86 | MLG16 | 1432 | 123.74 | LG16 | 2314 | 144.95 |
| FLG17 | 702 | 105.81 | MLG17 | 725 | 90.34 | LG17 | 1170 | 139.85 |
| FLG18 | 667 | 96.65 | MLG18 | 580 | 132.16 | LG18 | 1094 | 120.19 |
| FLG19 | 374 | 102.09 | MLG19 | 382 | 142.02 | LG19 | 615 | 137.16 |
| Total | 13,642 | 2193.78 | Total | 13,741 | 2381.93 | Total | 22,830 | 2239.71 |
| Maternal map . | Paternal map . | Consensus map . | ||||||
|---|---|---|---|---|---|---|---|---|
| Group . | Total marker . | Total distance (cM) . | Group . | Total marker . | Total distance (cM) . | Group . | Total marker . | Total distance (cM) . |
| FLG1 | 837 | 118.00 | MLG1 | 805 | 148.70 | LG1 | 1355 | 143.26 |
| FLG2 | 788 | 132.74 | MLG2 | 882 | 124.02 | LG2 | 1402 | 142.75 |
| FLG3 | 725 | 136.62 | MLG3 | 713 | 137.28 | LG3 | 1183 | 103.54 |
| FLG4 | 481 | 101.33 | MLG4 | 549 | 136.98 | LG4 | 869 | 142.71 |
| FLG5 | 782 | 128.83 | MLG5 | 793 | 104.40 | LG5 | 1296 | 110.57 |
| FLG6 | 872 | 129.78 | MLG6 | 794 | 125.72 | LG6 | 1387 | 96.87 |
| FLG7 | 602 | 138.12 | MLG7 | 618 | 140.52 | LG7 | 998 | 102.74 |
| FLG8 | 729 | 113.96 | MLG8 | 667 | 136.27 | LG8 | 1164 | 140.66 |
| FLG9 | 524 | 91.60 | MLG9 | 576 | 125.62 | LG9 | 912 | 98.54 |
| FLG10 | 775 | 91.70 | MLG10 | 827 | 107.81 | LG10 | 1377 | 101.10 |
| FLG11 | 800 | 129.95 | MLG11 | 827 | 128.47 | LG11 | 1353 | 116.95 |
| FLG12 | 512 | 127.17 | MLG12 | 612 | 103.98 | LG12 | 967 | 97.31 |
| FLG13 | 770 | 91.25 | MLG13 | 719 | 117.28 | LG13 | 1231 | 129.91 |
| FLG14 | 491 | 141.64 | MLG14 | 527 | 123.03 | LG14 | 874 | 145.29 |
| FLG15 | 810 | 105.68 | MLG15 | 713 | 133.58 | LG15 | 1269 | 125.36 |
| FLG16 | 1401 | 110.86 | MLG16 | 1432 | 123.74 | LG16 | 2314 | 144.95 |
| FLG17 | 702 | 105.81 | MLG17 | 725 | 90.34 | LG17 | 1170 | 139.85 |
| FLG18 | 667 | 96.65 | MLG18 | 580 | 132.16 | LG18 | 1094 | 120.19 |
| FLG19 | 374 | 102.09 | MLG19 | 382 | 142.02 | LG19 | 615 | 137.16 |
| Total | 13,642 | 2193.78 | Total | 13,741 | 2381.93 | Total | 22,830 | 2239.71 |
All 22,830 markers that segregated as ef×eg, hk×hk, lm×ll or nn×np were used to generate a consensus map for S. triandra. At the LOD threshold of 4.0, all of these markers were successfully grouped into 19 LGs (Fig. 2 and Fig. S1). The number of LGs was consistent with the haploid chromosome number of willows (2n = 38). The established consensus map covered a genetic distance of 2239.71 cM, with LG sizes varying from 96.87 cM (LG6) to 145.29 cM (LG14) (Table 3). The marker distribution along each LG was evaluated by counting the number of marker bins and all mapped markers using a sliding window of 10 cM. The average number of marker bins ranged from 18.00 to 69.87, with the average number of mapped markers varying from 43.93 to 154.27. The window with the highest density (26.7 markers per cM) was found in LG16 (Fig. 3).
The consensus genetic map of S. triandra
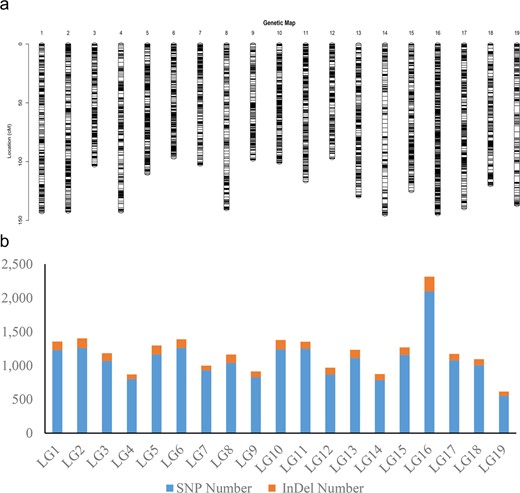
a Distribution of mapped markers within each linkage group of S. triandra. A black line indicates a SNP/InDel marker. b The number of SNPs and InDels in each linkage group
Marker distribution analysis of the consensus map
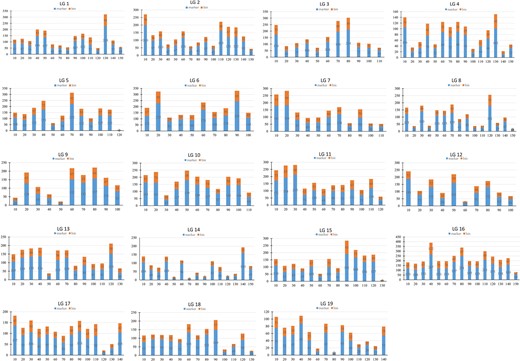
The numbers of markers and marker bins are indicated with blue and yellow bars, respectively
Haplotype maps, which revealed the missing data and recombination events of each individual intuitively, were generated for each LG in the 152 offspring (Supplementary Fig. S2). The percentages of missing data and double crossovers were less than 0.30% and 0.23%, respectively. Based on the pairwise recombination values of the markers grouped in each LG, heat maps were generated for the 19 LGs (Supplementary Fig. S3). All the heat maps demonstrated a clear trend in which the pairwise linkage generally decreased with an increase in genetic distance between the mapped markers, indicating that the markers in each LG were precisely mapped and ordered.
Collinearity between the genetic map and reference genome
All the mapped markers were aligned to the S. purpurea v1.0 genome to estimate the physical distances of the markers and to assess the collinearity between the genetic map and reference genome. In general, high collinearity was observed between the markers and the corresponding chromosomes (Fig. 4), with the Spearman rank correlation coefficient ranging from 0.99–1.00. However, there were also LGs showing discrepancies in some narrow regions (e.g., LGs 14, 18 and 19), which might have been due to different recombination rates, missing data or compromised marker orders in the consensus map.
Collinearity analysis between the integrated S. triandra genetic map and the S. purpurea v1.0. genome
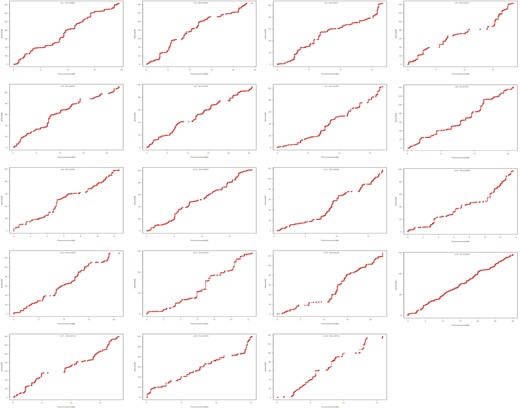
The marker position in the genome is shown on the x-axis; the marker position in each linkage group is shown on the y-axis
The mapped markers in the integrated genetic map covered at least 96.95% of the physical length of the reference genome (Table 4). The genetic-to-physical distance ratios ranged from 3.78 cM/Mb (LG6) to 11.65 cM/Mb (LG14) (Table 4). The marker density along each chromosome ranged from 28.81 to 70.64 markers/Mb, averaging 49.18 markers/Mb (Table 4).
Statistics of the collinearity analysis between the integrated S. triandra genetic map and the S. purpurea v1.0. genome
| LG . | Physical coverage (%) . | Genetic distance/physical distance (cM/Mb) . | Density (markers/Mb) . | Spearman correlation . |
|---|---|---|---|---|
| 1 | 99.54 | 7.35 | 55.10 | 0.99 |
| 2 | 99.34 | 5.91 | 65.73 | 1.00 |
| 3 | 99.85 | 6.19 | 57.08 | 0.99 |
| 4 | 99.72 | 7.67 | 42.89 | 0.99 |
| 5 | 98.75 | 4.93 | 33.47 | 1.00 |
| 6 | 99.63 | 3.78 | 28.81 | 1.00 |
| 7 | 99.78 | 6.03 | 35.61 | 1.00 |
| 8 | 98.98 | 8.94 | 53.54 | 0.99 |
| 9 | 99.21 | 7.83 | 40.35 | 0.99 |
| 10 | 99.62 | 5.32 | 46.90 | 1.00 |
| 11 | 96.95 | 6.95 | 65.70 | 1.00 |
| 12 | 99.38 | 7.12 | 51.26 | 0.99 |
| 13 | 99.87 | 6.18 | 42.68 | 0.99 |
| 14 | 99.75 | 11.65 | 60.42 | 0.99 |
| 15 | 99.91 | 5.55 | 42.30 | 1.00 |
| 16 | 99.96 | 4.81 | 70.64 | 1.00 |
| 17 | 99.11 | 7.73 | 58.53 | 0.99 |
| 18 | 99.62 | 7.93 | 52.38 | 0.99 |
| 19 | 99.76 | 8.66 | 31.05 | 0.99 |
| Mean | 99.41 | 6.87 | 49.18 | 0.99 |
| LG . | Physical coverage (%) . | Genetic distance/physical distance (cM/Mb) . | Density (markers/Mb) . | Spearman correlation . |
|---|---|---|---|---|
| 1 | 99.54 | 7.35 | 55.10 | 0.99 |
| 2 | 99.34 | 5.91 | 65.73 | 1.00 |
| 3 | 99.85 | 6.19 | 57.08 | 0.99 |
| 4 | 99.72 | 7.67 | 42.89 | 0.99 |
| 5 | 98.75 | 4.93 | 33.47 | 1.00 |
| 6 | 99.63 | 3.78 | 28.81 | 1.00 |
| 7 | 99.78 | 6.03 | 35.61 | 1.00 |
| 8 | 98.98 | 8.94 | 53.54 | 0.99 |
| 9 | 99.21 | 7.83 | 40.35 | 0.99 |
| 10 | 99.62 | 5.32 | 46.90 | 1.00 |
| 11 | 96.95 | 6.95 | 65.70 | 1.00 |
| 12 | 99.38 | 7.12 | 51.26 | 0.99 |
| 13 | 99.87 | 6.18 | 42.68 | 0.99 |
| 14 | 99.75 | 11.65 | 60.42 | 0.99 |
| 15 | 99.91 | 5.55 | 42.30 | 1.00 |
| 16 | 99.96 | 4.81 | 70.64 | 1.00 |
| 17 | 99.11 | 7.73 | 58.53 | 0.99 |
| 18 | 99.62 | 7.93 | 52.38 | 0.99 |
| 19 | 99.76 | 8.66 | 31.05 | 0.99 |
| Mean | 99.41 | 6.87 | 49.18 | 0.99 |
Statistics of the collinearity analysis between the integrated S. triandra genetic map and the S. purpurea v1.0. genome
| LG . | Physical coverage (%) . | Genetic distance/physical distance (cM/Mb) . | Density (markers/Mb) . | Spearman correlation . |
|---|---|---|---|---|
| 1 | 99.54 | 7.35 | 55.10 | 0.99 |
| 2 | 99.34 | 5.91 | 65.73 | 1.00 |
| 3 | 99.85 | 6.19 | 57.08 | 0.99 |
| 4 | 99.72 | 7.67 | 42.89 | 0.99 |
| 5 | 98.75 | 4.93 | 33.47 | 1.00 |
| 6 | 99.63 | 3.78 | 28.81 | 1.00 |
| 7 | 99.78 | 6.03 | 35.61 | 1.00 |
| 8 | 98.98 | 8.94 | 53.54 | 0.99 |
| 9 | 99.21 | 7.83 | 40.35 | 0.99 |
| 10 | 99.62 | 5.32 | 46.90 | 1.00 |
| 11 | 96.95 | 6.95 | 65.70 | 1.00 |
| 12 | 99.38 | 7.12 | 51.26 | 0.99 |
| 13 | 99.87 | 6.18 | 42.68 | 0.99 |
| 14 | 99.75 | 11.65 | 60.42 | 0.99 |
| 15 | 99.91 | 5.55 | 42.30 | 1.00 |
| 16 | 99.96 | 4.81 | 70.64 | 1.00 |
| 17 | 99.11 | 7.73 | 58.53 | 0.99 |
| 18 | 99.62 | 7.93 | 52.38 | 0.99 |
| 19 | 99.76 | 8.66 | 31.05 | 0.99 |
| Mean | 99.41 | 6.87 | 49.18 | 0.99 |
| LG . | Physical coverage (%) . | Genetic distance/physical distance (cM/Mb) . | Density (markers/Mb) . | Spearman correlation . |
|---|---|---|---|---|
| 1 | 99.54 | 7.35 | 55.10 | 0.99 |
| 2 | 99.34 | 5.91 | 65.73 | 1.00 |
| 3 | 99.85 | 6.19 | 57.08 | 0.99 |
| 4 | 99.72 | 7.67 | 42.89 | 0.99 |
| 5 | 98.75 | 4.93 | 33.47 | 1.00 |
| 6 | 99.63 | 3.78 | 28.81 | 1.00 |
| 7 | 99.78 | 6.03 | 35.61 | 1.00 |
| 8 | 98.98 | 8.94 | 53.54 | 0.99 |
| 9 | 99.21 | 7.83 | 40.35 | 0.99 |
| 10 | 99.62 | 5.32 | 46.90 | 1.00 |
| 11 | 96.95 | 6.95 | 65.70 | 1.00 |
| 12 | 99.38 | 7.12 | 51.26 | 0.99 |
| 13 | 99.87 | 6.18 | 42.68 | 0.99 |
| 14 | 99.75 | 11.65 | 60.42 | 0.99 |
| 15 | 99.91 | 5.55 | 42.30 | 1.00 |
| 16 | 99.96 | 4.81 | 70.64 | 1.00 |
| 17 | 99.11 | 7.73 | 58.53 | 0.99 |
| 18 | 99.62 | 7.93 | 52.38 | 0.99 |
| 19 | 99.76 | 8.66 | 31.05 | 0.99 |
| Mean | 99.41 | 6.87 | 49.18 | 0.99 |
Mapping the sex locus and gene content in the confined genetic interval
The mapping results showed that the sex locus could only be mapped in the maternal map 27.07 cM from the telemetric end of chromosome XV (Fig. 5a), and no linkage with sex was detected in the paternal map. Using the sequences of the SNP markers co-segregating with sex, we obtained genome sequences in the confined genetic interval and developed upstream and downstream sex-linked simple sequence repeat (SSR) markers. In total, seven SSR markers co-segregating with the sex locus were generated in the SDR on chromosome XV of the female (Fig. 5c), and the confined interval encompassing the sex locus (IESL) was bounded by SSR markers wssr304 and wssr470, with spacing of 5.9 cM (Fig. 5b). Based on the reference genome of S. purpurea, the confined IESL corresponded to a 6.5-Mb genomic region on chromosome 15. On average, a 1 cM genetic length contains a 140 kb sequence in the willow genome. Thus, the recombination rate in the confined IESL of the female is approximately eight-fold lower than the genome-wide average.
Locating the sex locus of S. triandra

a Linkage analysis mapping the sex locus of S. triandra within LGXV of the female map. b Fine positioning of the SSR markers in the vicinity of the sex locus in S. triandra. c The physical positions of SSR markers in the confined IESL
The target region harbored 249 genes. Gene Ontology (GO) terms clarified that genes involved in metabolic processes, cellular processes, single organism processes, reproductive processes and biological organization were the most represented groups (Fig. 6). Six genes were associated with microtubule motor activity (GO:0003777), and six genes were associated with microtubule binding activity (GO:0008017). EVM0038350 has a methyltransferase domain that may be related to DNA methylation. Salix_newGene_2 is a homologous gene of LRR receptor-like serine/threonine-protein kinase ERL1 in Arabidopsis thaliana, which is important for anther lobe formation. The EVM0005130 gene contains a Myb-like DNA-binding domain that plays an important role in regulating anther and pollen development; EVM0045351 contains a mitogen-activated protein kinase (MAP kinase) domain that may function as a regulator of pollen development and germination.
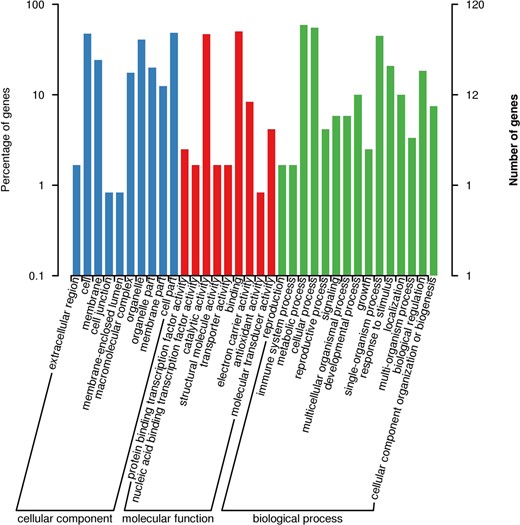
Numbers of willow unigenes in each functional category in the confined IESL in S. triandra
In addition to the genes involved in flower organ development, flower development-associated miR156 was also found in the confined IESL. miR156 has emerged as the most important regulator in the vegetative phase change and the vegetative-to-reproductive transition in both Arabidopsis and maize. The candidate genes related to flower development are listed in Table 5.
Classification and function of important genes in the confined IESL in chromosome XV of S. triandra
| Gene ID . | Start . | End . | Strand . | Function distribution annotation . |
|---|---|---|---|---|
| EVM0002598 | 12,380,227 | 12,385,512 | − | Microtubule motor activity |
| EVM0006532 | 13,615,059 | 13,624,340 | − | Microtubule motor activity |
| EVM0048283 | 13,638,084 | 13,638,395 | − | Microtubule motor activity |
| Salix_newGene_14 | 14,399,846 | 14,402,709 | + | Microtubule motor activity |
| Salix_newGene_6 | 13,351,098 | 13,354,415 | + | Microtubule motor activity |
| Salix_newGene_7 | 13,354,476 | 13,357,328 | + | Microtubule motor activity |
| EVM0038350 | 12,867,149 | 12,869,132 | + | Methyltransferase activity |
| EVM0005130 | 13,655,785 | 13,658,118 | − | Myb-like DNA-binding domain |
| Salix_newGene_28 | 14,013,632 | 14,016,788 | − | RNA-directed RNA polymerase activity |
| EVM0008632 | 13,406,181 | 13,414,288 | − | RNA processing and modification |
| EVM0045351 | 11,991,112 | 12,004,061 | + | Mitogen-activated protein kinase (MAP kinase) domain |
| Salix_newGene_2 | 12,380,227 | 12,385,512 | − | LRR receptor-like serine/threonine-protein kinase |
| Gene ID . | Start . | End . | Strand . | Function distribution annotation . |
|---|---|---|---|---|
| EVM0002598 | 12,380,227 | 12,385,512 | − | Microtubule motor activity |
| EVM0006532 | 13,615,059 | 13,624,340 | − | Microtubule motor activity |
| EVM0048283 | 13,638,084 | 13,638,395 | − | Microtubule motor activity |
| Salix_newGene_14 | 14,399,846 | 14,402,709 | + | Microtubule motor activity |
| Salix_newGene_6 | 13,351,098 | 13,354,415 | + | Microtubule motor activity |
| Salix_newGene_7 | 13,354,476 | 13,357,328 | + | Microtubule motor activity |
| EVM0038350 | 12,867,149 | 12,869,132 | + | Methyltransferase activity |
| EVM0005130 | 13,655,785 | 13,658,118 | − | Myb-like DNA-binding domain |
| Salix_newGene_28 | 14,013,632 | 14,016,788 | − | RNA-directed RNA polymerase activity |
| EVM0008632 | 13,406,181 | 13,414,288 | − | RNA processing and modification |
| EVM0045351 | 11,991,112 | 12,004,061 | + | Mitogen-activated protein kinase (MAP kinase) domain |
| Salix_newGene_2 | 12,380,227 | 12,385,512 | − | LRR receptor-like serine/threonine-protein kinase |
Classification and function of important genes in the confined IESL in chromosome XV of S. triandra
| Gene ID . | Start . | End . | Strand . | Function distribution annotation . |
|---|---|---|---|---|
| EVM0002598 | 12,380,227 | 12,385,512 | − | Microtubule motor activity |
| EVM0006532 | 13,615,059 | 13,624,340 | − | Microtubule motor activity |
| EVM0048283 | 13,638,084 | 13,638,395 | − | Microtubule motor activity |
| Salix_newGene_14 | 14,399,846 | 14,402,709 | + | Microtubule motor activity |
| Salix_newGene_6 | 13,351,098 | 13,354,415 | + | Microtubule motor activity |
| Salix_newGene_7 | 13,354,476 | 13,357,328 | + | Microtubule motor activity |
| EVM0038350 | 12,867,149 | 12,869,132 | + | Methyltransferase activity |
| EVM0005130 | 13,655,785 | 13,658,118 | − | Myb-like DNA-binding domain |
| Salix_newGene_28 | 14,013,632 | 14,016,788 | − | RNA-directed RNA polymerase activity |
| EVM0008632 | 13,406,181 | 13,414,288 | − | RNA processing and modification |
| EVM0045351 | 11,991,112 | 12,004,061 | + | Mitogen-activated protein kinase (MAP kinase) domain |
| Salix_newGene_2 | 12,380,227 | 12,385,512 | − | LRR receptor-like serine/threonine-protein kinase |
| Gene ID . | Start . | End . | Strand . | Function distribution annotation . |
|---|---|---|---|---|
| EVM0002598 | 12,380,227 | 12,385,512 | − | Microtubule motor activity |
| EVM0006532 | 13,615,059 | 13,624,340 | − | Microtubule motor activity |
| EVM0048283 | 13,638,084 | 13,638,395 | − | Microtubule motor activity |
| Salix_newGene_14 | 14,399,846 | 14,402,709 | + | Microtubule motor activity |
| Salix_newGene_6 | 13,351,098 | 13,354,415 | + | Microtubule motor activity |
| Salix_newGene_7 | 13,354,476 | 13,357,328 | + | Microtubule motor activity |
| EVM0038350 | 12,867,149 | 12,869,132 | + | Methyltransferase activity |
| EVM0005130 | 13,655,785 | 13,658,118 | − | Myb-like DNA-binding domain |
| Salix_newGene_28 | 14,013,632 | 14,016,788 | − | RNA-directed RNA polymerase activity |
| EVM0008632 | 13,406,181 | 13,414,288 | − | RNA processing and modification |
| EVM0045351 | 11,991,112 | 12,004,061 | + | Mitogen-activated protein kinase (MAP kinase) domain |
| Salix_newGene_2 | 12,380,227 | 12,385,512 | − | LRR receptor-like serine/threonine-protein kinase |
Discussion
The Salicaceae family is a valuable model system for revealing the origin and evolution of plant sex chromosomes. These genera are widely distributed around the globe, representing a diverse assemblage of subtrees, shrubs37 and catkin-bearing trees. Nearly all species in genera Salix (Ca. 500 species)38 and Populus (Ca. 30 species)39 are dioecious, though obvious heteromorphic sex chromosomes have not yet evolved. Furthermore, the two lineages share a well-preserved whole-genome duplication7,8 and show an ongoing propensity toward polyploid formation40,41, which facilitates the exploration of the relationship between polyploidy and sex chromosome evolution42.
In the past decade, there have been many reports on the sex determination mechanisms of poplars. In both P. deltoides and P. nigra, which are from section Aigeiros, the SDR is located at the proximal telomeric end of chromosome XIX12,13. In P. tremuloides, P. tremula and P. alba, all of which belong to section Populus, a pericentromeric region of chromosome XIX14,15,43 was determined to be the SDR. Both female heterogamety12,15 and male heterogamety have been reported13,43. Recently, sex determination was explored in 52 P. trichocarpa (section Tacamahaca) and 34 P. balsamifera (section Populus) individuals by using the genome-wide association study. A total of 650 sex-associated SNPs were found to be heterozygous in males, indicating an XY sex determination system in these two species44. In genus Salix, the SDR is confined to the centromeric region of chromosome XV in S. viminalis (section Viminella)22,23,45 and S. suchowensis (section Helix)22,23,45. Female heterozygosis predominates in the SDRs of these species, suggesting a ZW sex determination system in Salix. Furthermore, no candidate genes in the willow SDR are orthologous to those in the poplar SDR22,23. Current research is not sufficient to demonstrate whether the sex determination mechanisms of the two lineages are related.
In this study, we sought to explore the SDR in an additional willow species, S. triandra (section Amygdalinae). In the S. triandra SDR, obvious recombination suppression was observed in the female. Recombination suppression, which means that homologous chromosomes cannot pair and undergo recombination, is an important component of sex chromosome evolution46. In both advanced and primitive sex chromosomes, the suppression of recombination in chromosomal regions is observed in numerous plant species. It has been reported that both the X and Y chromosomes of Actinidia chinensis var. chinensis exhibit a similar pattern of restricted recombination: approximately one-third of the sex chromosome (terminal ~6 Mb of chromosome XXV) spans the SDR and shows severe recombination suppression, while the remaining section undergoes normal recombination47. Recombination suppression is also observed in the SDR in papaya, and the nonrecombining region of the Y chromatid differed greatly from the corresponding region of the X chromatid due to two large inversions48. In hop (Humulus lupulus), an approximately four-fold reduction in recombination is found on the Y chromosome compared with the X chromosome linkage map49. A study on Silene alba also found that almost the entire SDR of the sex chromosome showed heavily suppressed recombination50. Chromosomal inversion, heterochromatinization, and DNA methylation may be the underlying mechanisms of recombination suppression51. Large-scale heterochromatinization and loss-of-function regions are found in the Y chromosome of sorrel52. DNA methylation may defend against the insertion of DNA repeats derived from transposons and speed up heterochromatinization in specific regions of sex determination53. The methylation and heterochromatin levels of the male-specific DNA region of the Y chromosome are higher than those of the corresponding region of the X chromosome in Papaya53. The reduced recombination in sex chromosomes leads to the differentiation of their structure and function; male- or female-specific sequences accumulate in chromosomes, leading to a high degree of degeneration in sex chromosomes51,54. After a long period of evolutionary accumulation, autosomes may ultimately evolve into morphologically and functionally different sex chromosomes. In this study, we performed fine local mapping with SSR markers designed with sequences from the confined IESL of S. triandra, and severe recombination repression in the SDR was observed between the two sexes. Similar features are observed in S. suchowensis22, S. viminalis23 and S. purpurea55.
Dioecy has evolved hundreds of times from a hermaphroditic ancestor, and different genes may be involved in this process56,57, leading to great challenges in identifying the particular sex determination genes of different taxa of plants. The identification of sex-determining genes is of great significance to reveal the mechanism underlying sex determination in flowering plants. Therefore, genes involved in floral development located in the SDR are good putative candidates. Akagi et al.58 found that the SyGI gene, located in the Y-specific region, was involved in carpel development. In Diospyros lotus (XY system), the only identified sex-determining gene, OGI, which is located in a male-specific region, encodes 21-bp small RNAs targeting the autosomal gene MeGI, which acts as a maleness suppressor59.
In this study, a single SDR was physically located within a physical interval of 6.5 Mb in chromosome XV in S. triandra, which shows clear female heterogamety. This observation is consistent with findings in other willow species22,23. However, the estimated size of the SDR should not be considered definitive, due to the limited resolution of the genetic maps. In the confined IESL of S. triandra, six genes exhibit the molecular function of microtubule motor activity, which is involved in male reproductive development and function60. Similar gene groups are found in the S. purpurea SDR55. Another gene, EVM0005130, which contains a Myb-like DNA-binding domain, deserves special attention because it plays an important role in regulating anther and pollen development61. We also detected the interesting gene EVM0045351, containing a mitogen-activated protein kinase (MAP kinase) domain, which has been proposed to function as a regulator of both pollen development and germination62. Increasing numbers of studies have demonstrated that miRNAs play critical roles in regulating plant growth and stress responses as well as plant reproductive development. miRNA156 and 159, which are related to plant flower development, have been identified in the SDR.
In conclusion, the present study developed high-density linkage maps for S. triandra. The mapping of the sex locus revealed female heterogamety, indicating that sex in this willow species is determined through a ZW determination system. We confined the sex determination locus of S. triandra to a 6.5 Mb genomic region that harbors 249 genes and 22 miRNAs. The region contains several promising sex determination candidates, which are worthy of special attention in future studies.
Acknowledgements
This work was funded by the National Key Research and Development Plan of China (2016YFD0600101), the National Natural Science Foundation (31800562), and the Youth Elite Science Sponsorship Program of CAST (YESS). This work was also supported by the Priority Academic Program Development Program of Jiangsu Province.
Author contributions
W.L., H.W. and Y.C. participated in all the experiments and data analyses and drafted the manuscript. X.L. established the mapping pedigree and conducted phenotyping. Y.C. and T.Y. finalized the manuscript.
Conflict of interest
The authors declare that they have no conflict of interest.
References
Author notes
These authors contributed equally: Wei Li, Huaitong Wu
Supplementary data
Table S1
Figure S1
Figure S2
Figure S3



