-
PDF
- Split View
-
Views
-
Cite
Cite
Massimo Mezzavilla, Chiara Ottavia Navarra, Roberto Di Lenarda, Paolo Gasparini, Lorenzo Bevilacqua, Antonietta Robino, Runs of homozygosity are associated with staging of periodontitis in isolated populations, Human Molecular Genetics, Volume 30, Issue 12, 15 June 2021, Pages 1154–1159, https://doi.org/10.1093/hmg/ddab085
Close - Share Icon Share
Abstract
Periodontitis is a common inflammatory disease characterized by a complex etiology, which is the result of a combination of genetic and environmental factors. Genetic variants linked to the periodontitis disease were already investigated, however, little was known regarding the severity of this disease. Recently, long runs of homozygosity (ROH) were associated with several multifactorial diseases. Therefore, in our work, we tried to assess the role of ROH and periodontitis status. We found an association between the excess of homozygosity owing to ROH and staging of periodontitis. More in detail, the total amount of homozygosity owing to ROH is positively associated with an increased severity of periodontitis (P = 0.0001). Regression tree analysis showed the impact of ROH burden in discriminating individuals with mild periodontitis stages I and II and periodontitis stages III and IV (P < 0.001). Furthermore, ROH mapping highlights several regions associated with a severe status of periodontitis (odds ratio > 1). Among them, we found a total of 33 genes. Interestingly, some of these genes were previously associated with granulocyte or platelet measures, both linked to the onset and the progression of periodontal disease. Our results suggest the not only single variants association test could help to risk assessment but even individual genomic features; furthermore, our ROH mapping highlighted the possible role of multiple genes in periodontal development.
Introduction
Periodontitis is a common inflammatory disease leading to the destruction of the supporting structures of the teeth (the periodontal ligament and alveolar bone) and eventually to the tooth loss. It has a multifactorial etiology with the involvement of a combination of genetic and environmental determinants. Although oral bacteria are necessary for the progression and clinical manifestations of the disease, each individual may have an individual response and a different susceptibility to periodontitis, supporting the role of a genetic contribution (1).
To date, genetic factors leading to susceptibility to periodontitis have been studied through candidate or genome-wide association (GWA) studies. Polymorphisms in genes coding for inflammatory mediators have been widely investigated. Moreover, other genes involved in host defense against bacteria, bone metabolism, remodeling and degradation of connective tissues have been suggested as factors that influence the risk of developing the disease (2–5). The GWA approach, scanning a multitude of genetic variants across the entire genome, has also contributed to identify novel genes or single nucleotide polymorphisms (SNPs) contributing to periodontitis or periodontitis-related phenotypes (6–11).
As for other multifactorial diseases, a large number of polymorphisms have been identified in recent years; however, generally, their effect is moderate, and the specific genetic variants that account for the majority of the heritability of periodontitis remain to be discovered. Moreover, another challenge in the genetics of periodontitis is the identification of variants underlying periodontitis severity. Therefore, additional studies are required to identify other genetic factors that influence periodontitis and its progression.
Recently, different multifactorial diseases have been tested for association with autozygosity. Autozygosity arises when two chromosomal segments that are identical from a common ancestor are inherited from each parent. It can occur from several different phenomena, such as genetic drift, consanguineous matings, population bottleneck as well as natural and artificial selection. Using high-density SNP arrays, autozygosity can be inferred using runs of homozygosity (ROH) (long stretches of homozygous SNPs). Interestingly, evidence support that ROH may be an additional type of genomic variability, and some studies have investigated the association of ROH with multifactorial diseases such as schizophrenia, depressive disorder, heart disease, osteoporosis and cancer (12–18). Also, several quantitative traits such as cognitive ability, height, educational attainment, reduced reproductive success as well as alcohol intake or smoke have been associated with ROH (19–21).
To our knowledge, no studies have been already conducted on the possible association between autozygosity and periodontitis. Therefore, in this work, we used ROHs in 602 individuals of genetically isolated populations to test their association with periodontitis severity.
Results
A total of 594 individuals were analyzed, and they were subdivided as follows: 152 healthy (healthy), 273 with mild periodontitis (mild) and 169 with severe periodontitis (severe). Average total homozygosity is significantly higher in severe individuals with respect to mild (Mann Whitney P-value = 5E-8) and healthy (Mann Whitney P-value = 2E-7). On the other hand, no significant difference was found between mild and healthy with a P-value = 0.61 for total homozygosity and P-value = 0.98 for the average number of segments.
Table 1 summarizes all the cohort characteristics.
| . | Healthy . | Mild periodontitis . | Severe periodontitis . |
|---|---|---|---|
| Male, n | 102 | 142 | 86 |
| Females, n | 50 | 131 | 83 |
| Age, mean (SD) | 37 (14) | 51 (12) | 59 (11) |
| % Smokers (% male, % female) | 22% (22%, 24%) | 22% (24%, 20%) | 19% (23%, 14%) |
| Total homozygosity in Mb, mean (SD) | 35 (40) | 37 (40) | 59 (43) |
| Number of ROH segments, mean (SD) | 7.5 (6.9) | 7.5 (6.4) | 10.7 (7.2) |
| . | Healthy . | Mild periodontitis . | Severe periodontitis . |
|---|---|---|---|
| Male, n | 102 | 142 | 86 |
| Females, n | 50 | 131 | 83 |
| Age, mean (SD) | 37 (14) | 51 (12) | 59 (11) |
| % Smokers (% male, % female) | 22% (22%, 24%) | 22% (24%, 20%) | 19% (23%, 14%) |
| Total homozygosity in Mb, mean (SD) | 35 (40) | 37 (40) | 59 (43) |
| Number of ROH segments, mean (SD) | 7.5 (6.9) | 7.5 (6.4) | 10.7 (7.2) |
Participants were classified as healthy (score = 0), mild periodontitis stages I and II (score = 3–4) and severe periodontitis stages III and IV (score = 5–6). SD, standard deviation.
| . | Healthy . | Mild periodontitis . | Severe periodontitis . |
|---|---|---|---|
| Male, n | 102 | 142 | 86 |
| Females, n | 50 | 131 | 83 |
| Age, mean (SD) | 37 (14) | 51 (12) | 59 (11) |
| % Smokers (% male, % female) | 22% (22%, 24%) | 22% (24%, 20%) | 19% (23%, 14%) |
| Total homozygosity in Mb, mean (SD) | 35 (40) | 37 (40) | 59 (43) |
| Number of ROH segments, mean (SD) | 7.5 (6.9) | 7.5 (6.4) | 10.7 (7.2) |
| . | Healthy . | Mild periodontitis . | Severe periodontitis . |
|---|---|---|---|
| Male, n | 102 | 142 | 86 |
| Females, n | 50 | 131 | 83 |
| Age, mean (SD) | 37 (14) | 51 (12) | 59 (11) |
| % Smokers (% male, % female) | 22% (22%, 24%) | 22% (24%, 20%) | 19% (23%, 14%) |
| Total homozygosity in Mb, mean (SD) | 35 (40) | 37 (40) | 59 (43) |
| Number of ROH segments, mean (SD) | 7.5 (6.9) | 7.5 (6.4) | 10.7 (7.2) |
Participants were classified as healthy (score = 0), mild periodontitis stages I and II (score = 3–4) and severe periodontitis stages III and IV (score = 5–6). SD, standard deviation.
Results of the regression tree analysis are shown in Figure 1. The binary tree revealed that ROH homozygosity interacted with age to influence periodontal severity. The tree model distinguished those individuals carrying more than 44 Mb of homozygosity owing to ROHs and being more than 54 years old, who showed a predisposition for severe periodontitis, from individuals carrying less than 44 Mb of homozygosity, who showed a predisposition for mild periodontitis. This cut-off of 44 Mb for ROH homozygosity was obtained from the regression tree analysis. Individuals with more than 44 Mb of homozygosity fall over the 57th percentile of the population distribution (Supplementary Material, Fig. S1).
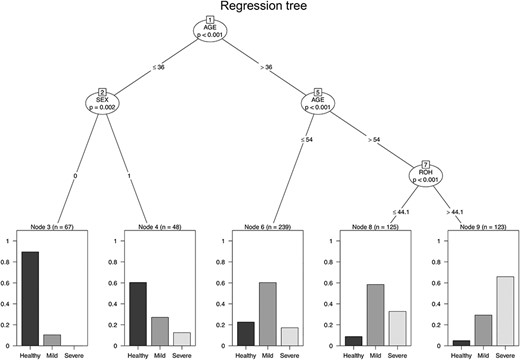
Regression tree. Binary tree computed by conditional recursive partitioning of the effect of the age, sex and the total homozygosity due to ROH on periodontal severity. Node 8 shows the following fractions of healthy (9%), mild (59%) and severe (33%), whereas in Node 9 (characterized by individuals with higher homozygosity) the fraction of healthy is 5%, mild is 29% and severe 66%. The figure shows only the variables having a significant effect on periodontal status classification after Bonferroni correction. Sex is coded 1 for males and 0 for females.
The model predicts the value of a response variable based on several input variables by searching for the best split criteria to divide the subjects into binary subgroups. Homozygosity owing to ROH did not show a significant effect on younger individuals, whereas the difference between sexes was detected. None of the other indicators used in the analysis had significant effects in the model (Bonferroni corrected P-value > 0.05). In particular, nodes 8 and 9 contain individuals above 55 years old and are composed by both smokers and non-smokers and both sexes. Linear regression analysis, using as covariates age, sex, smoking status, number of cigarettes smoked and homozygosity owing to ROH, confirmed the results obtained from the partitioning tree analysis. Age and ROH homozygosity were the only variables with significant P-value (b = 0.027, P-value < 2e-16 for age and b = 0.0022, P-value = 0.0001 for ROH), and the other predictor variables showed a P-value>0.05. Furthermore, the ROH burden showed no effect in discriminating healthy individuals and individuals affected by periodontitis (score from 3 to 6); instead, it shows the ability to discriminate between individuals with mild (score from 3 to 4) and severe periodontitis (score from 5 to 6).
Considering that homozygosity owing to ROH showed its effect on individuals older than 54 years, we calculated the area under the receiver operating characteristic (ROC) curve (AUC) metrics of the ROC curves using age and homozygosity as predictors and the periodontal status (mild vs. severe) as the response. As shown in Supplementary Material, Figure S2, ROH showed an AUC metric of 0.67, while age showed an AUC metric of 0.54 (Supplementary Material, Fig. S3), showing that homozygosity owing to ROH is a better predictor of periodontal severity than age in individuals more than 54 years old.
We also compared the regions of shared homozygosity between individuals with severe periodontitis and mild periodontitis. Then the frequencies of pools in severe and mild individuals of overlapping ROHs were used to estimate the odds ratio (Supplementary Material, Table S1). A total of 39 regions were found, among them, 19 have an odds ratio higher than 1 (including confidence intervals), and 5 of them have odds ratios lower than 1 (including confidence intervals). Then, we analyzed the genes found in the top 10 regions with the highest odds ratio, resulting in a total of 33 coding genes (Supplementary Material, Table S2). We collected the Sum singleton score (SSC) score in Europeans, probability of LOF intolerance (pLI) and residual variation intolerance score (RVIS) for all of them.
Interestingly, 9 of them showed loss of function (LOF) intolerance (pLi > 0.9), and 19 of them showed LOF tolerance (pLI < 0.1). Regarding the SSC score, only seven of them resulted as outliers (SSC score < −2), which indicated genetic constraints. Finally, for RVIS, two of them were under the fifth percentile of the genome-wide distribution indicating a high level of conservation and selection, and nine are under the 25th percentile.
Discussion
In this work, we found that total homozygosity is significantly higher in individuals with severe periodontitis stages III and IV with respect to both individuals with mild periodontitis stages I and II and healthy subjects. Moreover, from regression tree analysis, it emerged that only in individuals older than 54 years, ROH homozygosity influences periodontal severity. In particular, a higher percentage of individuals with severe periodontitis (compared with mild periodontitis) emerge among individuals with higher homozygosity owing to ROHs. To our knowledge, no other works on periodontitis and ROH have been conducted. Furthermore, most of the studies on the genetics of periodontitis were case–control studies on the risk to develop the disease, while very few studies on severity have been carried out. The most established studies have shown an association between moderate–to-severe chronic periodontitis and variations in the IL1B gene, a regulator of the host responses to microbial infection and osteoclastic bone resorption (22–24). In another work, polymorphisms in FcγR, coding for the Fc-gamma receptor crucial in the bacteria phagocytosis, have been associated with severity of chronic periodontitis in a Japanese population (25).
In the present work, comparing the regions of shared homozygosity between individuals with severe and mild periodontitis, we detect some genes of interest. For example, the region on chromosome 17 includes the cytokine receptor-like factor 3 (CRLF3) gene, which is ubiquitously expressed throughout the hematopoietic system and previously associated with the mean platelet volume (26).
Moreover, in the region of chromosome 3, we identify the TRANK1 gene, earlier linked to platelet component distribution width (26). None of these genes has been previously associated with periodontitis or its severity. However, an increased platelet count, possibly due to an amplified systemic inflammation, has been already associated with both the development and progression of periodontitis (27,28). Moreover, in past works, other genes linked to platelet have been linked to periodontitis. More in detail, the PF4/PPBP/CXCL5 gene cluster was reported as a genetic risk factor of human periodontitis (29). Platelet factor 4 (PF4) is released from the alpha granules of activated platelets and is involved in platelet aggregation and hematopoiesis, and it has antimicrobial activity. Pro-platelet basic protein (PPBP) is a platelet-derived growth factor and potent chemoattractant and activator of neutrophils. It is also an antimicrobial protein with bactericidal and antifungal activity. CXCL5 is stored in the alpha-granules of platelets and encodes a member of the CXC subfamily of chemokinesis, which recruits neutrophils, promotes angiogenesis and remodels connective tissues.
Besides, in regions of shared homozygosity between individuals with severe and mild periodontitis, we also detect genes such as AZIN1 and ATAD5 that were previously associated with granulocyte or neutrophil count (26). Until now, no studies have reported an association between these genes and periodontitis. However, neutrophils are considered as the primary protective cells of the periodontal tissues, and alterations in the neutrophil homeostasis can lead to periodontal disease onset and progression (30).
Another intriguing gene we found is SUZ12 located on chromosome 17. This gene was found to bind with ANRIL; the first published genetic risk factor for aggressive periodontitis (31). In support of our findings, the dysregulation of ANRIL was reported in the peripheral blood of patients with periodontitis (32). Moreover, polymorphism in ANRIL was associated with inter-individual variation in high-sensitive C-reactive protein (hsCRP) levels in periodontitis patients (33).
This study is subject to certain limitations. For instance, one possible weakness of our study is the limited sample size. However, it could be mitigated by the use of isolated populations, which are characterized by genetic and environmental homogeneity. Isolated villages arise when a relatively small number of individuals found a new population, then rare deleterious polymorphisms already present in the founders can grow in frequency, thus increasing power for association studies. Finally, the reduced effective population size leads to increased levels of homozygosity and reduces the effect of purifying selection purging detrimental alleles (34).
Moreover, the small sample size prevented subgroups’ analyses. For example, future studies with increased sample size for all age categories could help to better understand the interaction between age and ROH in periodontal severity and eventual risk factors for younger individuals.
Finally, a possible confounding factor could be consanguinity affecting the socio-economic status, which could be a risk factor for severe periodontitis; however, we do not have information of evident socio-economic differences owing to consanguinity between individuals from these villages. Furthermore, we checked for any possible correlation between other pathologies and severe periodontitis, and we found no significant ones, and this could be owing to the limited number of affected individuals by other disease (e.g. we have only seven individuals with type II diabetes in our sample).
In conclusion, our results suggest a possible role of the ROH in periodontal severity development. In particular, we highlight the possibility that variants with small deleterious effect that happen to be in homozygous state and accumulate in specific genomic regions could increase the risk of a specific disease.
Our works show the necessity of future sequencing studies to analyze rare variants and their cumulative effect in homozygosity regions.
Besides, the use of individual genomic features (such as ROH) to assess the genetic risk of disease could be a useful addition to the already well-established methodology of personalized medicine.
Materials and Methods
We analyzed the genetic data of a total of 826 individuals coming from six isolated villages from the Friuli Venezia Giulia project. Written informed consent was obtained, and the ethical committee of IRCCS Burlo Garofolo and the Ethics Committee of the University of Trieste approved the study (Prot. ce/V − 78, 06/08/2007), that was performed following the ethical standards of the 1975 Declaration of Helsinki (seventh revision, 2013).
Information regarding medical and individual data was recorded, and the periodontal disease was diagnosed as reported in our previous articles, following the criteria of the American Academy of Periodontology and the European Federation of Periodontology (35). Periodontitis score was used to classify each individual as healthy (score = 0), mild periodontitis stages I and II (score = 3–4) and severe periodontitis stages III and IV (score = 5–6). We did not consider individuals with a score of 1 and 2, resulting in a total of 602 individuals analyzed. Covariates such as age, sex and smoke were collected as well. The DNA was extracted from peripheral blood with the EZ1 DNA investigator kit (Qiagen, Milan, Italy) according to the manufacturer’s instructions and then was analyzed with the Illumina 370k high-density SNP array (Illumina, Inc., San Diego, CA, USA). Genotype calling was performed with the GenomeStudio software (Illumina, Inc.). Markers were filtered according to minor allele frequency (MAF < 0.01) and genotyping rate > 0.99. After quality control, a total of 188 757 autosomal SNPs and 602 individuals with call rare >90% were analyzed.
Statistical analysis
ROHs for each individual were estimated with PLINK v.1.9 (36), using the command –homozyg, selecting only the ROHs with a length of at least 1.5 Mb. We performed a regression tree analysis using total homozygosity owing to ROH, age, sex and smoke as predictor variables in periodontitis severity. This analysis was done using the R package party (37).
In detail, recursive partitioning is used in medicine, genetics and other fields when a graphical representation of different predictor variables' interaction is needed. The procedure utilizes the variable with the lowest P-value (after Bonferroni correction) as the decision tree’s first node. Following this, two subgroups are created (I and II). For subgroup I, the variable with the lowest P-value (if there is one) is taken as the second or third node. The same is done for subgroup II. The final model is based on the splitting variables in each node with the highest statistical significance.
An ROC curve was created to assess the performance of total homozygosity owing to ROH in predicting periodontal severity (mild vs. severe); ROC curves were analyzed using the R package PRROC. Pools of overlapping ROH in healthy, mild and severe individuals were identified using PLINK using the command —homoyzg-group and default parameters. Genomic regions were then compared between individuals with a mild and severe phenotype, and the odds ratio for each region was then calculated.
Constraints metrics for genes found in pools of overlapping ROHs were obtained, more in detail, we collected pLI (38), RVIS score (39) and SSC score in Europeans (40). In particular, a gene with pLI > 0.9 is considered LOF-intolerant, whereas a gene with pLI < 0.1 is considered LOF-tolerant; regarding the RVIS score and SSC score, the more negative the value on a gene is, the higher the evolutionary genetic constrains are on that gene.
Acknowledgements
We thank all the participants to the project. This work was supported by Italian Ministry of Health and IRCCS Burlo Garofalo of Trieste (RC 35/2017).
Conflict of Interest Statement. None declared.
Funding
This work was supported by Italian Ministry of Health and IRCCS Burlo Garofalo of Trieste (RC 35/2017).


