-
PDF
- Split View
-
Views
-
Cite
Cite
Daria Radchenko, Ines Teichert, Stefanie Pöggeler, Ulrich Kück, A Hippo Pathway-Related GCK Controls Both Sexual and Vegetative Developmental Processes in the Fungus Sordaria macrospora, Genetics, Volume 210, Issue 1, 1 September 2018, Pages 137–153, https://doi.org/10.1534/genetics.118.301261
Close - Share Icon Share
Abstract
The supramolecular striatin-interacting phosphatases and kinases (STRIPAK) complex is conserved from yeast to human, and regulates a variety of key biological processes. In animals, this complex consists of the scaffold protein striatin, the protein phosphatase 2A, and kinases, such as germinal center kinase (GCK) III and GCKIV family members, as well as other associated proteins. The STRIPAK complex was identified as a negative regulator of the Hippo pathway, a large eukaryotic signaling network with a core composed of a GCK and a nuclear Dbf2-related kinase. The signaling architecture of the Hippo core resembles the fungal septation initiation network (SIN) that regulates cytokinesis in fission yeast as well as septation in filamentous fungi. In the filamentous model fungus Sordaria macrospora, core components of the STRIPAK complex have been functionally described and the striatin homolog PRO11 has been shown to interact with the GCK SmKIN3. However, the exact role of SmKIN3 in fungal development has not yet been fully elucidated. Here, we provide comprehensive genetic and functional analysis of SmKIN3 from S. macrospora. Using deletion mutants and site-directed mutagenesis, along with phenotypic and phylogenetic analysis, we provide compelling evidence that SmKIN3 is involved in fruiting body formation, hyphal fusion, and septation. Strains carrying the ATP-binding mutant SmKIN3K39R, as well as a double-deletion strain lacking SmKIN3 and the core STRIPAK subunit PRO11, also revealed severe developmental defects. Collectively, this study suggests that SmKIN3 links both the SIN and STRIPAK complex, thereby regulating multiple key cellular processes.
GERMINAL center kinases (GCKs) are Ste20-related serine/threonine kinases (STKs) with a conserved catalytic domain located at the N-terminus. In mammals, flies, and worms, eight GCK subfamilies (I–VIII) are known, and are distinguished according to their structure and biological function (Delpire 2009). Some members of these subfamilies transmit extracellular signals to mitogen-activated protein kinase (MAPK) cascades, while others act as signaling hubs within conserved eukaryotic complexes. Five out of eight GCK subfamilies (GCK-I, II, III, IV, and VIII) were shown to functionally interact with either the Hippo pathway, the supramolecular striatin-interacting phosphatases and kinases (STRIPAK) complex, or with both (Couzens et al. 2013; Madsen et al. 2015; Meng et al. 2015, 2016; Thompson and Sahai 2015). GCKs regulate key developmental processes, such as cytoskeleton organization, the cell cycle, and apoptosis. In humans, malfunction of GCKs is associated with different medical conditions, including autoimmune diseases and cancer, which highlights them as potential therapeutic targets (Delpire 2009; Yin et al. 2012; Thompson and Sahai 2015).
The STRIPAK complex is highly conserved in eukaryotes, and its core consists of the protein phosphatase 2A (PP2A) B’’’-regulatory subunit striatin, the scaffold and catalytic subunits PP2AA and PP2AC, the striatin-interacting protein STRIP1/2, the monopolar spindle one-binder (Mob) protein Mob3, and—in vertebrates and invertebrates—the cerebral cavernous malformation 3 (CCM3) protein. In mammals, additional proteins, such as the sarcolemmal membrane-associated protein (SLMAP), the suppressor of IKKε, and the fibroblast growth factor receptor oncogene partner 2, or the cortactin-binding protein 2, can interact with the core STRIPAK in a mutually exclusive manner. Moreover, it is highly likely that STRIPAK-association of GCK-III and GCK-IV family kinases is also mutually exclusive. GCK-III kinases mammalian Ste20-like (MST) 3 (STK24), MST4 (STK26; MASK), and YSK1 (STK25; SOK1) bind to striatin family members via CCM3. However, whether GCK-IV kinases Misshapen (Msn)-like kinase 1 (MINK1; MAP4K6) and MAP4K4 interact with striatin directly or via CCM3 is as yet not completely understood (Hwang and Pallas 2014). Finally, GCK-III kinases are thought to be downregulated by PP2A (Gordon et al. 2011).
Drosophila melanogaster possesses only one homolog of each of the mammalian GCK families, thereby indicating partial redundancy of vertebrate GCKs. GCK-III kinase Wheezy (GckIII) and GCK-IV kinase Misshapen (Msn) were found in affinity capture-mass spectrometry with the striatin homolog Cka and the MOB3 homolog Mob4, confirming these factors to be a part of the dSTRIPAK complex (Ribeiro et al. 2010). Interestingly, D. melanogaster Msn and GCK-I kinase Happyhour (Hppy) were shown as alternative activators of the nuclear Dbf2-related (NDR) kinase Warts of the Hippo pathway (Li et al. 2015; Zheng et al. 2015), and the same conclusion was drawn for the human homologs MINK1, MAP4K4, and MAP4K1/2/3/5 (Meng et al. 2015).
The Hippo pathway in D. melanogaster, as well as its human counterpart, represent large signaling networks. Its core consists of GCK-II kinase Hippo (MST1/2 in human, also known as STK4/3) associated with the adaptor protein Salvador (SAV1 in human), and the NDR kinase Warts (large tumor suppressor1/2 or LATS1/2 in human) associated with the kinase activator Mob1 (MOB1). Of note is that malfunction of the Hippo pathway leads to tissue overgrowth and tumor formation (Pan 2007, 2010). In particular, lack of MST1/2 in mammalian cells is connected with the development of carcinoma, adenoma, and acute leukemia (Pan 2010; Harvey et al. 2013; Richardson and Portela 2017). GCK-II kinases interact with STRIPAK by binding via their C-terminal linker to a forkhead-associated domain of SLMAP, thereby enabling their dephosphorylation by PP2AC. At the same time, SAV1 inhibits the phosphatase activity of PP2A via binding to the STRIPAK core, thus disabling dephosphorylation of the GCKII activation-loop. This mechanism is responsible for the reciprocal downregulation of STRIPAK and Hippo (Couzens et al. 2013; Bae et al. 2017). Even though Hippo kinases MST1 and MST2 are capable of autophosphorylation-dependent activation, they can also be activated by an upstream regulator, namely GCK-VIII kinases TAO1/2/3 (Fallahi et al. 2016; Meng et al. 2016). Collectively, these facts provide evidence for a complex GCK signaling network.
Although animal GCKs have been extensively investigated, their evolutionary history remains unclear (Sebé-Pedrós et al. 2012, 2016). Molecular mechanisms, underlying multiple GCK-related disease phenotypes in mammals, also require further investigation (Maugeri-Saccà and De Maria 2018; Xiang et al. 2018). Moreover, new GCK targets, regulators, and interaction partners are being steadily identified (Johnson and Halder 2014; Meng et al. 2016).
Interestingly, signaling pathways from metazoans share more similarities with those from fungi, rather than with those from plants or protists. Indeed, fungal model organisms have been highly helpful in advancing our understanding of conserved eukaryotic signaling pathways in general, and the structure and function of these conserved pathways in particular (Glotzer 2017). The animal Hippo pathway, which resembles the septation initiation network (SIN) from Schizosaccharomyces pombe, is a prominent example. Both, the Hippo and the SIN are kinase cascades, which have a core consisting of a highly conserved GCK and an NDR kinase. In yeast, SIN is required for the intricate coordination of mitosis, septation, and cytokinesis (Simanis 2015). Similar to the homologous networks in animals, the kinase activity of SIN is inhibited by the SIN-inhibitory phosphatase complex, a homolog of STRIPAK (Singh et al. 2011). In filamentous fungi, this complex has already been extensively investigated (Kück et al. 2016), although detailed knowledge about STRIPAK-associated fungal kinases is still lacking. Further, interplay of STRIPAK and SIN has not yet been described for filamentous fungi. Recently, two GCKs—SmKIN3 and SmKIN24—from Sordaria macrospora, an ascomycete model organism, were reported as interaction partners of PRO11, the homolog of mammalian striatin (Frey et al. 2015). These authors further showed a high sequence similarity of SmKIN24 and SmKIN3 within their catalytic domains, and demonstrated that they are orthologs of the Neurospora crassa kinases MST-1 and septation initiation-deficient-1 (SID-1), respectively. The latter was previously shown to be part of SIN, the fungal counterpart of the mammalian Hippo pathway (Heilig et al. 2013; Frey et al. 2015).
Here, we investigate whether SmKIN3 links SIN with the STRIPAK complex. Therefore, we provide a detailed functional analysis of SmKIN3, using gene deletion and ATP-binding site variants of the SmKIN3 kinase. Further phenotypic analysis of single and double mutants was done to demonstrate a genetic interaction (GI) between SIN and the STRIPAK complex in filamentous fungi.
Materials and Methods
Strains, media, and growth conditions
All S. macrospora strains, as listed in Table 1, were grown under standard conditions (Nowrousian et al. 1999; Dirschnabel et al. 2014), unless otherwise described. Transformation of S. macrospora strains with recombinant plasmids was performed according to Nordzieke et al. (2015), but without caylase. Cloning was performed in Saccharomyces cerevisiae strain PJ69-4α (James et al. 1996) using the homologous recombination system described previously (Colot et al. 2006). For propagation of recombinant plasmids, Escherichia coli strains XL1-Blue MRF’ (Jerpseth 1992) and NEB5α (New England Biolabs, Beverly, MA) were used under standard conditions (Sambrook and Russell 2001).
S. macrospora strains used in this study
| Strain . | Relevant genotype . | Relevant phenotype . | Reference source . |
|---|---|---|---|
| R19027 | Wild-type, sos1+ | F | Culture collection of the Department of General and Molecular Botany |
| S147487 | Wild-type, sos1− | F | This study |
| S70823 | fus, sos1+ | F | Culture collection of the Department of General and Molecular Botany |
| S149233 | fus, sos1− | F | This study |
| S96888 | ∆ku70::natr, sos1− | F | Pöggeler and Kück (2006) |
| D2273 | ∆Smkin3::hygr, sos1− | S, RS, HFD | This study |
| D2201 | ∆Smkin3::hygr, sos1+ | F | This study |
| D2387 | ∆Smkin3::hygr/fus, sos1− | S, RS, HFD | This study |
| D2227 | ∆Smkin3::hygr/fus, sos1+ | F | This study |
| D2694 | ∆Smkin3::hygr:: Smkin3(p)::Smkin3::Smkin3(t)::natr | F | This study |
| D2704 | ∆Smkin3::hygr:: gpd(p)::mrfp::Smkin3::trpC(t)::natr | F, HS | This study |
| D2606 | ∆Smkin3::hygr::Smkin3(p)::Smkin3K26R::Smkin3(t)::natr | F | This study |
| D160A-10 | ∆Smkin3::hygr::Smkin3(p)::Smkin3K39R::Smkin3(t)::natr | S, NS, HFD, GD | This study |
| S63685 | pro11 | S, HFD | Pöggeler and Kück (2004) |
| S5.7 | ∆pro11::hygr | S, HFD | Bloemendal et al. (2012) |
| S141923 | pro11/fus | S, HFD | Culture collection of the Department of General and Molecular Botany |
| ∆pro11/fus | ∆pro11::hygr/fus | S, HFD | Culture collection of the Department of General and Molecular Botany |
| S150712 | ∆Smkin3::hygr/pro11 | S, NS, HFD | This study |
| S150772 | ∆Smkin3::hygr/∆pro11::hygr | S, NS, HFD | This study |
| S140253 | ∆pro22::hygr/pro11 | S, HFD | This study |
| S140062 | ∆pro22::hygr/∆pro11::hygr | S, HFD | This study |
| S56 | ∆pro22::hygr | S, HFD | Bloemendal et al. (2012) |
| AB2854 | ∆pro22::hygr::pro22::natr | F | Beier (2017) |
| Strain . | Relevant genotype . | Relevant phenotype . | Reference source . |
|---|---|---|---|
| R19027 | Wild-type, sos1+ | F | Culture collection of the Department of General and Molecular Botany |
| S147487 | Wild-type, sos1− | F | This study |
| S70823 | fus, sos1+ | F | Culture collection of the Department of General and Molecular Botany |
| S149233 | fus, sos1− | F | This study |
| S96888 | ∆ku70::natr, sos1− | F | Pöggeler and Kück (2006) |
| D2273 | ∆Smkin3::hygr, sos1− | S, RS, HFD | This study |
| D2201 | ∆Smkin3::hygr, sos1+ | F | This study |
| D2387 | ∆Smkin3::hygr/fus, sos1− | S, RS, HFD | This study |
| D2227 | ∆Smkin3::hygr/fus, sos1+ | F | This study |
| D2694 | ∆Smkin3::hygr:: Smkin3(p)::Smkin3::Smkin3(t)::natr | F | This study |
| D2704 | ∆Smkin3::hygr:: gpd(p)::mrfp::Smkin3::trpC(t)::natr | F, HS | This study |
| D2606 | ∆Smkin3::hygr::Smkin3(p)::Smkin3K26R::Smkin3(t)::natr | F | This study |
| D160A-10 | ∆Smkin3::hygr::Smkin3(p)::Smkin3K39R::Smkin3(t)::natr | S, NS, HFD, GD | This study |
| S63685 | pro11 | S, HFD | Pöggeler and Kück (2004) |
| S5.7 | ∆pro11::hygr | S, HFD | Bloemendal et al. (2012) |
| S141923 | pro11/fus | S, HFD | Culture collection of the Department of General and Molecular Botany |
| ∆pro11/fus | ∆pro11::hygr/fus | S, HFD | Culture collection of the Department of General and Molecular Botany |
| S150712 | ∆Smkin3::hygr/pro11 | S, NS, HFD | This study |
| S150772 | ∆Smkin3::hygr/∆pro11::hygr | S, NS, HFD | This study |
| S140253 | ∆pro22::hygr/pro11 | S, HFD | This study |
| S140062 | ∆pro22::hygr/∆pro11::hygr | S, HFD | This study |
| S56 | ∆pro22::hygr | S, HFD | Bloemendal et al. (2012) |
| AB2854 | ∆pro22::hygr::pro22::natr | F | Beier (2017) |
F, fertile; S, sterile; RS, rare septa; HFD, hyphal fusion defect; HS, hyperseptation; NS, no septa; GD, germination defect.
| Strain . | Relevant genotype . | Relevant phenotype . | Reference source . |
|---|---|---|---|
| R19027 | Wild-type, sos1+ | F | Culture collection of the Department of General and Molecular Botany |
| S147487 | Wild-type, sos1− | F | This study |
| S70823 | fus, sos1+ | F | Culture collection of the Department of General and Molecular Botany |
| S149233 | fus, sos1− | F | This study |
| S96888 | ∆ku70::natr, sos1− | F | Pöggeler and Kück (2006) |
| D2273 | ∆Smkin3::hygr, sos1− | S, RS, HFD | This study |
| D2201 | ∆Smkin3::hygr, sos1+ | F | This study |
| D2387 | ∆Smkin3::hygr/fus, sos1− | S, RS, HFD | This study |
| D2227 | ∆Smkin3::hygr/fus, sos1+ | F | This study |
| D2694 | ∆Smkin3::hygr:: Smkin3(p)::Smkin3::Smkin3(t)::natr | F | This study |
| D2704 | ∆Smkin3::hygr:: gpd(p)::mrfp::Smkin3::trpC(t)::natr | F, HS | This study |
| D2606 | ∆Smkin3::hygr::Smkin3(p)::Smkin3K26R::Smkin3(t)::natr | F | This study |
| D160A-10 | ∆Smkin3::hygr::Smkin3(p)::Smkin3K39R::Smkin3(t)::natr | S, NS, HFD, GD | This study |
| S63685 | pro11 | S, HFD | Pöggeler and Kück (2004) |
| S5.7 | ∆pro11::hygr | S, HFD | Bloemendal et al. (2012) |
| S141923 | pro11/fus | S, HFD | Culture collection of the Department of General and Molecular Botany |
| ∆pro11/fus | ∆pro11::hygr/fus | S, HFD | Culture collection of the Department of General and Molecular Botany |
| S150712 | ∆Smkin3::hygr/pro11 | S, NS, HFD | This study |
| S150772 | ∆Smkin3::hygr/∆pro11::hygr | S, NS, HFD | This study |
| S140253 | ∆pro22::hygr/pro11 | S, HFD | This study |
| S140062 | ∆pro22::hygr/∆pro11::hygr | S, HFD | This study |
| S56 | ∆pro22::hygr | S, HFD | Bloemendal et al. (2012) |
| AB2854 | ∆pro22::hygr::pro22::natr | F | Beier (2017) |
| Strain . | Relevant genotype . | Relevant phenotype . | Reference source . |
|---|---|---|---|
| R19027 | Wild-type, sos1+ | F | Culture collection of the Department of General and Molecular Botany |
| S147487 | Wild-type, sos1− | F | This study |
| S70823 | fus, sos1+ | F | Culture collection of the Department of General and Molecular Botany |
| S149233 | fus, sos1− | F | This study |
| S96888 | ∆ku70::natr, sos1− | F | Pöggeler and Kück (2006) |
| D2273 | ∆Smkin3::hygr, sos1− | S, RS, HFD | This study |
| D2201 | ∆Smkin3::hygr, sos1+ | F | This study |
| D2387 | ∆Smkin3::hygr/fus, sos1− | S, RS, HFD | This study |
| D2227 | ∆Smkin3::hygr/fus, sos1+ | F | This study |
| D2694 | ∆Smkin3::hygr:: Smkin3(p)::Smkin3::Smkin3(t)::natr | F | This study |
| D2704 | ∆Smkin3::hygr:: gpd(p)::mrfp::Smkin3::trpC(t)::natr | F, HS | This study |
| D2606 | ∆Smkin3::hygr::Smkin3(p)::Smkin3K26R::Smkin3(t)::natr | F | This study |
| D160A-10 | ∆Smkin3::hygr::Smkin3(p)::Smkin3K39R::Smkin3(t)::natr | S, NS, HFD, GD | This study |
| S63685 | pro11 | S, HFD | Pöggeler and Kück (2004) |
| S5.7 | ∆pro11::hygr | S, HFD | Bloemendal et al. (2012) |
| S141923 | pro11/fus | S, HFD | Culture collection of the Department of General and Molecular Botany |
| ∆pro11/fus | ∆pro11::hygr/fus | S, HFD | Culture collection of the Department of General and Molecular Botany |
| S150712 | ∆Smkin3::hygr/pro11 | S, NS, HFD | This study |
| S150772 | ∆Smkin3::hygr/∆pro11::hygr | S, NS, HFD | This study |
| S140253 | ∆pro22::hygr/pro11 | S, HFD | This study |
| S140062 | ∆pro22::hygr/∆pro11::hygr | S, HFD | This study |
| S56 | ∆pro22::hygr | S, HFD | Bloemendal et al. (2012) |
| AB2854 | ∆pro22::hygr::pro22::natr | F | Beier (2017) |
F, fertile; S, sterile; RS, rare septa; HFD, hyphal fusion defect; HS, hyperseptation; NS, no septa; GD, germination defect.
Generation of plasmids
All plasmids used in this study are listed in Table 2. To generate the knockout plasmid pKO4490 for the deletion of Smkin3, the yeast homologous recombination system was used. Yeast strain PJ69-4α was transformed with two 1-kb PCR fragments carrying the 5′ or 3′ flanking sequences of Smkin3, respectively, together with a hygromycin B resistance cassette from pDrivehph and EcoRI/XhoI-linearized vector pRS426. Similarly, the yeast recombination system was used to obtain complementation plasmids pNAkin3 and pOEkin3.
Plasmids used in this study
| Plasmid . | Feature . | Reference . |
|---|---|---|
| pRS426 | URA3, lacZ_a, bla | Christianson et al. (1992) |
| pDrivehph | trpC(p)::hph, lacZ_a, bla, kann | Nowrousian and Cebula (2005) |
| pKO4490 | Deletion cassette for Smkin3, 1 kb 5′ flank, hph from pDrivehph and 1 kb 3′ flank in pRS426 | This study |
| pRSnat | trpC(p)::nat, URA3, bla | Klix et al. (2010) |
| pDS23 | gpd(p)::egfp::trpC(t), trpC(p)::nat, URA3, bla | Schindler and Nowrousian (2014) |
| pMHN2 | gpd(p)::mrfp::trpC(t), trpC(p)::hph, bla | Rech (2007) |
| pNAkin3 | Smkin3 with 1,9 kb upstream and 1,1 kb downstream sequence in pRSnat | This study |
| pOEkin3 | gpd(p)::mrfp::SmKin3::trpC(t) in pRSnat | This study |
| p4490-K26R | pNAkin3 carrying Smkin3K26R mutation | This study |
| p4490-K39R | pNAkin3 carrying Smkin3K39R mutation | This study |
| Plasmid . | Feature . | Reference . |
|---|---|---|
| pRS426 | URA3, lacZ_a, bla | Christianson et al. (1992) |
| pDrivehph | trpC(p)::hph, lacZ_a, bla, kann | Nowrousian and Cebula (2005) |
| pKO4490 | Deletion cassette for Smkin3, 1 kb 5′ flank, hph from pDrivehph and 1 kb 3′ flank in pRS426 | This study |
| pRSnat | trpC(p)::nat, URA3, bla | Klix et al. (2010) |
| pDS23 | gpd(p)::egfp::trpC(t), trpC(p)::nat, URA3, bla | Schindler and Nowrousian (2014) |
| pMHN2 | gpd(p)::mrfp::trpC(t), trpC(p)::hph, bla | Rech (2007) |
| pNAkin3 | Smkin3 with 1,9 kb upstream and 1,1 kb downstream sequence in pRSnat | This study |
| pOEkin3 | gpd(p)::mrfp::SmKin3::trpC(t) in pRSnat | This study |
| p4490-K26R | pNAkin3 carrying Smkin3K26R mutation | This study |
| p4490-K39R | pNAkin3 carrying Smkin3K39R mutation | This study |
| Plasmid . | Feature . | Reference . |
|---|---|---|
| pRS426 | URA3, lacZ_a, bla | Christianson et al. (1992) |
| pDrivehph | trpC(p)::hph, lacZ_a, bla, kann | Nowrousian and Cebula (2005) |
| pKO4490 | Deletion cassette for Smkin3, 1 kb 5′ flank, hph from pDrivehph and 1 kb 3′ flank in pRS426 | This study |
| pRSnat | trpC(p)::nat, URA3, bla | Klix et al. (2010) |
| pDS23 | gpd(p)::egfp::trpC(t), trpC(p)::nat, URA3, bla | Schindler and Nowrousian (2014) |
| pMHN2 | gpd(p)::mrfp::trpC(t), trpC(p)::hph, bla | Rech (2007) |
| pNAkin3 | Smkin3 with 1,9 kb upstream and 1,1 kb downstream sequence in pRSnat | This study |
| pOEkin3 | gpd(p)::mrfp::SmKin3::trpC(t) in pRSnat | This study |
| p4490-K26R | pNAkin3 carrying Smkin3K26R mutation | This study |
| p4490-K39R | pNAkin3 carrying Smkin3K39R mutation | This study |
| Plasmid . | Feature . | Reference . |
|---|---|---|
| pRS426 | URA3, lacZ_a, bla | Christianson et al. (1992) |
| pDrivehph | trpC(p)::hph, lacZ_a, bla, kann | Nowrousian and Cebula (2005) |
| pKO4490 | Deletion cassette for Smkin3, 1 kb 5′ flank, hph from pDrivehph and 1 kb 3′ flank in pRS426 | This study |
| pRSnat | trpC(p)::nat, URA3, bla | Klix et al. (2010) |
| pDS23 | gpd(p)::egfp::trpC(t), trpC(p)::nat, URA3, bla | Schindler and Nowrousian (2014) |
| pMHN2 | gpd(p)::mrfp::trpC(t), trpC(p)::hph, bla | Rech (2007) |
| pNAkin3 | Smkin3 with 1,9 kb upstream and 1,1 kb downstream sequence in pRSnat | This study |
| pOEkin3 | gpd(p)::mrfp::SmKin3::trpC(t) in pRSnat | This study |
| p4490-K26R | pNAkin3 carrying Smkin3K26R mutation | This study |
| p4490-K39R | pNAkin3 carrying Smkin3K39R mutation | This study |
To obtain pNAkin3, a 5.8-kb fragment carrying the Smkin3 gene, together with 5′ and 3′ flanking regions and overlapping sequences for pRSnat, was amplified using 5′na4490-fw and 3′na4490-rv oligonucleotides (Table 3) from S. macrospora genomic DNA. The amplified fragment was recombined into BamHI-linearized pRSnat.
Oligonucleotides used in this study
| Oligonucleotide . | Sequence (5′–3′) . | Specificity . |
|---|---|---|
| KO-4490-5fw | gtaacgccagggttttcccagtcacgacggaa | SmKin3 5′ flank with |
| ttccttccccatcgcgctaccaggtaca | pRS426 overlap | |
| KO-4490-5rv | cgagggcaaaggaatagggttccgttgaggct | SmKin3 5′ flank with |
| tttggttacagaagggtgattgt | hph overlap | |
| KO-4490-3fw | gcccaaaaatgctccttcaatatcagttgctgag | hph with SmKin3 3′ flank |
| gtgatgaatggtgaagagaag | overlap | |
| KO-4490-3rv | gcggataacaatttcacacaggaaacagcgaatt | SmKin3 3′ flank with |
| cctgttttggttactgtaacagccgt | pRS426 overlap | |
| SmKin3_F | atggccgacgaaggagtcgc | SmKin3 start |
| SmKin3_R | ctaagatccggcaacagccc | SmKin3 end |
| kin3- 5-2DR | aaccgtgtacttcgattggc | SmKin3 5′ flank |
| kin3-3-DR2 | aagcttgatcgccatggca | SmKin3 3′ flank |
| hph1MN | cgatggctgtgtagaagtactcgc | hph |
| hph2MN | atccgcctggacgactaaaccaa | hph |
| 5′na4490-fw | gacggtatcgataagcttgatatcgggtcctcaacggcgcacttg | pRS426, SmKin3 5′ UTR |
| 3′na4490-rv | cggccgctctagaactagtgcaacgtaggta | SmKin33′ UTR, pRS426 |
| tgtacgtagctgc | ||
| Pgpd-mrfp-fw | catcgcagcttgactaacagctacatggcctcctccgaggacgtcatc | gpd(p), mrfp |
| mrfp-kin3-rv | gtcggccatagaaccaccaccaccggcgccg | mrpf, SmKin3 |
| gtggagtggcg | ||
| mrfp-kin3-fw | cggcgccggtggtggtggttctatggccgacgaaggagtcgccaac | mrpf, SmKin3 |
| kin3-Ttrpc-rv | gtggatccactagttctagactaagatccggc | SmKin3, trpc(t) |
| aacagccccccaccg | ||
| kin3K26R-Q5-fw | cgtttacaggggaattgacagg | SmKin3 K26 codon |
| kin3K26R-Q5-rv | acaccaaaactgcctcctgtg | SmKin3 K26 codon |
| kin3K39R-Q5-2fw | gtggccatcagacatgtacg | SmKin3 K39 codon |
| kin3K39R-Q5-2rv | tgtttcgcccgttgtcctg | SmKin3 K39 codon |
| pro11mut-fw | cgtatgggaaagaggaaggg | pro11 before pro11 mutation |
| pro11mut-rv | cggggccttgtgtttgatc | pro11 after pro11 mutation |
| pro11-21 | aagcgcgcttgccagtcgctgc | pro11 5′ flank |
| pro11-kor | acgatcagcctcggaaagaccgc | pro11 3′ flank |
| pro22-ClaI-for | gcggggtttttcagtatctacgattcatctgcagctaaccaaacattgag | pro22 gene |
| atcaaccccag | ||
| pro22-SacI-rv | cctccctagcgatgaggatgatgacatggtaaccattattggacctcaag | pro22 gene |
| accacgtg | ||
| pro22vp1 | ccaagttcagcaacaagaggatgg | pro22 5′ flank |
| pro22vp2 | cttacggtagctacaacccgtaca | pro22 3′ flank |
| Oligonucleotide . | Sequence (5′–3′) . | Specificity . |
|---|---|---|
| KO-4490-5fw | gtaacgccagggttttcccagtcacgacggaa | SmKin3 5′ flank with |
| ttccttccccatcgcgctaccaggtaca | pRS426 overlap | |
| KO-4490-5rv | cgagggcaaaggaatagggttccgttgaggct | SmKin3 5′ flank with |
| tttggttacagaagggtgattgt | hph overlap | |
| KO-4490-3fw | gcccaaaaatgctccttcaatatcagttgctgag | hph with SmKin3 3′ flank |
| gtgatgaatggtgaagagaag | overlap | |
| KO-4490-3rv | gcggataacaatttcacacaggaaacagcgaatt | SmKin3 3′ flank with |
| cctgttttggttactgtaacagccgt | pRS426 overlap | |
| SmKin3_F | atggccgacgaaggagtcgc | SmKin3 start |
| SmKin3_R | ctaagatccggcaacagccc | SmKin3 end |
| kin3- 5-2DR | aaccgtgtacttcgattggc | SmKin3 5′ flank |
| kin3-3-DR2 | aagcttgatcgccatggca | SmKin3 3′ flank |
| hph1MN | cgatggctgtgtagaagtactcgc | hph |
| hph2MN | atccgcctggacgactaaaccaa | hph |
| 5′na4490-fw | gacggtatcgataagcttgatatcgggtcctcaacggcgcacttg | pRS426, SmKin3 5′ UTR |
| 3′na4490-rv | cggccgctctagaactagtgcaacgtaggta | SmKin33′ UTR, pRS426 |
| tgtacgtagctgc | ||
| Pgpd-mrfp-fw | catcgcagcttgactaacagctacatggcctcctccgaggacgtcatc | gpd(p), mrfp |
| mrfp-kin3-rv | gtcggccatagaaccaccaccaccggcgccg | mrpf, SmKin3 |
| gtggagtggcg | ||
| mrfp-kin3-fw | cggcgccggtggtggtggttctatggccgacgaaggagtcgccaac | mrpf, SmKin3 |
| kin3-Ttrpc-rv | gtggatccactagttctagactaagatccggc | SmKin3, trpc(t) |
| aacagccccccaccg | ||
| kin3K26R-Q5-fw | cgtttacaggggaattgacagg | SmKin3 K26 codon |
| kin3K26R-Q5-rv | acaccaaaactgcctcctgtg | SmKin3 K26 codon |
| kin3K39R-Q5-2fw | gtggccatcagacatgtacg | SmKin3 K39 codon |
| kin3K39R-Q5-2rv | tgtttcgcccgttgtcctg | SmKin3 K39 codon |
| pro11mut-fw | cgtatgggaaagaggaaggg | pro11 before pro11 mutation |
| pro11mut-rv | cggggccttgtgtttgatc | pro11 after pro11 mutation |
| pro11-21 | aagcgcgcttgccagtcgctgc | pro11 5′ flank |
| pro11-kor | acgatcagcctcggaaagaccgc | pro11 3′ flank |
| pro22-ClaI-for | gcggggtttttcagtatctacgattcatctgcagctaaccaaacattgag | pro22 gene |
| atcaaccccag | ||
| pro22-SacI-rv | cctccctagcgatgaggatgatgacatggtaaccattattggacctcaag | pro22 gene |
| accacgtg | ||
| pro22vp1 | ccaagttcagcaacaagaggatgg | pro22 5′ flank |
| pro22vp2 | cttacggtagctacaacccgtaca | pro22 3′ flank |
| Oligonucleotide . | Sequence (5′–3′) . | Specificity . |
|---|---|---|
| KO-4490-5fw | gtaacgccagggttttcccagtcacgacggaa | SmKin3 5′ flank with |
| ttccttccccatcgcgctaccaggtaca | pRS426 overlap | |
| KO-4490-5rv | cgagggcaaaggaatagggttccgttgaggct | SmKin3 5′ flank with |
| tttggttacagaagggtgattgt | hph overlap | |
| KO-4490-3fw | gcccaaaaatgctccttcaatatcagttgctgag | hph with SmKin3 3′ flank |
| gtgatgaatggtgaagagaag | overlap | |
| KO-4490-3rv | gcggataacaatttcacacaggaaacagcgaatt | SmKin3 3′ flank with |
| cctgttttggttactgtaacagccgt | pRS426 overlap | |
| SmKin3_F | atggccgacgaaggagtcgc | SmKin3 start |
| SmKin3_R | ctaagatccggcaacagccc | SmKin3 end |
| kin3- 5-2DR | aaccgtgtacttcgattggc | SmKin3 5′ flank |
| kin3-3-DR2 | aagcttgatcgccatggca | SmKin3 3′ flank |
| hph1MN | cgatggctgtgtagaagtactcgc | hph |
| hph2MN | atccgcctggacgactaaaccaa | hph |
| 5′na4490-fw | gacggtatcgataagcttgatatcgggtcctcaacggcgcacttg | pRS426, SmKin3 5′ UTR |
| 3′na4490-rv | cggccgctctagaactagtgcaacgtaggta | SmKin33′ UTR, pRS426 |
| tgtacgtagctgc | ||
| Pgpd-mrfp-fw | catcgcagcttgactaacagctacatggcctcctccgaggacgtcatc | gpd(p), mrfp |
| mrfp-kin3-rv | gtcggccatagaaccaccaccaccggcgccg | mrpf, SmKin3 |
| gtggagtggcg | ||
| mrfp-kin3-fw | cggcgccggtggtggtggttctatggccgacgaaggagtcgccaac | mrpf, SmKin3 |
| kin3-Ttrpc-rv | gtggatccactagttctagactaagatccggc | SmKin3, trpc(t) |
| aacagccccccaccg | ||
| kin3K26R-Q5-fw | cgtttacaggggaattgacagg | SmKin3 K26 codon |
| kin3K26R-Q5-rv | acaccaaaactgcctcctgtg | SmKin3 K26 codon |
| kin3K39R-Q5-2fw | gtggccatcagacatgtacg | SmKin3 K39 codon |
| kin3K39R-Q5-2rv | tgtttcgcccgttgtcctg | SmKin3 K39 codon |
| pro11mut-fw | cgtatgggaaagaggaaggg | pro11 before pro11 mutation |
| pro11mut-rv | cggggccttgtgtttgatc | pro11 after pro11 mutation |
| pro11-21 | aagcgcgcttgccagtcgctgc | pro11 5′ flank |
| pro11-kor | acgatcagcctcggaaagaccgc | pro11 3′ flank |
| pro22-ClaI-for | gcggggtttttcagtatctacgattcatctgcagctaaccaaacattgag | pro22 gene |
| atcaaccccag | ||
| pro22-SacI-rv | cctccctagcgatgaggatgatgacatggtaaccattattggacctcaag | pro22 gene |
| accacgtg | ||
| pro22vp1 | ccaagttcagcaacaagaggatgg | pro22 5′ flank |
| pro22vp2 | cttacggtagctacaacccgtaca | pro22 3′ flank |
| Oligonucleotide . | Sequence (5′–3′) . | Specificity . |
|---|---|---|
| KO-4490-5fw | gtaacgccagggttttcccagtcacgacggaa | SmKin3 5′ flank with |
| ttccttccccatcgcgctaccaggtaca | pRS426 overlap | |
| KO-4490-5rv | cgagggcaaaggaatagggttccgttgaggct | SmKin3 5′ flank with |
| tttggttacagaagggtgattgt | hph overlap | |
| KO-4490-3fw | gcccaaaaatgctccttcaatatcagttgctgag | hph with SmKin3 3′ flank |
| gtgatgaatggtgaagagaag | overlap | |
| KO-4490-3rv | gcggataacaatttcacacaggaaacagcgaatt | SmKin3 3′ flank with |
| cctgttttggttactgtaacagccgt | pRS426 overlap | |
| SmKin3_F | atggccgacgaaggagtcgc | SmKin3 start |
| SmKin3_R | ctaagatccggcaacagccc | SmKin3 end |
| kin3- 5-2DR | aaccgtgtacttcgattggc | SmKin3 5′ flank |
| kin3-3-DR2 | aagcttgatcgccatggca | SmKin3 3′ flank |
| hph1MN | cgatggctgtgtagaagtactcgc | hph |
| hph2MN | atccgcctggacgactaaaccaa | hph |
| 5′na4490-fw | gacggtatcgataagcttgatatcgggtcctcaacggcgcacttg | pRS426, SmKin3 5′ UTR |
| 3′na4490-rv | cggccgctctagaactagtgcaacgtaggta | SmKin33′ UTR, pRS426 |
| tgtacgtagctgc | ||
| Pgpd-mrfp-fw | catcgcagcttgactaacagctacatggcctcctccgaggacgtcatc | gpd(p), mrfp |
| mrfp-kin3-rv | gtcggccatagaaccaccaccaccggcgccg | mrpf, SmKin3 |
| gtggagtggcg | ||
| mrfp-kin3-fw | cggcgccggtggtggtggttctatggccgacgaaggagtcgccaac | mrpf, SmKin3 |
| kin3-Ttrpc-rv | gtggatccactagttctagactaagatccggc | SmKin3, trpc(t) |
| aacagccccccaccg | ||
| kin3K26R-Q5-fw | cgtttacaggggaattgacagg | SmKin3 K26 codon |
| kin3K26R-Q5-rv | acaccaaaactgcctcctgtg | SmKin3 K26 codon |
| kin3K39R-Q5-2fw | gtggccatcagacatgtacg | SmKin3 K39 codon |
| kin3K39R-Q5-2rv | tgtttcgcccgttgtcctg | SmKin3 K39 codon |
| pro11mut-fw | cgtatgggaaagaggaaggg | pro11 before pro11 mutation |
| pro11mut-rv | cggggccttgtgtttgatc | pro11 after pro11 mutation |
| pro11-21 | aagcgcgcttgccagtcgctgc | pro11 5′ flank |
| pro11-kor | acgatcagcctcggaaagaccgc | pro11 3′ flank |
| pro22-ClaI-for | gcggggtttttcagtatctacgattcatctgcagctaaccaaacattgag | pro22 gene |
| atcaaccccag | ||
| pro22-SacI-rv | cctccctagcgatgaggatgatgacatggtaaccattattggacctcaag | pro22 gene |
| accacgtg | ||
| pro22vp1 | ccaagttcagcaacaagaggatgg | pro22 5′ flank |
| pro22vp2 | cttacggtagctacaacccgtaca | pro22 3′ flank |
pOEkin3 is a derivative of pDS23, in which the egfp gene was substituted by the mrfp::Smkin3 fusion construct. Fragments of mrfp and Smkin3, with overlapping sequences to the gpd promoter and trpC terminator, were amplified with oligonucleotides Pgpd-mrfp-fw/mrfp-kin3-rv and mrfp-kin3-fw/kin3-Ttrpc-rv from pMHN2 and pNAkin3, respectively.
To generate point mutations in the Smkin3 sequence encoding the ATP-binding site, we used the Q5 Site-Directed Mutagenesis Kit (New England Biolabs). Oligonucleotide pairs kin3K26R-Q5-fw/kin3K26R-Q5-rv and kin3K39R-Q5-2fw/kin3K39R-Q5-2rv were used. This resulted in plasmids p4490-K26R and p4490-K39R, which are both derivatives of pNAkin3, carrying A to G nucleotide substitution at positions 173 and 212 of the Smkin3 gene, respectively.
Generation and verification of Smkin3 deletion strain and double-deletion mutants
To generate a ΔSmkin3 deletion strain, pKO4490 was linearized with EcoRI. The 3.4-kb ΔSmkin3 deletion cassette was transformed into Δku70 (Pöggeler and Kück 2006). The primary transformants were selected for hygromycin B resistance and verified by PCR. Ascospore isolates (AIs) of the ΔSmkin3 strain with the wild-type genetic background were obtained by crosses with the spore color mutant fus1-1, as described previously (Kück et al. 2009; Nowrousian et al. 2012), and verified by PCR and Southern Blot analysis (Supplemental Material, Figure S1).
For generation of ΔSmkin3/pro11 and ΔSmkin3Δpro11, sterile pro11/fus and Δpro11/fus spore color mutants were crossed with the fertile black-spored complementation strain ΔSmkin3::NAkin3 (D2694). For generation of Δpro22/pro11 and Δpro22Δpro11, pro11/fus and Δpro11/fus were crossed with the fertile black-spored complementation strain Δpro22komp (AB2854). Single spores were isolated from recombinant asci, and selected for hygromycin B resistance and nourseothricin sensitivity to outcross the complementation plasmid. Gene deletions were verified by PCR and Southern blot analysis (Figures S2 and S3). For verification of the pro11 point mutation, a 0.6-kb fragment was amplified with pro11mut-fw and pro11mut-rv oligonucleotides (Figure S2A), and sequenced with pro11mut-bw (data not shown).
Microscopic investigations
Microscopic investigations were performed with an AxioImager microscope (Zeiss [Carl Zeiss], Thornwood, NY) equipped with a CoolSnap HQ camera (Roper Scientific) and a SpectraX LED lamp (Lumencor). Images were acquired and edited with MetaMorph (version 7.7.0.0; Universal Imaging).
To investigate sexual development by differential interference contrast microscopy, glass slides were covered with 5 ml solid malt–cornmeal fructification medium (BMM) and incubated for 2–7 days. Within this time period, different stages of fruiting body development can be observed (Engh et al. 2007; Teichert et al. 2014). The generation of ascogonia per square centimeter was examined after 3 days. Ascogonial coils were counted on BMM-coated glass slides (n = 4), on the surface covered with a 22 × 32 mm coverslip.
Hyphal fusion assays were performed after 2 days of growth on minimal medium containing soluble starch overlaid with a cellophane layer (Bio-Rad, Hercules, CA) (Rech et al. 2007; Bloemendal et al. 2012).
Septa in vegetative mycelia and ascogonial coils were visualized by staining the cell wall with Calcofluor White M2R (CFW; Sigma [Sigma Chemical], St. Louis, MO), a 1,3-β-glucan-binding agent (Roncero and Duran 1985). The 1 μg/ml CFW stock solution was diluted 1:400 in a 0.9% NaCl solution and applied on 2-day-grown samples on BMM-coated glass slides, unless otherwise described. CFW fluorescence was analyzed using Chroma filter set 31000v2 (excitation filter D350/50, emission filter D460/50, and beam splitter 400 dclp; Chroma Technology, Bellows Falls, VT). The generation of septa per millimeter of hyphae was examined after 2 days of incubation on BMM-coated glass slides (n = 4). In each sample, one hypha was examined from the hyphal tip to the colony interior. Septa were quantified over the entire observed length (30 mm), including the bases of hyphal branches. For quantification of hyphal branch base septation, 100 branch bases were analyzed in two independent samples for each strain. Hyphal length measurements were taken in Adobe Photoshop.
Phylogenetic analysis
Catalytic STK domain sequences were used as a base for the phylogenetic analysis. Multiple protein sequence alignments were performed using the ClustalW program (Larkin et al. 2007). The evolutionary history was inferred by using the maximum likelihood method (Jones et al. 1992). Obtained results were reproduced using the neighbor-joining method (Saitou and Nei 1987). To evaluate the statistical significance, a bootstrap analysis with 1000 iterations of bootstrap samplings was performed. Evolutionary analyses were conducted in MEGA7 (Kumar et al. 2016). The generated phylogenetic tree was exported to Adobe Illustrator and edited to optimize graphical representation.
Data availability
Strains and plasmids are available upon request. The authors affirm that all data necessary for confirming the conclusions of the article are present within the article, figures, and tables. Supplemental material available at Figshare: https://doi.org/10.25386/genetics.6741290.
Results
GCKs from animals and fungi share highly homologous primary sequences
BLAST (basic local alignment search tool) analysis (Altschul et al. 1997) of full-length protein sequences confirms SmKIN3 as a homolog of SIN kinases Sid1p from Sc. pombe, SID-1 from N. crassa, and SEPL from Aspergillus nidulans, whereas no homologs were found in Sa. cerevisiae. In humans, there are 22 partially redundant GCKs described (Delpire 2009), while the S. macrospora genome carries only four genes for GCKs: SMAC_04490 (Smkin3), SMAC_01456 (Smkin24) (Frey et al. 2015), SMAC_04024 coding for the homolog of SEPH from A. nidulans, and SMAC_05827 coding for the homologs of Nak1p/Kic1p from Sc. pombe and Sa. cerevisiae, respectively. Protein sequence alignment shows that GCKs from different organisms are highly conserved only within the STK catalytic domain (amino acid positions 10–260 in S. macrospora). Therefore, an alignment of regions corresponding to this conserved domain (Figure S4) was used as a basis for the generation of a phylogenetic tree. As shown in Figure 1, maximum likelihood analysis of fungal GCKs and animal STRIPAK/Hippo-associated GCKs revealed the closest relationship between SmKIN3/SmKIN24 and the GCK-III subfamily, whereas the SEPH-like protein clade as well as the Nak1p/Kic1p homolog clade are more fungal-specific.
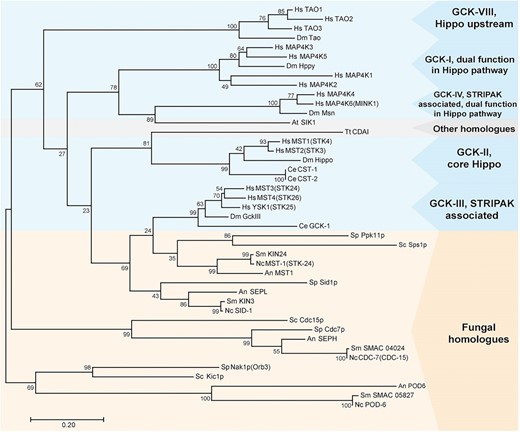
Phylogenetic analysis of GCKs from animals (blue box), fungi (orange box), and plants and protozoans (gray box). Amino acid sequences were taken from the National Center for Biotechnology Information database. The phylogenetic tree was built using the maximum likelihood method based on the sequence alignment of conserved serine/threonine kinase domains from GCKs. The percentage of trees is given in which the associated taxa clustered together next to the branches (n = 1000). An, A. nidulans; At, Arabidopsis thaliana; Dm, D. melanogaster; GCK, germinal center kinase; Hs, Homo sapiens; Nc, N. crassa; Sc, Sa. cerevisiae; Sm, Sordaria macrospora; Sp, Sc. pombe; STRIPAK, striatin-interacting phosphatases and kinases; Tt, Tetrahymena thermophila.
Generation and verification of a ΔSmkin3 deletion strain
To functionally characterize SmKIN3, we generated a ΔSmkin3 deletion strain, as described in the Materials and Methods section. The Smkin3 gene was substituted by a hygromycin B resistance cassette (Figure S1) in the nonhomologous end-joining-deficient Δku70 strain carrying a nourseothricin resistance marker gene. Primary transformants were able to grow on selection media containing both antibiotics. To outcross the Δku70 deletion and to obtain homokaryotic ΔSmkin3 mutants, ΔSmkin3Δku70 primary transformants were crossed with the brown-spored fus1-1 mutant, which is fertile like the wild type (Nowrousian et al. 2012). Recombinant AIs were subsequently selected for hygromycin B resistance (hygr) and nourseothricin sensitivity (nats), which indicate a ΔSmkin3 genotype. Interestingly, some of the hygr strains were sterile, while others were fertile, and thus able to develop wild-type-like mature fruiting bodies. PCR and Southern blot analysis showed that both sterile and fertile hygr strains lacked the Smkin3 gene (Figure S1).
We next crossed a sterile ΔSmkin3 (DR2273) to fus1-1 and performed tetrad analysis. Hygr strains corresponding to ΔSmkin3 and hygromycin B-sensitive (hygs) strains corresponding to wild-type occurred with a ratio of ∼1:1. As expected, all hygs strains were fertile; however, the hygr progeny showed a 1:1 ratio of fertile and sterile strains. This observation led to the hypothesis that an unknown genetic determinant rescued the developmental defect in the fertile ΔSmkin3 strains. We named this genetic determinant suppressor of sterility 1 (sos1). Accordingly, sterile and fertile ΔSmkin3 strains were designated ΔSmkin3sos1− and ΔSmkin3sos1+, respectively.
To further test the hypothesis of the presence of a suppressor, we performed tetrad analysis with different parental strains as detailed in Figure 2. Crosses of sterile ΔSmkin3sos1− with fus1-1 produced hygs fertile isolates corresponding to wild-type, as well as sterile and fertile hygr isolates corresponding to ΔSmkin3sos1− and ΔSmkin3sos1+, respectively (Figure S5, cross 1). The ΔSmkin3sos1−:ΔSmkin3sos1+ ratio was ∼1:1. In contrast, crosses of fertile ΔSmkin3sos1+ with fus1-1 produced wild-type isolates as well as fertile ΔSmkin3sos1+, but no sterile ΔSmkin3sos1− strains, indicating that fus1-1 carries the sos1+ allele (Figure S5, cross 2). To perform a cross in which both parental strains carry the sos1− allele, we obtained wtsos1− strain S147487 from the cross of ΔSmkin3sos1− with fus1-1sos1+ (Figure S5, cross 1). Strain S147487 was taken from a complete tetrad of this cross, in which all strains were fertile (Figure S5, cross 1), suggesting that the fertile ΔSmkin3 strains and not the wild-type strains carried the sos1+ allele. Wtsos1− was fertile and generated fruiting bodies like our laboratory wild-type strain, indicating that the suppressor mutation alone does not affect fruiting body development. Crossing sterile ΔSmkin3sos1− with wtsos1− (S147487) generated only sterile ΔSmkin3sos1− and wild-type (Figure S5, cross 3), while crossing fertile ΔSmkin3sos1+ × wtsos1− (S147487) resulted in a 1:1:2 distribution of sterile ΔSmkin3sos1−:fertile ΔSmkin3sos1+:wt (Figure 2 and Figure S5, cross 4). This result confirmed that sos1 is a single gene that is inherited in a Mendelian manner independently from the Smkin3 locus.
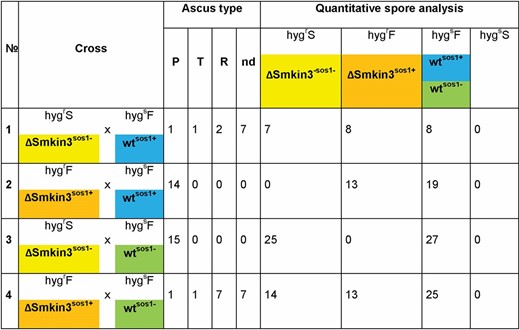
Tetrad analysis to demonstrate the inheritance of the suppressor of sterility 1 (sos1) in S. macrospora. Sterile (ΔSmkin3sos1−, color code yellow) or fertile (ΔSmkin3sos1+, color code orange) deletion strains lacking Smkin3 were crossed with wild-type (wt) strains carrying different alleles of sos1 (S70823 wtsos1+, color code blue and S147487 wtsos1−, color code green). In all strains, we have neglected the fus1-1 mutation since it is only a marker to recognize recombinant asci during ascospore isolation. Ascus type designation: P, parental; T, tetratype; R, recombinant; and nd, not determined due to a reduced spore germination. Phenotype designation: hygrS, hygromycin B-resistant, sterile (ΔSmkin3sos1−); hygrF, hygromycin B-resistant, fertile (ΔSmkin3sos1+); and hygsF, hygromycin B-sensitive, fertile (wtsos1− and wtsos1+). HygsS strains, which would indicate sterile wt strains, were never observed.
The fungal SmKIN3 kinase is involved in the septation and fusion of hyphae, as well as fruiting body formation
For further characterization, the ΔSmkin3sos1− strain was tested for septation and fusion of hyphae, as well as fruiting body formation. In parallel, the functional analysis of SmKIN3 was completed by ectopic integration of the wild-type Smkin3 gene in ΔSmkin3sos1−. In this analysis, two different complementation constructs were used with the Smkin3 gene under the control of the weak native promoter, as known from RNA-sequencing data (Teichert et al. 2012), or a strong constitutive promoter (gpd) (Punt et al. 1992). The corresponding strains were designated ΔSmkin3::NAkin3 and ΔSmkin3::OEkin3, respectively.
To examine the septation of vegetative mycelia, mycelium grown on rich medium was investigated microscopically after 16, 24, and 48 hr. We observed three different types of septa in this investigation: septa in trunk hyphae, in the base of branches, and in ascogonia (Figure 3A). As shown in Figure 3B, S. macrospora wild-type generated septa that were equally distributed over leading and branching hyphae. In contrast, the ΔSmkin3sos1− strain tended to form septa predominantly in the branch bases. These septa were visible directly after 16 hr of growth and the septation pattern remained constant after growth for ≥ 2 days. The septation defect was rescued by ectopic integration of complementation constructs, which is evident in Figure 3C. Strains carrying a construct with the native promoter (ΔSmkin3::NAkin3) showed a wild-type septation pattern, while the overexpression construct caused a hyperseptation phenotype in the bases of hyphal branches (Figure 3C). We quantified the hyperseptation phenotype (Figure 3D) as described in the Materials and Methods section. In ΔSmkin3::OEkin3, two and three narrowly (2–12 μm apart) spaced septa occurred in 25 ± 4% and 4 ± 1% of branches, respectively. In contrast, the wild-type and ΔSmkin3::NAkin3 strains generated double septa in hyphal branch bases only in 5 ± 2% and 5 ± 0.5% cases, respectively. In a total of 200 observed branches, we never found any triple septa.
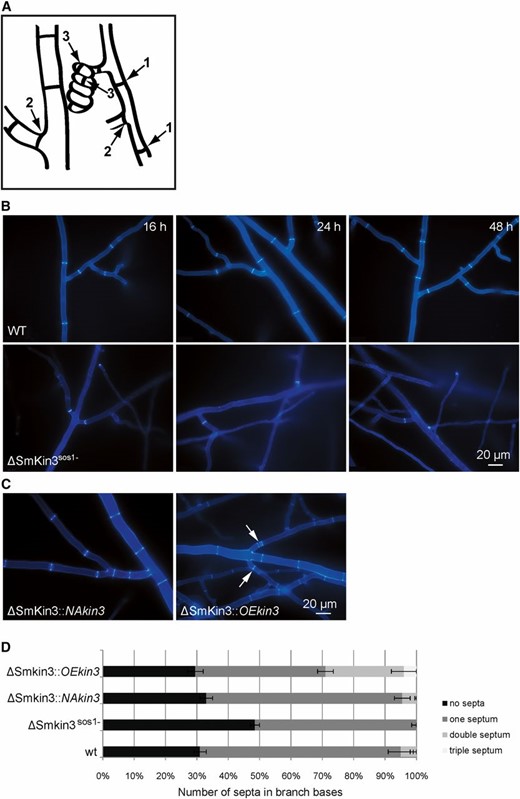
Septation of the vegetative mycelium in wild-type (WT) and mutant strains as indicated. (A) Cartoon to indicate the three types of septa described in this investigation: septa in trunk hyphae (1), in the base of branches (2), and in ascogonia (3). (B) Septation in vegetative mycelium of the wild-type and ΔSmKin3sos1− strains after 16, 24, and 48 hr of incubation on BMM (solid malt–cornmeal fructification medium). (C) Complementation strains show WT-like hyphal septation. ΔSmkin3::NAkin3 and ΔSmkin3::OEkin3 indicate transformants, where fertility was restored by ectopic integration of the WT Smkin3 gene under the control of a native (NA) or overexpression (OE) promoter. The arrows indicate multiple narrow-spaced septa in the strain overexpressing Smkin3. Mycelium samples were grown on BMM medium for 2 days and stained with Calcofluor White M2R. (D) Quantification of the hypersepation phenotype in strains as indicated. Error bars show SD of two experiments (n = 100).
Lack of STRIPAK subunits in S. macrospora leads to strictly correlating defects in the fusion of hyphae and fruiting body formation (Kück et al. 2016). Using light microscopy, we found that all ΔSmkin3sos1− strains have a hyphal fusion defect, which can be rescued by the ectopic integration of pNAkin3, but not pOEkin3 (Figure S6). Further, we analyzed the sexual development of the ΔSmkin3sos1− mutant and the two complementation strains. Unlike wild-type, the ΔSmkin3sos1− mutant forms aseptate female gametangia (ascogonia) and only a few immature unpigmented prefruiting bodies (protoperithecia) (Figure 4). Remarkably, ascogonial coils are extremely elongated, as was similarly observed in another sterile mutant pro22 (Bloemendal et al. 2010). With both constructs, all complementation strains regained the fertile phenotype. Thus, generation of homokaryotic AIs was enabled via the isolation of ascospores from the selfing perithecia. Unlike the vegetative mycelium, ascogonia produced by the overexpressing strain did not visibly differ from those in the native expression strain with regard to the amount of septa and number of ascogonial coils.
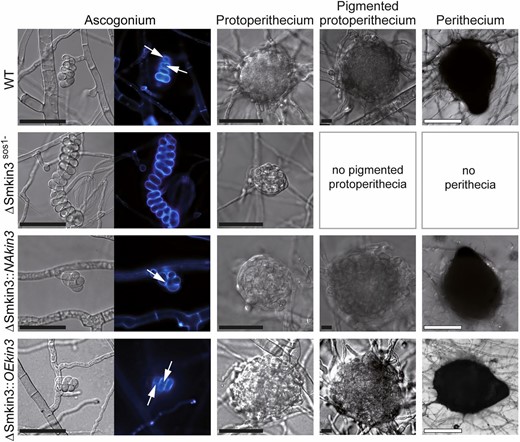
Sexual development of wild-type (WT) and mutant strains. Strain denomination is the same as in Figure 2. Ascogonia, unpigmented and pigmented protoperithecia, as well as perithecia develop after 2, 3, 4, and 7 days, respectively, on complete solid malt–cornmeal fructification medium. Ascogonial coils were stained with Calcofluor White M2R and white arrows indicate septa. Bars: black represents 20 μm and white represents 100 μm.
Functional analysis of double-mutant strains
Since SmKIN3 was previously shown to interact with PRO11, the B’’’-regulatory subunit of PP2A within STRIPAK (Frey et al. 2015), we generated double mutants ΔSmkin3Δpro11 and ΔSmkin3/pro11. Δpro11 was generated by genetic engineering and lacks the whole open reading frame for the PRO11 protein (Bloemendal et al. 2012), while the pro11 mutant was discovered in a mutant screen and contains a stop codon at position 547. This results in a premature translational termination of the open reading frame, and consequently pro11 encodes only for the N-terminal part of the protein (Pöggeler and Kück 2004). We compared the morphology of both double mutants with the corresponding single mutants. Both double mutants showed distinct morphological features compared to either wild-type or single mutants. Hyphal septation and fruiting body formation of the wild-type and ΔSmkin3sos1− strains was already shown in Figure 3 and Figure 4. As now seen in Figure 5, both pro11 and Δpro11 strains generate equally distributed septa in the vegetative mycelium, but only pro11 is able to generate septa in ascogonial coils. This morphological difference is particularly remarkable since the truncated protein in pro11 seems to be sufficient for regulating septum formation. On the other hand, both double mutants have completely aseptate mycelium and do not generate ascogonia at all. The severe septation defect renders both double mutants extremely sensitive to mechanical stress, so that any small damage causes complete leakage of hyphal contents (data not shown).
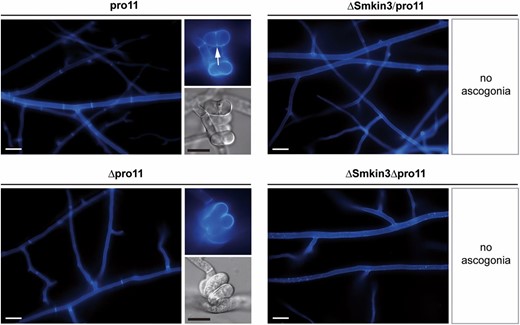
Septation of the vegetative mycelium and ascogonial coils in pro11 and Δpro11 single mutants, as well as in ΔSmkin3/pro11 and ΔSmkin3Δpro11 double mutants. Arrow indicates septum in the ascogonial coil. Mycelium samples were grown on complete solid malt–cornmeal fructification medium for 2 days and stained with Calcofluor White M2R. Bars: white represent 20 μm and black represent 10 μm.
The observed changes in morphology were then quantified to chart the GI of Smkin3 with pro11. GI refers to a double-mutant phenotype that is not easily explained by combining the effect of both single mutants. Negative GI results in severely compromised fitness and, in some cases, even synthetic lethality. In contrast, positive GI refers to a higher than expected fitness and a partial restoration of the wild-type phenotype. While negative GI usually occurs between members of different pathways, both of which regulate one essential biological process, positive GI can connect members of the same pathway (Costanzo et al. 2011; van Leeuwen et al. 2016). For the GI studies, double mutants Δpro22/pro11 and Δpro22Δpro11 served as controls. Both PRO22 and PRO11 belong to the core of the STRIPAK complex and interact physically with each other, a phenomenon that should be indicated by a positive interaction (Bloemendal et al. 2012). As depicted in Figure 6, ascogonia formation per square centimeter as well as septum formation per millimeter in vegetative hyphae was used as the phenotypic readout to map the GI. From values obtained for single mutants, the expected fitness of the resultant double mutants (ΔSmkin3/pro11, ΔSmkin3Δpro11, Δpro22/pro11, and Δpro22Δpro11) was calculated based on a multiplicative model (value double mutant = value mutant 1 × value mutant 2) (Costanzo et al. 2011). These expected values (light gray bars) were compared to the experimentally obtained values (Table 4). In the case of ascogonia formation, the absolute value for the S. macrospora wild-type is 142.9 ± 10.7 ascogonia/cm2. The following values for mutant strains are relative to the wild-type, which was set to 1. The corresponding values for ΔSmkin3, Δpro22, pro11, and Δpro11 are 0.06 ± 0.01, 0.77 ± 0.06, 0.69 ± 0.03, and 0.05 ± 0.02, respectively. From these data, the expected values for double mutants are as follows: ΔSmkin3/pro11, 0.05 (0.06 × 0.77); ΔSmkin3Δpro11, 0.003 (0.06 × 0.05); Δpro22/pro11, 0.53 (0.77 × 0.69); and Δpro22Δpro11, 0.04 (0.77 × 0.05). However, when we examined the ascogonia in the double mutants, we found significant deviations from the expected values. In detail, ΔSmkin3/pro11 and ΔSmkin3Δpro11 showed no ascogonium formation at all, while Δpro22/pro11 (0.74 ± 0.06) and Δpro22Δpro11 (0.05 ± 0.006), the two STRIPAK double mutants, had values that were significantly higher than expected (Figure 6A). Similar tendencies were found when septum formation per millimeter was taken as the phenotypic readout. The wild type generates 19 ± 1.9 septa per millimeter vegetative mycelium, and this value was set to 1. The corresponding values for ΔSmkin3, Δpro22, pro11, and Δpro11 were 0.26 ± 0.05, 0.96 ± 0.11, 0.94 ± 0.12, and 0.70 ± 0.11, respectively. The expected values for the double mutants are 0.25 for ΔSmkin3/pro11, 0.18 for ΔSmkin3Δpro11, 0.91 for Δpro22/pro11, and 0.68 for Δpro22Δpro11. However, both Smkin3 double mutants showed no septation at all. This negative deviation from the expected values has to be considered as negative GI, and according to Costanzo et al. (2011), these data point toward an interaction between members of different biological pathways. For the two STRIPAK double mutants (Δpro22/pro11 and Δpro22Δpro11), the experimentally obtained values (0.93 ± 0.11 and 0.73 ± 0.09) did not significantly deviate from the expected ones (Figure 6B).
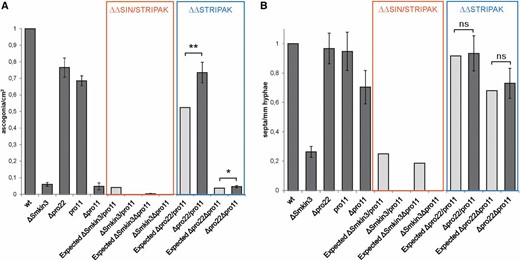
Evaluation of the genetic interaction between SmKIN3 and PRO11 based on phenotypes of ΔSmkin3/pro11 and ΔSmkin3Δpro11 double mutants (orange frame). Δpro22/pro11 and Δpro22Δpro11 double mutants are taken as a control (blue frame). Dark gray bars indicate experimentally obtained values for single and double mutants; light gray bars indicate predicted values for double mutants based on single-mutant values (see Results for details). All values are given in relation to the wild-type (WT) absolute values, which are set to 1. For absolute values, see Table 4. Brackets indicate significant difference according to Student’s t-test: * P ≤ 0.05; ** P ≤ 0.01; and ns, not significant, P > 0.05. (A) Generation of ascogonia per square centimeter compared to WT. Samples were incubated for 3 days on solid malt–cornmeal fructification medium (BMM)-coated glass slides (n = 4) (B) Generation of septa per millimeter hyphae compared to WT. Samples were incubated for 2 days on BMM-coated glass slides (n = 4).
Absolute values for verification of the genetic interactions
| Strain . | Ascogonia/cm2 . | Septa/mm hyphae . |
|---|---|---|
| Wild-type | 142.9 ± 10.7 | 19.0 ± 1.9 |
| ΔSmkin3 | 8.8 ± 1.7 | 5.0 ± 0.7 |
| Δpro22 | 109.3 ± 8.2 | 18.4 ± 1.9 |
| pro11 | 97.8 ± 4.2 | 18.0 ± 2.4 |
| Δpro11 | 6.9 ± 3.0 | 13.4 ± 2.2 |
| Expected ΔSmkin3/pro11 | 5.7 | 4.8 |
| ΔSmkin3/pro11 | 0 | 0 |
| Expected ΔSmkin3Δpro11 | 0.4 | 3.4 |
| ΔSmkin3Δpro11 | 0 | 0 |
| Expected Δpro22/pro11 | 74.7 | 17.3 |
| Δpro22/pro11 | 105.0 ± 8.9 | 17.7 ± 2.2 |
| Expected Δpro22Δpro11 | 5.2 | 12.9 |
| Δpro22Δpro11 | 6.6 ± 0.9 | 13.9 ± 1.9 |
| Strain . | Ascogonia/cm2 . | Septa/mm hyphae . |
|---|---|---|
| Wild-type | 142.9 ± 10.7 | 19.0 ± 1.9 |
| ΔSmkin3 | 8.8 ± 1.7 | 5.0 ± 0.7 |
| Δpro22 | 109.3 ± 8.2 | 18.4 ± 1.9 |
| pro11 | 97.8 ± 4.2 | 18.0 ± 2.4 |
| Δpro11 | 6.9 ± 3.0 | 13.4 ± 2.2 |
| Expected ΔSmkin3/pro11 | 5.7 | 4.8 |
| ΔSmkin3/pro11 | 0 | 0 |
| Expected ΔSmkin3Δpro11 | 0.4 | 3.4 |
| ΔSmkin3Δpro11 | 0 | 0 |
| Expected Δpro22/pro11 | 74.7 | 17.3 |
| Δpro22/pro11 | 105.0 ± 8.9 | 17.7 ± 2.2 |
| Expected Δpro22Δpro11 | 5.2 | 12.9 |
| Δpro22Δpro11 | 6.6 ± 0.9 | 13.9 ± 1.9 |
| Strain . | Ascogonia/cm2 . | Septa/mm hyphae . |
|---|---|---|
| Wild-type | 142.9 ± 10.7 | 19.0 ± 1.9 |
| ΔSmkin3 | 8.8 ± 1.7 | 5.0 ± 0.7 |
| Δpro22 | 109.3 ± 8.2 | 18.4 ± 1.9 |
| pro11 | 97.8 ± 4.2 | 18.0 ± 2.4 |
| Δpro11 | 6.9 ± 3.0 | 13.4 ± 2.2 |
| Expected ΔSmkin3/pro11 | 5.7 | 4.8 |
| ΔSmkin3/pro11 | 0 | 0 |
| Expected ΔSmkin3Δpro11 | 0.4 | 3.4 |
| ΔSmkin3Δpro11 | 0 | 0 |
| Expected Δpro22/pro11 | 74.7 | 17.3 |
| Δpro22/pro11 | 105.0 ± 8.9 | 17.7 ± 2.2 |
| Expected Δpro22Δpro11 | 5.2 | 12.9 |
| Δpro22Δpro11 | 6.6 ± 0.9 | 13.9 ± 1.9 |
| Strain . | Ascogonia/cm2 . | Septa/mm hyphae . |
|---|---|---|
| Wild-type | 142.9 ± 10.7 | 19.0 ± 1.9 |
| ΔSmkin3 | 8.8 ± 1.7 | 5.0 ± 0.7 |
| Δpro22 | 109.3 ± 8.2 | 18.4 ± 1.9 |
| pro11 | 97.8 ± 4.2 | 18.0 ± 2.4 |
| Δpro11 | 6.9 ± 3.0 | 13.4 ± 2.2 |
| Expected ΔSmkin3/pro11 | 5.7 | 4.8 |
| ΔSmkin3/pro11 | 0 | 0 |
| Expected ΔSmkin3Δpro11 | 0.4 | 3.4 |
| ΔSmkin3Δpro11 | 0 | 0 |
| Expected Δpro22/pro11 | 74.7 | 17.3 |
| Δpro22/pro11 | 105.0 ± 8.9 | 17.7 ± 2.2 |
| Expected Δpro22Δpro11 | 5.2 | 12.9 |
| Δpro22Δpro11 | 6.6 ± 0.9 | 13.9 ± 1.9 |
A functional ATP-binding pocket of SmKIN3 is required for fungal development
Previous mutation analysis of conserved MST kinases showed that substitution of either of two conserved lysine residues by arginine residues results in variants lacking kinase activity in vitro (Lin et al. 2001; Huang et al. 2002). In an amino acid sequence alignment of SmKIN3 with diverse MST kinases, we found that the abovementioned lysine residues are highly conserved and located within an ATP-binding pocket (Figure 7A). To investigate whether catalytic activity of SmKIN3 is essential for its function in fungal development, we generated two single-point mutants at amino acid codons 26 or 39 of the Smkin3 gene (Smkin3K26R and Smkin3K39R, respectively). These mutations result in lysine to arginine residue substitutions, which are depicted in Figure 7A. Ectopic integration of these mutant genes into ΔSmkin3sos1− resulted in recombinant strains, which were designated either ΔSmkin3::kin3K26R or ΔSmkin3::kin3K39R. As shown in Figure 7B, ΔSmkin3::kin3K26R was phenotypically similar to wild-type. However, transfer of SmkinK39R into ΔSmkin3sos1− resulted in transformants with severe developmental defects, clearly distinct from ΔSmkin3sos1− and more similar to ΔSmkin3/pro11 and ΔSmkin3Δpro11. This result provides clear evidence for the requirement of SmKIN3 kinase activity. To obtain homokaryotic AIs, ΔSmkin3::kin3K39R primary transformants were crossed with fus1-1. AIs from the recombinant perithecia were selected for nourseothricin resistance provided by a p4490-K39R plasmid. However, none of the isolated ascospores were able to grow on corresponding medium. Thus, we assume Smkin3K39R to be a dosage-dependent lethal mutation that cannot be tolerated in a homokaryotic background, but only in a heterokaryotic recipient one.
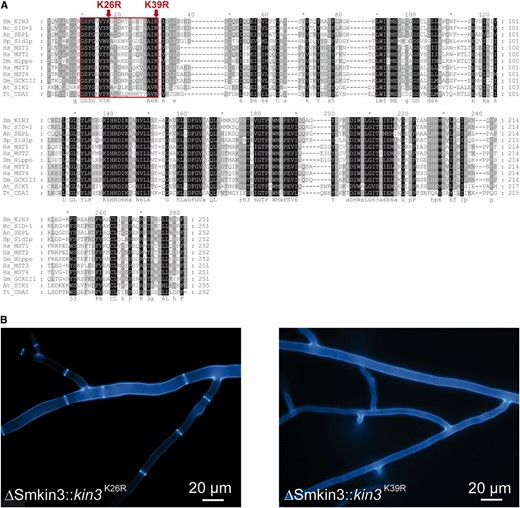
Conserved lysine 39 within the ATP-binding pocket is essential for the biological function of SmKIN3. (A) Alignment of the conserved catalytic domains of SmKIN3 homologs from different organisms. The frame highlights the ATP-binding site. Two lysine to arginine substitutions of SmKIN3 are indicated by arrows. (B) Hyphal septation of transformants carrying either the K26R or K39R mutation, namely ΔSmkin3::kin3K26R or ΔSmkin3::kin3K39R, respectively. Mycelium samples were grown on solid malt–cornmeal fructification medium for 2 days and stained with Calcofluor White M2R. An, A. nidulans; At, Arabidopsis thaliana; Dm, D. melanogaster; Hs, Homo sapiens; Nc, N. crassa; Sc, Sa. cerevisiae; Sm, Sordaria macrospora; Sp, Sc. pombe; Tt, Tetrahymena thermophila.
Discussion
Since the discovery of the first fungal striatin homolog PRO11 in S. macrospora, this fungus has been used as a model to study the related STRIPAK signaling complex (Pöggeler and Kück 2004; Bloemendal et al. 2012). The core of the STRIPAK complex is highly conserved and its architecture in S. macrospora resembles that in animals. PP2AA and PP2Ac1 are scaffolding and catalytic subunits of protein phosphatase 2A, respectively. PRO11 represents the B’’’-regulatory subunit of PP2A that recruits a striatin-interacting protein 1/2 homolog PRO22, an SLMAP homolog PRO45, and a kinase activator SmMOB3 to the STRIPAK complex (Kück et al. 2016). SmKIN3 and SmKIN24 from S. macrospora were the first STRIPAK-associated GCKs ever described in fungi. They both were shown to interact directly with PRO11 (Frey et al. 2015), and our comparative sequence analysis reveals a phylogenetic relationship between SmKIN3 and the GCK-III subfamily of striatin-associated kinases from animals. Further, SmKIN3 is an ortholog of other characterized fungal SIN kinases, namely Sid1p from Sc. pombe and SEPL from A. nidulans. From this, we predict SmKIN3 to be part of SIN. The phenotypic analysis of the ΔSmkin3 deletion mutant has shown a lack of septation, typical phenotypes of SIN mutants in other fungal systems. Thus, the role of SmKIN3 as a possible link between the fungal STRIPAK complex and SIN is very intriguing.
In this study, we provide detailed insight into SmKIN3 kinase function in diverse fungal developmental processes, such as septation and fusion of hyphae, as well as sexual propagation. Sterility, as well as hyphal fusion defects, are significant features already observed in diverse developmental mutants from S. macrospora, including those having a defect in STRIPAK subunits (Kück et al. 2016). Another feature, which was observed in ΔSmkin3sos1− and in some but not all STRIPAK mutants—namely pro22, Δpro22, Δpp2Ac1, and Δpro11 (Bloemendal et al. 2010; Beier et al. 2016, Figure 5)—is the generation of aseptate ascogonia. We have previously speculated that the lack of septa prevents the accumulation of signaling molecules, and thus, prevents further sexual development (Bloemendal et al. 2010). A further consequence of this developmental arrest may be the formation of overly elongated ascogonial coils, as observed in Δpro22 and in this study for ΔSmkin3sos1− (Bloemendal et al. 2012). As described previously, protoperithecium development starts with the enveloping of an ascogonium with hyphae, which grow from ascogonium-bearing and adjacent hyphae (Mai 1976; Lord and Read 2011). We propose that mistargeting of cell wall material and a defect of cell wall synthesis may initiate the extreme elongation of ΔSmkin3sos ascogonia instead of proper protoperithecium formation.
The phenotype described here for the ΔSmkin3sos1− mutant is significantly different from the N. crassa strain lacking the Smkin3 homolog sid-1. Deletion of sid-1 did not affect sexual propagation at all and triggered only a mild septation defect. Interestingly, the mutant strain generated aseptate hyphae after 18 hr of incubation, but after 36 hr, those reverted to the wild-type phenotype (Heilig et al. 2013). In contrast, the septation defect of ΔSmkin3sos1− is stable over a longer time interval, which indicates that SmKIN3 is a positive regulator of the septation process. Although there is high sequence similarity between catalytic domains from SmKIN24 and SmKIN3, ΔSmkin24 and ΔSmkin3 deletion strains have distinctly different phenotypes. Deletion of the Smkin24 gene resulted in a developmental arrest at the late stage of the protoperithecium formation, as well as in the hyperseptation of the entire vegetative mycelium (Frey et al. 2015). This result led us to assume that both GCKs regulate septation in distinct ways in S. macrospora.
In Sc. pombe, GCK Sid1p was first discovered in a screen of sid temperature-sensitive mutants (Balasubramanian et al. 1998). The corresponding gene sid1 is essential for viability, and strains lacking sid1 fail to initiate medial ring constriction and septum formation. As a result, elongated multicellular cells are generated that will eventually lyse (Guertin et al. 2000). Sid1p and homologous kinases in other fungi are responsible for septation and cytokinesis as a part of the conserved SIN. The SIN core in fission yeasts consists of GCK Sid1p with kinase adaptor Cdc14p and NDR kinase Sid2p, with a Mob1p kinase activator. This complex is similar to the mammalian MST1/2 and LATS1/2 GCK/NDR Hippo kinase cascade. In addition, fission yeasts and filamentous fungi possess an extra upstream GCK Cdc7p, which can activate Sid1p. Deletion and loss-of-function of SIN subunits in Sc. pombe causes the formation of elongated multicellular cells without septa. Moreover, overexpression, as well as deletion, of negative SIN regulators leads to a hyperseptation phenotype (Krapp and Simanis 2008; Simanis 2015). In our study, a similar effect was also observed for S. macrospora. SIN architecture in N. crassa is very similar to that of Sc. pombe. In N. crassa, GCK CDC-7 activates GCK SID-1, which in turn activates downstream NDR kinase DBF-2 (Heilig et al. 2013). Furthermore, MST-1, another GCK, has a dual function in activating two NDR kinases, namely DBF-2 in the SIN and COT-1 in the morphogenesis Orb6 (MOR) network (Seiler et al. 2006; Heilig et al. 2014). BLAST analysis revealed the presence of all putative SIN subunits in S. macrospora, wherein SmKIN3 is a homolog of SID-1 and SmKIN24 is a homolog of MST-1.
We previously observed that STRIPAK double mutant Δpp2Ac1Δpro22 has a phenotype that indicates only a slight reduction in fitness when compared with the corresponding single mutants. From this analysis, we concluded a positive GI of the corresponding genes (Beier et al. 2016). Here, we attained the same conclusion after analyzing Δpro22/pro11 and Δpro22Δpro11 mutants. However, the double mutants ΔSmkin3/pro11 and ΔSmkin3Δpro11 show a more severe defect than predicted. We consider this as indication of a negative GI, which can be explained when both SmKIN3 and PRO11 belong to different pathways and/or protein complexes. This assumption is consistent with data from N. crassa. There, it was shown that SID-1, the SmKIN3 homolog, is part of the SIN complex (Heilig et al. 2013). Our overall conclusion is that SmKIN3 is also a member of the SIN in S. macrospora and is connected with the STRIPAK complex by interacting with PRO11 (Frey et al. 2015).
The quantitative fitness analysis of double mutants has revealed clear indications of the negative GI of SIN subunit SmKIN3 and STRIPAK subunit PRO11, identifying their function in distinct biological pathways that are essential for septation and sexual development. However, the weak physical interaction between SmKIN3 and PRO11, demonstrated by Frey et al. (2015), hints toward the enzyme–substrate relationship. Whether PRO11 is a phosphorylation target of SmKIN3, or SmKIN3 is being recruited to the STRIPAK by PRO11 and dephosphorylated by PP2Ac, will be the subject of future studies.
To the best of our knowledge, this is the first study to provide in vivo evidence that GCK activity is essential for proper fungal cellular development, namely hyphal septation, fertility, and spore germination. Substitution of the conserved lysine 39 to arginine, which was predicted to alter the ATP-binding pocket of the kinase domain, resulted in a kinase-dead variation of SmKIN3. Moreover, since we never succeeded in isolating homokaryotic Smkin3K39R strains, Smkin3K39R seems to be a recessive lethal mutation. Thus, we propose that lethality is dependent on the dosage of the mutated protein SmKIN3K39R in the cell. We also propose that such a mutation is tolerated in dikaryotic primary transformants, which carry nuclei from the recipient. Due to ectopic integration of Smkin3K39R and its expression from the weak native promoter in some nuclei, the number of kinase-dead molecules remains low enough to enable viability of the fungus. Although the fitness of the recipient strain (ΔSmkin3sos1−) is already reduced, the obtained transformants exhibit an even more reduced fitness. Expression of Smkin3K39R in all nuclei prevents basic survival functions, such as the germination of ascospores. An example of a point mutation that causes more severe developmental defects when compared to the complete deletion of the same gene is a phosphatase-dead mutant of PP2Ac1 in S. macrospora. Expression of the inactive phosphatase version in Δpp2Ac1 was not only unable to restore the developmental defect of the deletion strain, but also decreased its growth rate and the size of generated protoperithecia. However, the viability of AIs was not affected (Beier et al. 2016). We speculate that lack of SmKIN3 in the SIN signaling cascade is at least partially compensated by another kinase. A possible candidate is SmKIN24, since its N. crassa homolog MST-1 was previously shown to have an additional function in SIN (Heilig et al. 2014). Similarly, dual-function GCKs have been reported in animal systems. For example, STRIPAK-associated MAP4K4/6 in human and Hppy/Msn in fly are capable of Hippo pathway NDR kinase phosphorylation (Meng et al. 2015; Zheng et al. 2015). However, in S. macrospora, the physical presence of a kinase-dead SmKIN3 in a signaling cascade may prevent such a bypass. Indeed, kinase-dead SmKIN3 might bind all free target molecules, preventing interaction with an alternative activator (Figure 8).
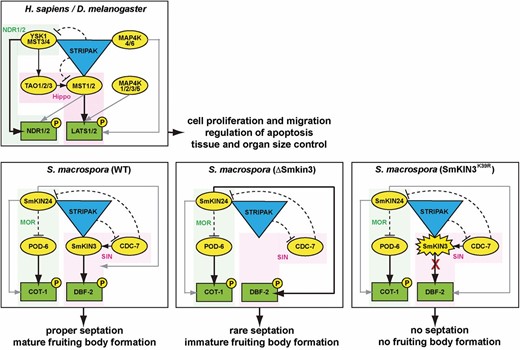
Schematic representation of the GCK signaling network in animals and fungi. The animal model is generated based on the data obtained from the H. sapiens and D. melanogaster studies (referenced in the Introduction). The S. macrospora model is based on the data from Sc. pombe (Singh et al. 2011) and N. crassa (Heilig et al. 2013, 2014), as well as previous data from S. macrospora (Frey et al. 2015) and this study. STRIPAK corresponds to a striatin-interacting phosphatase and kinase complex. GCKs are represented as yellow ovals. NDR kinases are represented as green rectangles. Corresponding pathways are highlighted in green/pink. Solid black arrows indicate canonical kinase activation; solid gray arrows indicate optional bypass kinase activation; and dashed blunt-ended black lines indicate negative regulation. GCK, germinal center kinase; MOR, morphogenesis Orb6; NDR, nuclear Dbf2-related; SIN, septation initiation network; STRIPAK, striatin-interacting phosphatases and kinases.
In this study, we made the unexpected observation that dependent on the genetic background, fertility is restored in sterile ΔSmkin3 mutants. Moreover, we provide strong evidence that a suppressor mutation derived from strain S70823 is responsible for this phenotype. Genetic suppression, as a particular case of a positive GI, occurs when a phenotype caused by a mutation in one gene is rescued by a mutation in another one (Costanzo et al. 2011; van Leeuwen et al. 2016). Mechanistic suppression classes, previously described in Sa. cerevisiae, are very diverse. Mutation within one complex can be suppressed by the gain-of-function of another member of the same complex, whereas the loss-of-function of the negative regulator in the same pathway or a specific mutation would allow another protein to acquire a novel function and bypass the query mutation. In some rare cases, suppression can also be a result of alternative mRNA processing and protein degradation (van Leeuwen et al. 2016). In the mitotic exit network (MEN), the homolog of the SIN from Sa. cerevisiae, multiple suppressor mutants are known as telophase arrest bypassed (tab). One of the tab mutations results in gain-of-function of GCK Cdc15p, while other tabs are loss-of-function mutations in MEN negative regulators, such as GTPase-activating proteins Bub2p and Bfa1p, or PP2A subunits Cdc55p and Sit4p (Shou and Deshaies 2002). Similarly, a loss-of-function mutation in the Cdc55p homolog from N. crassa is suppressed by phosphomimetic mutations in COT-1, the NDR kinase of the MOR network (Shomin-Levi and Yarden 2017). In A. nidulans, loss-of-function mutations in genes coding for GCK SEPH and NDR kinase activator MOBA were suppressed by random mutations in five gene loci, called suppressor of MobA (smo) A–E (Kim et al. 2006). Further investigation of smoA and smoB revealed their putative function in a phosphoribosyl pyrophosphate synthesis pathway, whose function in the SIN remains ambiguous (Zhong et al. 2012). In the case of ΔSmkin3sos1+, we propose that sos1 is a genetic suppressor of the phenotype caused by the Smkin3 deletion. Wherever the suppressor mutation might be located, its knowledge will improve our currently incomplete understanding of the architecture of eukaryotic signaling pathways and their regulation.
Finally, this study suggests that SmKIN3 links both the SIN and STRIPAK complex, thereby regulating multiple cellular processes. Further affinity chromatography studies combined with mass spectrometry analysis are currently underway to identify interaction partners of SmKIN3.
Acknowledgments
We thank Susanne Schlewinski and Ingeborg Godehardt for their excellent technical assistance, Tim Dahlmann and Dominik Terfehr for their help in the bioinformatics analysis, M. Nowrousian for fruitful discussions, and G. Frenßen-Schenkel for assistance with graphical work. This work was funded by grants KU517/11-2, KU517/16-1, and PO523/4-2 from the Deutsche Forschungsgemeinschaft (Bonn Bad-Godesberg, Germany). D.R. received a scholarship from the Friedrich-Ebert-Stiftung (Bonn, Germany).
Footnotes
Supplemental material available at Figshare: https://doi.org/10.25386/genetics.6741290.
Communicating editor: A. Gladfelter
Literature Cited
Beier, A. M., 2017 The STRIPAK-associated catalytic subunit 1 of protein phosphatase 2A governs sexual development in the filamentous fungus Sordaria macrospora. Dissertation, Ruhr-Universität Bochum, Germany.
Rech, C., 2007 Molekulargenetische Charakterisierung des pro22-Gens aus Sordaria macrospora: die funktionelle Beteiligung von PRO22 an der Zelldifferenzierung bei Ascomyceten. Dissertation, Ruhr-Universität Bochum, Germany



