-
PDF
- Split View
-
Views
-
Cite
Cite
Michael F Scott, Sarah P Otto, Haploid Selection Favors Suppressed Recombination Between Sex Chromosomes Despite Causing Biased Sex Ratios, Genetics, Volume 207, Issue 4, 1 December 2017, Pages 1631–1649, https://doi.org/10.1534/genetics.117.300062
Close - Share Icon Share
Abstract
To date, research on the evolution of sex chromosomes has focused on sexually antagonistic selection among diploids, which has been shown to be a potent driver of the strata and reduced recombination that characterize many sex chromosomes. However, significant selection can also occur on haploid genotypes during less conspicuous life cycle stages, e.g., competition among sperm/pollen or meiotic drive during gamete/spore production. These haploid selective processes are typically sex-specific, e.g., gametic/gametophytic competition typically occurs among sperm/pollen, and meiotic drive typically occurs during either spermatogenesis or oogenesis. We use models to investigate whether sex-specific selection on haploids could drive the evolution of recombination suppression on the sex chromosomes, as has been demonstrated for sex-specific selection among diploids. A potential complication is that zygotic sex-ratios become biased when haploid selected loci become linked to the sex-determining region because the zygotic sex ratio is determined by the relative number and fitness of X- vs. Y-bearing sperm. Despite causing biased zygotic sex-ratios, we find that a period of sex-specific haploid selection generally favors recombination suppression on the sex chromosomes. Suppressed recombination is favored because it allows associations to build up between haploid-beneficial alleles and the sex that experiences haploid selection most often (e.g., pollen beneficial alleles become strongly associated with the male determining region, Y or Z). Haploid selected loci can favor recombination suppression even in the absence of selective differences between male and female diploids. Overall, we expand our view of the sex-specific life cycle stages that can drive sex chromosome evolution to include gametic competition and meiotic drive. Based on our models, sex chromosomes should become enriched for genes that experience haploid selection, as is expected for genes that experience sexually antagonistic selection. Thus, we generate a number of predictions that can be evaluated in emerging sex chromosome systems.
IN organisms with diploid genetic sex determination, recombination is typically suppressed between the X and Y chromosomes, or Z and W chromosomes. Suppressed recombination appears to begin near the sex-determining region (SDR) and then expand to include larger segments of each sex chromosome (Bergero et al. 2007; Nam and Ellegren 2008; Lemaitre et al. 2009; Wang et al. 2012; Charlesworth 2013). In the absence of recombination, the sex-limited chromosome (Y or W) accumulates deleterious mutations and rearrangements within the nonrecombining region, and “genetic degeneration” occurs (Rice 1996; Charlesworth and Charlesworth 2000; Bachtrog 2006; Marais et al. 2008). Thus, the selective forces driving reduced recombination on sex chromosomes are fundamental to our understanding of sex chromosome evolution.
Typically, selective differences between males and females have been evoked to explain the suppression of recombination around established sex-determining regions (Fisher 1931; Bull 1983; Rice 1987). Considering species with separate diploid sexes, Charlesworth and Charlesworth (1980) showed that loci where males and females differ in equilibrium allele frequency due to selection (for example, sexually antagonistic selection) should evolve complete linkage with the sex-determining locus via translocations or fusions. Subsequently, Lenormand (2003) demonstrated that sex differences in allele frequencies at equilibrium are not required in order to favor reduced recombination with the SDR. In fact, recombination suppression can evolve around the SDR even if selection favors the same allele in both sexes, as long as that allele is favored more strongly in one sex than the other. Immler and Otto (2015) considered species, such as mosses and liverworts, where sex is determined genetically during the haploid phase (U and V sex chromosomes), finding that fitness differences between haploid sexes can also drive suppressed recombination around the SDR. In essence, these studies have demonstrated that suppressors of recombination can be favored because they strengthen the association between the sex in which an allele is most favored, and the chromosome that is present in that sex more often, e.g., between male beneficial alleles and the Y or Z and between female beneficial alleles and the X or W (Otto et al. 2011).
While differences in selection between the diploid sexes has attracted the most theoretical and empirical attention, the haploid gametes/gametophytes produced by male and female diploids also experience distinct selective environments. Intense competition typically occurs among pollen and sperm (Mulcahy et al. 1996; Bernasconi 2004; Joseph and Kirkpatrick 2004). To the extent that pollen and sperm success reflects differences in their haploid genotypes, competition among these gametes/gametophytes is qualitatively distinct from selection among diploid males (Immler et al. 2012). In plants, selection among haploid male gametophytes is thought to be pervasive (Skogsmyr and Lankinen 2002; Moore and Pannell 2011; Marshall and Evans 2016); in Arabidopsis, 60–70% of all genes are expressed during the haploid phase (Borg et al. 2009), and pollen-expressed genes exhibit stronger signatures of purifying selection and positive selection (Arunkumar et al. 2013; Gossmann et al. 2014). For agricultural breeding, pollen has been exposed to a variety of selection pressures in vivo and in vitro, including temperature (Clarke et al. 2004; Hedhly et al. 2004), herbicides (Frascaroli and Songstad 2001), metals (Searcy and Mulcahy 1985), water stress (Ravikumar et al. 2003), and pathogens (Ravikumar et al. 2012), resulting in an increased frequency of beneficial genotypes among the diploid sporophytic offspring. In animals, expression during the haploid sperm stage is traditionally thought to be suppressed (Hecht 1998), although recent evidence suggests that postmeiotic gene expression occurs (Zheng et al. 2001; Vibranovski et al. 2010), that hundreds of genes are haploid selected (Joseph and Kirkpatrick 2004), and that haploid selection can impact offspring fitness (Immler et al. 2014; Alavioon et al. 2017). Even without postmeiotic gene expression, meiotic drive can cause strong selection on haploid genotypes. As with gamete/gametophyte competition, meiotic drive is usually sex specific (Úbeda and Haig 2005), with biased segregation occurring either during polar body formation in females (Buckler et al. 1999; Fishman and Saunders 2008; Didion et al. 2015) or during male gamete/spore production (Jaenike 2001; Burt and Trivers 2006; Larracuente and Presgraves 2012).
In this study, we include haploid selection in models for the evolution of recombination, assuming separate diploid sexes and genetic sex determination (XY or ZW). Specifically, we include a period of selection among the gametes/gametophytes produced by one sex, for example, competition among sperm but not among eggs, see Figure 1. Thus, we investigate whether sex differences in the haploid selective environment can cause the evolution of suppressed recombination on sex chromosomes, as with sex differences in diploid selection. One reason to rigorously examine this hypothesis is that haploid selection can cause zygotic sex ratios to become biased. For example, sex-chromosome-linked meiotic drivers can alter the relative frequency of X- vs. Y-bearing gametes (Jaenike 2001). Similarly, sex-chromosome-linkage allows alleles with high pollen fitness to become associated with the Y, such that Y-bearing pollen outcompetes X-bearing pollen, and most zygotes will become males, see Figure 1. Sex ratio biases caused by meiotic drive or gametic competition have been found to favor more equal zygotic sex ratios via the evolution of new sex-chromosomes (Kozielska et al. 2010; Úbeda et al. 2015) or modifications to the haploid selective arena (Hough et al. 2013; Otto et al. 2015). Here, we find that meiotic drive or gametic competition typically favors suppressed recombination on the sex chromosomes, despite causing biased zygotic sex ratios.

XY and ZW diploid genetic sex determination systems. In our model, haploid selection occurs during gamete/gametophyte production (meiotic drive) and/or competition (gametic competition) in one sex. In this case, haploid selection occurs in males, as indicated by the dashed circle. In an XY system, male haploid selection causes the zygotic sex ratio to become biased when X- and Y-bearing gametes/gametophytes have different haploid fitnesses.
Model Background
Recombination evolution on sex chromosomes is usually modeled by considering a locus under selection, the SDR, and another locus that modifies the recombination rate between them. Recombination modifiers may be inversions, fusions, translocations, hotspot mutations, and changes to genes involved in double-strand breaks and repairs. A general model therefore includes three loci and the recombination rates between them, which is typically too complex to interpret without further simplifying assumptions (Otto and Day 2007). Lenormand (2003) assumed that the recombination rates between these loci are large relative to selection, such that the linkage disequilibria among loci equilibrate on a faster timescale than changes in allele frequencies (a “quasi-linkage equilibrium” approximation). This analysis is most appropriate for selected loci that are far from the SDR on sex chromosomes and when modifiers of recombination are weak and loosely linked (e.g., autosomal modifiers of recombination machinery). Charlesworth and Charlesworth (1980) assumed that the selected locus is initially autosomal and then considered fusions with (or translocations to) the SDR, where their analysis assumed these rearrangements became closely linked to the selected locus. Their model also corresponds to modifications on sex chromosomes (e.g., inversions) that change the recombination rate with the SDR from a very high to a very low level. Finally, Otto (2014) considered modifiers of recombination between the SDR and selected loci when the linkage between them is initially very tight.
Here, we study recombination evolution in a manner akin to Charlesworth and Charlesworth (1980) and Otto (2014), except that we include a period of selection upon haploid genotypes of one sex (gametic competition and/or meiotic drive). The model of Lenormand (2003) is very general and allows a period of gametic competition (assuming weak linkage); he recognizes but does not discuss the potential of such sex-specific gametic competition to favor suppressed recombination on sex chromosomes. The models we develop here, in which haploid selected loci and the SDR can become tightly linked, are particularly significant because strong associations between haploid selected alleles and the SDR (that can build up when linkage is tight) will cause zygotic sex ratios to become strongly biased.
Model
We consider a modifier model in which the recombination rate between a locus under selection (selected locus, A, with alleles A and a) and the SDR depends on the genotype at the modifier locus (M, with alleles M and m). In our model, haploids of one sex experience selection according to their A locus genotype. The appropriate nomenclature for the haploid stage of animals and plants is different. For simplicity, we will use the animal terms “egg,” “sperm,” and “gamete”; in angiosperms, the appropriate terms for these stages would be “embryo sac,” “pollen,” and “gametophyte,” respectively. We assume that the number of eggs fertilized does not depend on the strength of haploid selection (i.e., that there is no sperm limitation). Our model includes both “gametic competition” and “meiotic drive” in one sex; we use the term “haploid selection” to encompass both selective processes. In the case of gametic competition (e.g., sperm competition), all haploids produced by one sex compete before mating. In the case of meiotic drive, after recombination, a fraction α of spores/gametes produced by an heterozygote will inherit the A allele, whereas spores/gametes inherit the a allele (overall fertility is assumed to be unaffected). Therefore, the main distinction between meiotic drive and gametic competition in our model is that haploid selection via meiotic drive only occurs among gametes/spores produced by a heterozygote, whereas all gametes/gametophytes compete for fertilization during gametic competition. Under monogamous mating, gametic competition among sperm is equivalent to male meiotic drive because haploid allele frequencies would only change during matings with a heterozygous male.
Zygotes develop as diploid males or females depending on their genotype at the SDR. Diploid genetic sex determination systems are either male heterogametic (females XX and males XY) or female heterogametic (females ZW and males ZZ). There are therefore two important asymmetries in the model, the sex in which haploid selection occurs and the sex that is heterogametic. For simplicity, we primarily describe XY sex determination with male haploid selection (sperm competition or meiotic drive during spermatogenesis), although we also present results for ZW sex determination and male haploid selection. By interchanging “male” and “female” labels, this pair of models covers all four cases of haploid selection in males or females and heterogamety in males or females.
After a period of selection among diploid males and females (Table 1), recombination occurs to produce haploid gametes/gametophytes. Because females are homozygous at the SDR (with XY sex determination), the only recombination event of consequence in females is between the A and M locus, which occurs at rate In males, recombination similarly occurs between the selected locus A and the modifier locus M at rate Recombination can also occur between the SDR and the A locus in males; this recombination rate is controlled by the modifier locus, and is given by where is the genotype at the M locus ( or ), allowing this recombination rate to evolve. Recombination events between the SDR and M locus in males occur at rate ρ. Note that and ρ only impact offspring genotypes in males that are heterozygous at the M locus, so we take their values to be those in males in cases where the modifier has broader effects. Because we have three loci and three recombination parameters, any ordering of the loci or type of modifier (genic, inversion, and fusion) can be modeled with appropriate choices of ρ, and see Table A1. We track the frequencies of and genotypes among female eggs, male X-bearing sperm, and male Y-bearing sperm separately to allow sex-specific allele frequencies and disequilibria. The recursion equations describing the change in genotype frequencies after a single generation of this life cycle are provided in Appendix A.1.
Sex- and stage-specific selection terms
| Diploid Selection . | Male Fitness . | Female Fitnessa . |
|---|---|---|
| AA | ||
| Aa | ||
| aa |
| Diploid Selection . | Male Fitness . | Female Fitnessa . |
|---|---|---|
| AA | ||
| Aa | ||
| aa |
| Gametic Competition . | Male fitness . | Female fitness . |
|---|---|---|
| A | 1 | |
| a | 1 |
| Gametic Competition . | Male fitness . | Female fitness . |
|---|---|---|
| A | 1 | |
| a | 1 |
| Transmission During Meiosis . | Males . | Females . |
|---|---|---|
| A | 1/2 | |
| a | 1/2 |
| Transmission During Meiosis . | Males . | Females . |
|---|---|---|
| A | 1/2 | |
| a | 1/2 |
When assuming weak selection, we assume s⚥, , and are small.
| Diploid Selection . | Male Fitness . | Female Fitnessa . |
|---|---|---|
| AA | ||
| Aa | ||
| aa |
| Diploid Selection . | Male Fitness . | Female Fitnessa . |
|---|---|---|
| AA | ||
| Aa | ||
| aa |
| Gametic Competition . | Male fitness . | Female fitness . |
|---|---|---|
| A | 1 | |
| a | 1 |
| Gametic Competition . | Male fitness . | Female fitness . |
|---|---|---|
| A | 1 | |
| a | 1 |
| Transmission During Meiosis . | Males . | Females . |
|---|---|---|
| A | 1/2 | |
| a | 1/2 |
| Transmission During Meiosis . | Males . | Females . |
|---|---|---|
| A | 1/2 | |
| a | 1/2 |
When assuming weak selection, we assume s⚥, , and are small.
In our first analysis, we assume that selection is weak relative to the initial recombination rate (), such that allele frequency differences between males and females are initially small. We then evaluate the spread of modifiers of recombination (m) that cause recombination rates to become very small (assuming ρ, and are all small). These modifiers could be translocations or fusions from autosomes to sex chromosomes or, if the selected locus (A) begins on the sex chromosome, inversions or expansions of the nonrecombining region. We assume that chromosomes are still able to pair regularly with their homologs during meiosis, and we do not include any direct selection on the modifier (e.g., meiotic drive between acrocentric and metacentric chromosomes following a fusion, Yoshida and Kitano 2012).
Summary of cases considered, simplifying assumptions, and results ( indicates invasion by m)
| Selection Weak Relative to (s⚥, , of Order ) . | |
|---|---|
| Assumptions: R⚥ | if |
| if | |
| No sex differences in fitness in diploids (), variation maintained by ploidally antagonistic selection and/or overdominance | |
| Assumptions: R⚥ | if |
| if | |
| SDR-A linkage tight ( and of order ε) | |
| Assumptions: Arbitrarily assume A allele nearly fixed on Y | Typically (but not always) if |
| Necessary (not sufficient) conditions for when are that and that and/or | |
| Selection Weak Relative to (s⚥, , of Order ) . | |
|---|---|
| Assumptions: R⚥ | if |
| if | |
| No sex differences in fitness in diploids (), variation maintained by ploidally antagonistic selection and/or overdominance | |
| Assumptions: R⚥ | if |
| if | |
| SDR-A linkage tight ( and of order ε) | |
| Assumptions: Arbitrarily assume A allele nearly fixed on Y | Typically (but not always) if |
| Necessary (not sufficient) conditions for when are that and that and/or | |
| Selection Weak Relative to (s⚥, , of Order ) . | |
|---|---|
| Assumptions: R⚥ | if |
| if | |
| No sex differences in fitness in diploids (), variation maintained by ploidally antagonistic selection and/or overdominance | |
| Assumptions: R⚥ | if |
| if | |
| SDR-A linkage tight ( and of order ε) | |
| Assumptions: Arbitrarily assume A allele nearly fixed on Y | Typically (but not always) if |
| Necessary (not sufficient) conditions for when are that and that and/or | |
| Selection Weak Relative to (s⚥, , of Order ) . | |
|---|---|
| Assumptions: R⚥ | if |
| if | |
| No sex differences in fitness in diploids (), variation maintained by ploidally antagonistic selection and/or overdominance | |
| Assumptions: R⚥ | if |
| if | |
| SDR-A linkage tight ( and of order ε) | |
| Assumptions: Arbitrarily assume A allele nearly fixed on Y | Typically (but not always) if |
| Necessary (not sufficient) conditions for when are that and that and/or | |
In our second analysis, following Otto (2014), we assume that the A locus begins at equilibrium and in tight linkage with the SDR ( and ρ are on the order of a small term, ε). We then consider whether any modifiers can invade that increase this recombination rate slightly (where the change in recombination rate, is on the order of ε). The recombination rate between the modifier locus and the selected locus ( and ) is not constrained. This analysis focuses on the final stages of sex chromosome evolution, asking when complete recombination suppression is favored or not. Table 2 summarizes the results of these analyses.
Results
In a population fixed for the M allele, the frequencies of the A allele among X-bearing eggs, X-bearing sperm, and Y-bearing sperm are given by and respectively. The frequency of Y-bearing sperm among all sperm before gametic competition can deviate from due to meiotic drive, and is given by q. The spread of a rare mutant, m, that changes the recombination rate, can be evaluated using the leading eigenvalue, λ, of the system described by Equations (A.1c), (A.1d), (A.2c), (A.2d), (A.3c), and (A.3d). We first consider modifiers that completely suppress recombination. (In Appendix A.2B, we consider cases where carriers of the recombination suppressor have small, but nonzero, recombination rates, such that modifier alleles can recombine onto alternative A allele and SDR backgrounds, which tends to slow their spread.) Finally, we consider arbitrarily linked recombination modifiers and assume close linkage between A and the SDR.
Suppressors of recombination
Equilibrium allele frequencies:
Equation (1) and inequality (2) depend on the frequency of the A and a alleles. If either allele were fixed, recombination would have no effect, and recombination modifiers would be neutral. Furthermore, because recombination only occurs in double heterozygotes, suppressors of recombination generally have a larger effect when the frequency of heterozygous males is higher (XY– heterozygotes common). An allele that is directionally selected will segregate for a relatively brief time, during which a recombination modifier would have to arise and/or experience selection. We therefore focus on longer-lasting polymorphisms at the A locus that are maintained by selection. However, we expect similar results in cases where selection is not balanced. For example, we find below that suppressors of recombination are typically favored when they associate pollen-beneficial alleles (e.g., A) with the Y because the Y experiences pollen competition most often. We would predict that recombination suppressors would be similarly favored while the A allele were spreading to fixation, even if the A and a alleles were neutral during all other life cycle stages.
Invasion conditions:
In addition, Equation (6) shows that recombination suppressors are favored even when polymorphism is not maintained by differences in selection between the diploid sexes ( and such that ). Specifically, recombination suppressors that couple the Y and A allele spread () when the A allele is favored during male gametic competition ( see Equation 6) and/or meiotic drive (see Equations 5 and 6). That is, these recombination suppressors spread when the A allele is favored by haploid selection in males.
It may not be intuitively obvious why an association between the X and the allele that is less fit during meiotic drive or gametic competition should be favored. This result comes from the fact that the a allele is initially maintained at an equilibrium frequency by selection. At equilibrium, selection against a during meiotic drive or sperm competition in males must be balanced by selection in favor of a in diploids (given the equilibrium exists). The X chromosome experiences meiosis in males and sperm competition less often than an autosomal or loosely linked locus. Thus, linked Xa haplotypes are favored because the a allele experiences haploid selection less often and is beneficial during other life cycle stages. More intuitively, Equation (6) indicates that linkage between the Y, which experiences haploid selection most often, and a haploid beneficial allele is favored.
As with previous analyses (Charlesworth and Charlesworth 1980; Charlesworth and Wall 1999; Lenormand 2003), we find that the strength of selection in favor of recombination modifiers is strongest on Y chromosomes because these are always found in only one sex, whereas the X will sometimes be found in males and sometimes in females. In particular, (6) and (7) differ by a factor of once we account for the difference between the probability of linkage arising with the A allele, or the a allele, However, mutations causing linkage with the Y (e.g., fusions) should also arise at a lower rate because there are three times as many X chromosomes as Y chromosomes in the population, such that the overall establishment rate of recombination modifiers is the same on the X and Y, all else being equal (Pennell et al. 2015).
In Figure 3, Figure S3 in File S2, and Figure S4 in File S2, we numerically iterate the recursion Equations A.1–A.3. As expected from the above results, a recombination suppressor spreads and the haploid beneficial allele (A, green and purple curves in Figures) becomes more common on the Y and less common on the X (even without sex-differences in selection among diploids). These numerical simulations and our approximation (Appendix A.2.B) show that incomplete recombination suppressors are also favored by selection. Incomplete recombination suppressors spread less quickly than complete recombination suppressors because associations between loci are disrupted by continuing recombination between the A locus, M locus, and the SDR. Despite the fact that zygotic sex ratios become increasingly biased as recombination declines, recombination suppressors typically spread to fixation and new mutations that further suppress recombination are also favored (e.g., Supplemental Material, Figure S1 in File S2). Below, we consider whether recombination should evolve to become completely suppressed by considering selected loci that are closely linked to the SDR.
ZW sex determination:
Recombination evolution between SDR and closely linked selected loci
Finally, we evaluate the evolution of recombination during the final stages of sex chromosome evolution by considering the evolution of small amounts of recombination around the SDR. As discussed above, linkage allows favorable associations to build up between the SDR and selected loci. Therefore, we generally also expect that recombination rates near the SDR will also evolve to be lower. Considering diploid selection alone, Otto (2014) demonstrated that, while this prediction is usually true, a small amount of recombination around the SDR can be maintained by selection. Otto (2014) showed that certain forms of selection (particularly overdominance in males), combined with the asymmetrical inheritance patterns of sex chromosomes can favor loosely linked modifiers that increase recombination around the SDR. Here, our goal is to evaluate the conditions under which recombination can be selectively maintained when there is also haploid selection and also to confirm whether suppressed recombination generally continues to be favored when linkage is tight. For simplicity, we consider an XY sex determination system and gametic competition (not meiotic drive) among male gametes/gametophytes.
With tight linkage between the SDR and a selected locus, A, the Y chromosome always becomes fixed for one allele or the other (or nearly so if there is some recombination, see Appendix A.3). Without loss of generality, we will assume that selection on the Y favors the A allele, which becomes nearly fixed on the Y. X chromosomes will therefore be paired with a YA haplotype in diploid males; this alters selection experienced by X chromosomes found in diploid males vs. those found in diploid females. For example, the A locus will never be homozygous for the a allele in males but could be in females. When there is a polymorphism maintained, the X can either be fixed for the a allele or be polymorphic (both XA and Xa haplotypes present). In either case, the effect of increasing the recombination rate with the SDR is to produce more Ya and XA haplotypes among sperm. Ya haplotypes always have low fitness given that the Y was originally fixed for the A allele. However, the XA haplotypes produced by recombination can be favored because they are found in male gametes/gametophytes. X-bearing male gametes/gametophytes first experience sperm competition and then necessarily produce females in the next generation (see Figure 1). Thus, the XA haplotypes produced by recombination in males do not experience the same selective environment as X chromosomes from mothers, which do not experience sperm competition and can be inherited by daughters or sons. Interestingly, certain selection regimes favor XA haplotypes in sperm (even if the X is fixed for the a allele), which can counterintuitively favor modifiers that increase recombination around the SDR.
With diploid selection only, increased recombination around the SDR can evolve only if selection in females favors the A allele (which is fixed on the Y) because XA sperm produced by recombination will next be found in a female (Figure 1). For this to occur, selection in males must be overdominant (a necessary, but not sufficient, condition). With overdominance in males, the a allele has the highest fitness on the X chromosome in males because it is always paired with an A allele on the Y. Thus, the a allele can be maintained (or even fixed) on X chromosomes, and yet the A allele can be favored during selection in females. However, with sperm competition, it is possible for increases in recombination to be favored under a wider variety of selective regimes in diploids, including overdominance, underdominance, sexually antagonistic selection and ploidally antagonistic selection. In Appendix A.3, we show that the evolution of increased recombination requires either that the A allele is selected against on the X in males (), and/or that the A allele is favored during male gametic competition (). If the A allele is selected against on X chromosomes in males (), it is possible for it to be favored on X chromosomes in females and yet still maintain the a allele. In addition, XA haplotypes produced by recombination will be found in sperm and, thus, experience gametic competition before becoming diploid females. Therefore, if gametic competition favors the A allele (), XA haplotypes in sperm can have high fitness, easing the conditions under which increased recombination is favored.
Given that XA sperm have an advantage due to male gametic competition and/or high fitness in female diploids, the fitness advantage of XA sperm must outweigh the cost of producing low-fitness Ya sperm. Thus, increased recombination around the SDR only evolves in particular regions of parameter space (Figure A1). In addition, the evolution of increased recombination requires that the modifier is sufficiently loosely linked to the SDR ( and are sufficiently large), e.g., modifiers are autosomal. This allows the modifier to gain the short-term advantage described above while not remaining linked to the selected locus for long (over the long term, an association with XA or Ya is neutral or deleterious when averaged across all backgrounds). Thus, although haploid selection means that overdominance is not required for the selective maintenance of recombination, increased recombination around the SDR still only evolves under a small subset of possible selective regimes. Suppressed recombination is favored in most circumstances (e.g., locally acting recombination suppressors spread, Figure S1 in File S2).
Discussion
Even in predominantly diploid organisms such as animals and angiosperms, there is considerable potential for selection upon haploid genotypes during competition among male gametes (sperm/pollen) and/or meiotic drive. Here, we demonstrate that haploid selection typically favors linkage with the diploid SDR (XY or ZW). Thus, along with selective differences between diploid sexes, selection among haploids could be a potent driver of recombination suppression on sex chromosomes.
In ZW sex determination systems, the zygotic sex ratio is unaffected by haploid selection in males. However, in XY sex determination systems, the number of males and females in each generation depends on the frequency of X and Y gametes after haploid selection in males. Despite this, we find that selection on recombination modifiers is not primarily driven to balance the zygotic sex ratio but to strengthen genetic associations between selected alleles and the SDR when selection differs between males and females in the haploid and/or diploid phase. In fact, the evolution of recombination suppression should lead to zygotic sex ratios becoming biased (typically biased toward males but see Figure 2 and Figure S2 in File S2 for cases where female-bias develops because the allele favored during male haploid selection is even more favored in females).
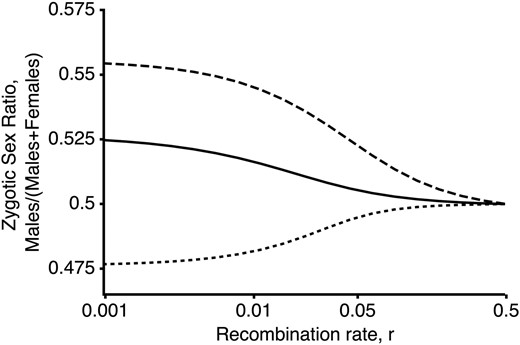
The zygotic sex ratio is biased by linkage between an XY SDR and a locus that experiences competition among male gametes. Here, we plot the zygotic sex ratio at equilibrium assuming that all individuals have the same recombination rate, r (fixed for modifier allele M). Male-biased sex ratios result when the Y becomes associated with alleles conferring high sperm fitness (solid line: dashed line: both with such that selection is ploidally antagonistic). Female-biased sex ratios can, however, arise if the haploid-beneficial allele is also strongly favored in females and becomes associated with the X (dotted line: such that selection in diploids is sexually antagonistic).
Although potentially caused by sex differences in survival, biased sex ratios at flowering are common among dioecious plants with 76/243 species exhibiting significantly male-biased sex ratios and 45/243 exhibiting female-biased ones (Field et al. 2013). We predict that, in the early stages of sex chromosome evolution, recombination suppression should allow associations between the Y and haploid-beneficial alleles leading to male-biased zygotic sex ratios. However, following recombination suppression, the Y-chromosome is expected to accumulate deleterious mutations and deletions, resulting in heteromorphic sex chromosomes (Charlesworth and Charlesworth 2000; Bachtrog 2013). Following Y-chromosome degeneration, Y-bearing gametophytes should have low haploid fitness, leading instead to female-biased zygotic sex ratios (Lloyd 1974; Stehlik and Barrett 2005). Indeed, plant species with heteromorphic rather than homomorphic sex chromosomes tend to have more female biased flowering sex ratios (Field et al. 2013). For example, in Rumex species with heteromorphic sex chromosomes and female biased flowering sex ratios, more intense pollen competition appears to result in more female biased sex-ratios among the progeny (Conn and Blum 1981; Stehlik and Barrett 2006; Field et al. 2012). Thus, while we predict that the Y should typically be associated with the allele that is beneficial during the male haploid stage when there are functional alleles present on both X and Y chromosomes, the overall fitness of the Y chromosome may become reduced due to degeneration. Therefore, the net effect of experimentally manipulating the intensity of haploid selection may depend on the stage of sex chromosome degeneration, as well as the alleles associated with the Y, where we predict that associations between haploid-beneficial alleles and the Y are favored when haploid selection occurs in males. The increasing availability of sex-linked markers should allow sexes to be identified before reproductive maturity in plants (e.g., McKown et al. 2017), thus allowing changes in the sex ratio to be directly evaluated across haploid and diploid phases in species with differing degrees of Y chromosome degeneration and recombination suppression.
The increasing study of both haploid expression profiles (Joseph and Kirkpatrick 2004; Borg et al. 2009) and a broader array of sex chromosome systems (Ming et al. 2011; Charlesworth 2013, 2015; Bachtrog et al. 2014; Vicoso and Bachtrog 2015) provides an excellent opportunity to evaluate whether sex chromosomes are enriched for genes selected during gametic competition, as predicted by our models. While Crowson et al. (2017) did not find evidence for an over-representation of haploid-expressed genes (as identified in Arabidopsis thaliana) on the sex chromosomes of Rumex rothschildianus, G. Sandler and colleagues (personal communication) recently sequenced the pollen transcriptome in R. rothschildianus and R. hastatulus directly and found that sex-linked genes show significantly higher expression in pollen, relative to autosomal genes, particularly so for Y-linked genes. We predict a stronger signal of association with SDRs should occur among loci explicitly shown to exhibit variation in haploid competitive ability (Travers and Mazer 2001) or loci where mutants affect fitness in both haploid and diploid phases (Muralla et al. 2011). Finally, we predict that the strength of gametic competition partly determines whether, and how fast, recombination suppression evolves. Selection on recombination suppression due to sexually antagonistic selection was recently investigated using populations that differ in the strength of sexually antagonistic selection (Wright et al. 2017); we predict that recombination suppression could be similarly correlated with the strength of haploid selection. Evaluating a related hypothesis, Lenormand and Dutheil (2005) correlate heterochiasmy (differences in autosomal recombination between sexes) with the degree of sex-specific haploid selection across species, using outcrossing rate as a proxy for male haploid selection. We would expect a similar pattern for recombination suppression around SDRs rising with the degree of outbreeding and polygamy.
Haploid expression and selection may occur during sperm competition in animals, particularly when sperm are long-lived (Immler et al. 2014; Alavioon et al. 2017). Genes expressed in the testes appear to accumulate on Y and Z chromosomes via translocations (Arunkumar et al. 2009; Mahajan and Bachtrog 2017), but it is not yet clear what proportion of these genes express their haploid vs. diploid genotype in sperm (Namekawa et al. 2006). Nevertheless, haploid selection in animals might occur via meiotic drive, of which there are many known examples (Jaenike 2001; Helleu et al. 2015; Lindholm et al. 2016). Meiotic drive in animals or plants is usually sex specific, either acting during spermatogenesis in males or polar body formation in females (Úbeda and Haig 2005). In the absence of diploid sex differences in selection, we predict that recombination suppression could evolve if it allows alleles favored by meiotic drive to become associated with the sex in which drive occurs (e.g., a male meiotic drive allele and the Y). This result is reflected in some other studies of meiotic drive. For example, reduced recombination is expected to evolve between an X chromosome that experiences drive and another selected locus (Feldman and Otto 1989; Rydzewski et al. 2016), and new male determining alleles can be favored when they appear in linkage to a locus that experiences drive in males (Úbeda et al. 2015). These studies reinforce the view that sex-specific meiotic drive can affect the evolution of recombination, often in a similar manner to sex-specific diploid selection.
Our model of meiotic drive is simple, involving a single locus with two alleles. However, many meiotic drive systems involve an interaction with another locus at which alleles may confer “susceptibility” or “resistance” to meiotic drive (Burt and Trivers 2006; Lindholm et al. 2016). Thus, the dynamics of meiotic drive alleles can be heavily dependent on the interaction between two loci and the recombination rate between them (Haig and Grafen 1991). After recombination suppression has evolved, sex chromosomes may therefore be more likely to facilitate the spread of new meiotic drive alleles (Hurst and Pomiankowski 1991). In addition, divergence between sex chromosomes may provide a particularly large source of suitable targets for drive, and meiotic drive on sex chromosomes is likely to be particularly easy to detect (Burt and Trivers 2006). Finally, meiotic drive can be strong, causing sex ratios to become extremely biased, which can have demographic consequences, especially where population growth depends predominantly on the number of females. This could mean that population extinction is more likely when meiotic drive alleles are linked to the Y (Hamilton 1967). These considerations should be taken into account when interpreting the genomic distribution of meiotic drive alleles.
As in a previous analysis by Otto (2014), we find that a small amount of recombination can be selectively maintained around the SDR. Otto (2014) considered only diploid selection and found that overdominance in males was required for recombination to be selectively maintained. Here, we include a period of competition among haploids and find that increased recombination can be favored with various forms of selection among diploids, including directional selection, sexually antagonistic selection, and ploidally antagonistic selection (Figure A1), as long as the allele fixed on the Y is favored in male haploids and/or females. However, increased recombination is never favored when modifiers of recombination act locally, such that they are also closely linked to the SDR. In a previous study, Feldman and Otto (1989) considered meiotic drive alleles that target the SDR and included no diploid selection. Numerically, Feldman and Otto (1989) also identified cases where loosely linked modifiers favor increased recombination, which parallels our results under gametic competition. Overall, while these dynamics may influence the maintenance of small amounts of recombination around SDRs when polymorphisms with the right form of selection arise (e.g., within the colored regions in Figure A1), suppressed recombination will be favored in most circumstances. Importantly, our results confirm that locally acting recombination modifiers continue to favor reduced recombination, even in cases where the zygotic sex ratio is initially strongly biased. Indeed, with initially biased zygotic sex ratios, the fact that reduced recombination typically evolves generally drives more extreme sex ratio biases (Figure S1 in File S2).
As well as providing several predictions, our model offers a new perspective on drivers of sex chromosome evolution. Traditionally, sex differences in selection are thought to provide the raw material driving recombination suppression on sex chromosomes. However, even where diploid sexes exhibit very few morphological or ecological differences, selection upon haploid genotypes may be very divergent. We have shown that sex-specific meiotic drive or gamete competition should typically also favor suppressed recombination despite causing the zygotic sex ratio to become biased. Consequently, our view of sex chromosome evolution is expanded to incorporate the degree of sex specific selection in haploids along with that in diploids.
Appendix A
In addition to this appendix, in File S1 we provide a supplementary Mathematica file (Wolfram Research Inc. 2017), which can be used to replicate our analyses.
A.1. Recursion Equations
In each generation, we census the genotype frequencies in male and female haploids before gametic competition. At this stage, the frequencies of X-bearing male and female haploids are given by and and the frequency of Y-bearing haploids is given by where the index i specifies genotypes and and Competition then occurs among male haploids according to the A locus allele, k, carried by individuals with genotype i. The genotype frequencies after gametic competition are and where is the mean fitness of haploid sperm. Random mating then occurs between gametes to produce diploid females with genotype at frequency and diploid males at frequency In females, individuals with genotype are equivalent to those with genotype For simplicity we denote the frequency of genotype in females to the average of these frequencies,
A.2. Invasion of Recombination Suppressors
A. General
B. Incomplete recombination suppression
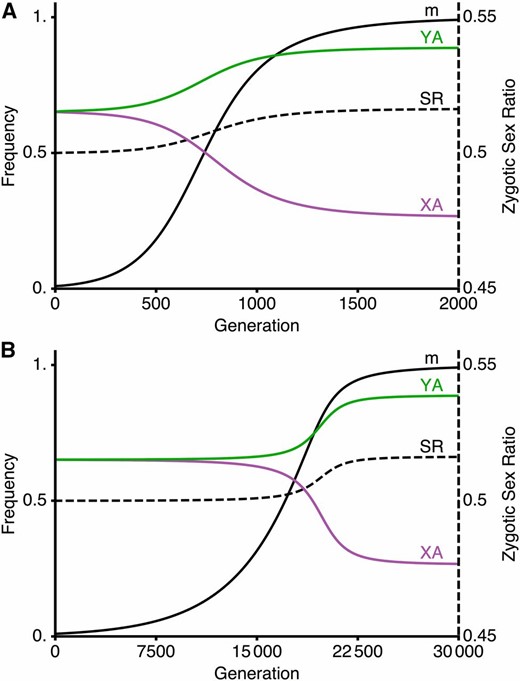
A modifier that reduces the recombination rate between the (A) locus and the SDR can spread to fixation despite causing sex ratios to become biased. We assume that the population initially has loose linkage between the (A) locus and the SDR ( where M is initially fixed), and allow allele frequencies to reach a polymorphic equilibrium. We then introduce a modifier allele m that reduces the recombination rate between (A) locus and the SDR ( ); in generation 0, m is at frequency 0.01 and in linkage equilibrium with M. In (A) the M locus lies between the (A) locus and the SDR (e.g., a fusion) with no crossover interference such that where In (B), the M locus is autosomal and unlinked to either the SDR or (A) locus: The autosomal recombination suppressor spreads more slowly (note change of x-axis scale), but it spreads despite an increasingly biased zygotic sex ratio. Fitness parameters are as in the solid curve in Figure 2. That is, selection is ploidally antagonistic with A favored during gametic competition (see Figure S3 in File S2 and Figure S4 in File S2 for meiotic drive). Curves show the frequencies of the recombination suppression mutant, m, among sperm (black curve), the A allele among Y-bearing sperm (green curve), the A allele among X-bearing sperm (purple curve), and male zygotes (dashed black curve, shown against the scale on the right hand side).
C. Ploidally antagonistic selection
A.3. Invasion of Modifiers that Increase Recombination from an Initially Low Level
We next calculate when and are locally stable for According to the “small parameter theory” (Karlin and McGregor 1972a,b), these stability properties are unaffected by small amounts of recombination between the SDR and A locus, although equilibrium frequencies may be slightly altered. For the A allele to be stably fixed on the Y requires that where the marginal fitnesses of YA and Ya haplotypes are (as above, Table A2) and respectively. Substituting from above, fixation of the A allele on the Y requires that where for equilibrium and for equilibrium Stability of a polymorphism on the X chromosome (equilibrium ) further requires that and Fixation of the a allele on the X (equilibrium ) is mutually exclusive with and requires that and that We will assume that these conditions are met, such that the population has reached a stable equilibrium at the A locus when considering evolution at the modifier locus.
Therefore, increased recombination is only favored if the A allele is favored during selection in females () and/or the A allele is favored during gametic competition (). Only under these conditions is it possible for recombination between the XA and Ya to produce XA sperm that are favored over the short term (in daughters and/or sperm competition, respectively).
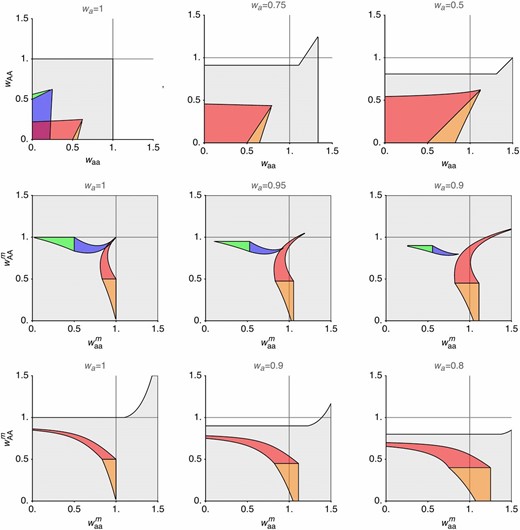
Selection can favor increased recombination between the SDR and a selected locus that is closely linked to the SDR (), even when selection in males is not overdominant. The gray regions show where one or more of the polymorphic equilibria are stable, and thus recombination modifiers can affect fitness. Colored regions show where increased recombination is favored in a population at equilibrium in blue, in green, in red, and in orange. Since this model is symmetrical, red/orange regions can be exchanged with blue/green regions if the labeling of A and a alleles is switched. Across columns, we vary the fitness of a-bearing haploids relative to the A-bearing haploids (). Gray lines show the fitness of heterozygous diploids In the first row, there are no differences in selection between male and female diploids (), where and are varied along the x and y axes, respectively. As haploid selection becomes stronger, increased recombination can evolve with weaker overdominance in diploids, and also with ploidally antagonistic selection (). In the second and third rows, we consider sex differences in selection, where and are varied along the x and y axes (). In the second row, where selection in females is overdominant ( ), increased recombination can be favored when selection is directional (or underdominant) in males, and haploid selection is moderately strong. In the third row, selection favors the A allele in females ( ) and increased recombination can be favored with overdominance in males or sexually antagonistic selection (). For this plot, we assume that the modifier of recombination is unlinked ().
ρ substitutions for different physical arrangements of the loci (assuming no crossover interference)
| Order of loci . | . |
|---|---|
| SDR-A-M | |
| SDR-M-A | |
| A-SDR-M |
| Order of loci . | . |
|---|---|
| SDR-A-M | |
| SDR-M-A | |
| A-SDR-M |
| Order of loci . | . |
|---|---|
| SDR-A-M | |
| SDR-M-A | |
| A-SDR-M |
| Order of loci . | . |
|---|---|
| SDR-A-M | |
| SDR-M-A | |
| A-SDR-M |
Marginal fitnesses of YA and Xa haplotypes and mean fitnesses in the resident population
| Marginal Fitnesses of YA and Xa Haplotypes . |
|---|
| Mean Fitnesses in Resident Population |
| Marginal Fitnesses of YA and Xa Haplotypes . |
|---|
| Mean Fitnesses in Resident Population |
| Marginal Fitnesses of YA and Xa Haplotypes . |
|---|
| Mean Fitnesses in Resident Population |
| Marginal Fitnesses of YA and Xa Haplotypes . |
|---|
| Mean Fitnesses in Resident Population |
Acknowledgments
We thank Jeannette Whitton, Loren Rieseberg, and two anonymous reviewers for helpful comments on this project. This work was supported by Natural Sciences and Engineering Research Council of Canada Discovery grant (RGPIN-2016-03711).
Footnotes
Supplemental material is available online at www.genetics.org/lookup/suppl/doi:10.1534/genetics.117.300062/-/DC1.
Communicating editor: S. Wright
Literature Cited
Wolfram Research, Inc., 2017 Mathematica, version 11. Champaign, IL. Available at: http://www.wolfram.com/mathematica/.



